Abstract
Introduction
Our objective was to systematically review and analyze published data on bone mineral density (BMD) and fracture rates in patients with phenylketonuria (PKU), and relationships between BMD and phenylalanine levels.
Methodology
We searched PubMed, CINAHL and Cochrane databases from January 1966 to November 2013 for studies of spine BMD or fracture in PKU and control subjects. We excluded studies assessing skeletal health by ultrasound or peripheral quantitative computer tomography. Both authors reviewed abstracts for inclusion, and read full text papers to extract data.
Results
Sixteen studies met eligibility criteria. Meta-analysis of 3 studies found that spine BMD was 0.100 g/cm2 lower (95% CI, −0.110, −0.090 g/cm2) in 67 subjects with PKU, compared to 161 controls. Among 6 studies, 20% (53 of 263) of PKU subjects experienced clinical fractures. In the single study with controls, the fracture rate was 2.6 fold higher (95% CI, 1.1–6.1) after age 8 in PKU subjects, compared to healthy sibling controls. When considering a total of 12 studies in 412 subjects, 9 or 75% of studies representing 71% of studied subjects reported no association between phenylalanine levels and BMD.
Summary
Spine BMD is lower in PKU than control subjects, but only one study controlled for smaller body size. Existing studies suggest a clinical fracture rate of 20% among PKU subjects, but fracture rates in controls are lacking. Finally, existing data shows no consistent relationship between phenylalanine levels and BMD. Future studies are needed to clarify the etiology and health consequences of low BMD in PKU.
Keywords: phenylketonuria, bone mineral density, fractures, osteoporosis, systematic review
INTRODUCTION
Phenylketonuria (PKU, OMIM 262600) is an autosomal recessive genetic disorder caused by reduced activity of hepatic phenylalanine hydroxylase (PAH, EC 1.14.16.1), preventing conversion of phenylalanine to tyrosine and resulting in elevated blood phenylalanine concentration with a typical diet (Vockley et al 2014). The disease was first described in 1934 (Fölling 1934), but low-phenylalanine medical foods were not developed until 1953 (Bickel et al 1953). In 1965, widespread neonatal screening for PKU allowed diagnosis and prescription of low-phenylalanine medical formula within days of birth, to prevent or mitigate devastating neurologic sequela of PKU including seizures and cognitive impairment.
As the first children diagnosed through newborn screening tests in the 1960’s reach their late 40’s, studies have described low bone mineral density (BMD) as part of the disorder. It is the purpose of this paper to summarize existing literature describing BMD and fracture rates in patients with PKU, and the association between serum phenylalanine levels and BMD. We conclude by suggesting potential directions for future research on causes of low BMD in patients with PKU.
MATERIALS and METHODS
We conducted this study using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al 2009). The primary study outcomes were spine BMD measurements and fracture rates in subjects with PKU, compared to these measures in age and gender-matched controls. The secondary outcome was the relationship between serum or plasma phenylalanine levels and spine BMD in subjects with PKU.
Eligible studies measured spine BMD via dual energy X-ray absorptiometry (DXA) or reported fractures in children or adults with PKU. BMD studies were only included when researchers also measured BMD in age and gender matched controls. We included studies written in any language. We excluded studies in which skeletal health was assessed by ultrasound or peripheral quantitative computer tomography, as normative data for these measures are not available. Additionally, the International Society for Clinical Densitometry recommends spine and total body (less head) measurements to assess BMD in children (Gordon et al 2008). We also excluded in vitro and murine studies.
To identify relevant articles, we searched PubMed, CINAHL and Cochrane databases from January 1, 1966 to November 18, 2013. We crossed the search term “phenylketonuria” with “bone mineral density,” “osteoporosis” and “fractures”. No limitations were applied to the searches. Two authors read all abstracts to determine eligibility for inclusion (level 1 review). Articles appearing to meet inclusion criteria underwent full text review by two authors (level 2 review). During level 3 review, data were extracted into tables by one author. The second author confirmed data extraction was accurate. Disagreement was resolved by consensus. The bibliographies of all level 3 publications were reviewed to identify additional studies for inclusion.
There was no independent funding for this project. The study review protocol was developed by both authors but was not registered or described on the web.
Data Analysis
We compared spine BMD (g/cm2) in subjects with and without PKU by Forest plot, using R software (version 3.0.2) and the package “meta” (R Core Development Team 2008). Heterogeneity between studies was assessed primarily by inspection of Forest plots and the I2 statistic, with 25%, 50% and 75% indicating low, moderate and high heterogeneity (Higgins et al 2003). Funnel plots (scatterplots of effect versus study size) were reviewed to detect publication bias. We used descriptive statistics to summarize fracture studies, and studies reporting associations between serum or plasma phenylalanine levels and BMD.
RESULTS
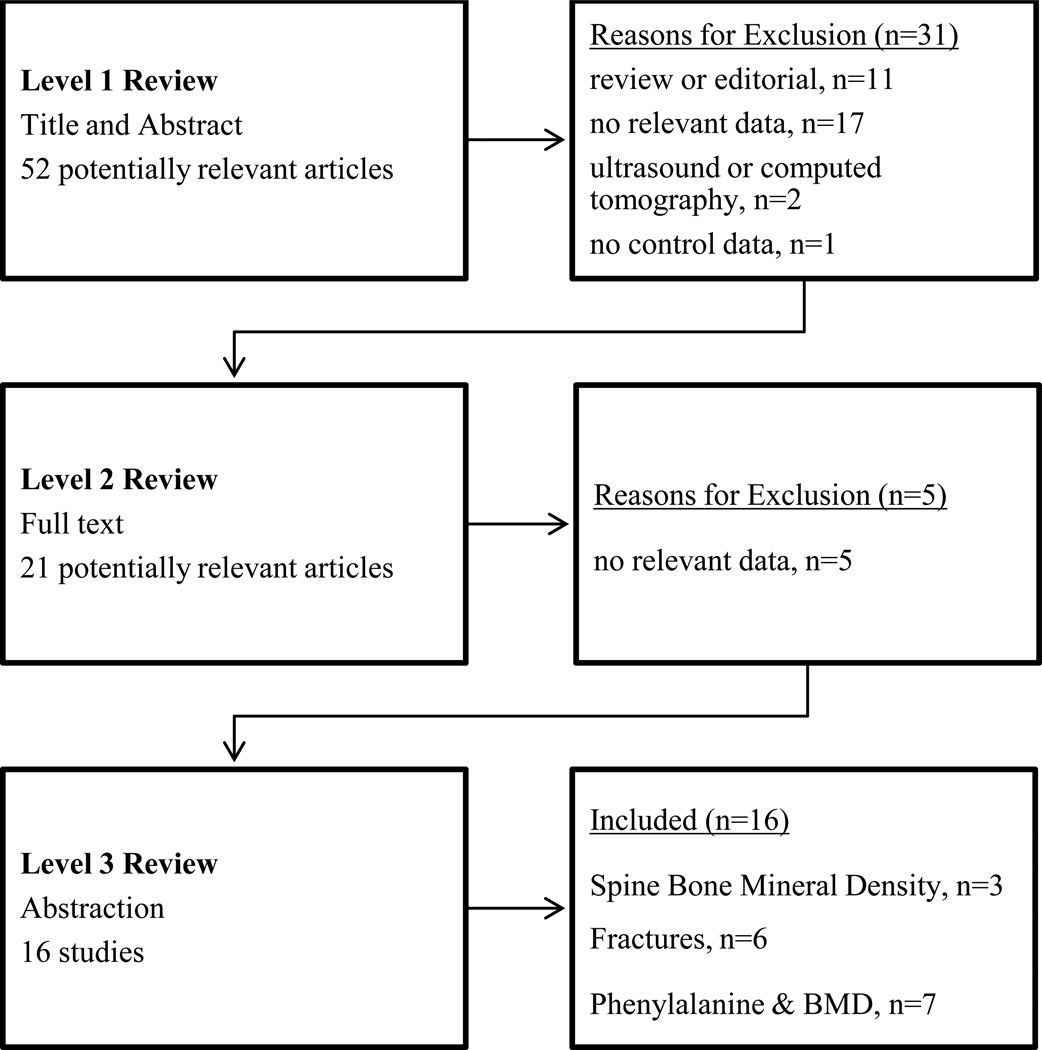
Fifty-two citations were identified from the electronic search and review of bibliographies. From 52 citations undergoing level 1 review (title and abstract), 21 articles underwent level 2 review (full text), leading to identification of 16 studies for level 3 review and inclusion. Figure 1 (on line supplemental material) summarizes the studies identified and reasons for exclusion.
Figure 1. Summary of Literature Review.
Three spine bone mineral density and two fracture studies also reported phenylalanine levels.
Bone Mineral Density in PKU
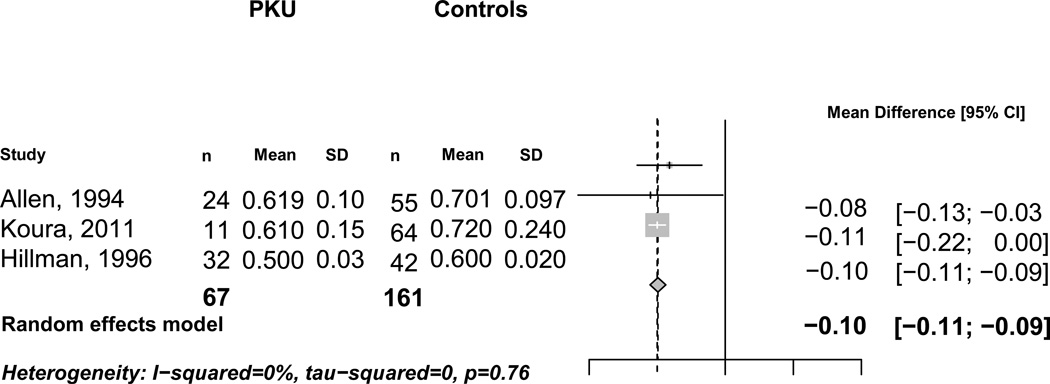
Three studies (Allen et al 1994; Hillman et al 1996; Koura et al 2011) reported spine BMD in 67 individuals with PKU and 161 healthy age and gender matched controls (Table 1). A Forest plot of these three studies (Figure 2) demonstrated that spine BMD was 0.100 g/cm2 lower (95% CI, −0.110, −0.090 g/cm2) in subjects with PKU, compared to controls. No significant heterogeneity was detected between studies (I2 = 0%).
Table 1.
Spine Bone Mineral Density in Subjects with and without PKU
| Country Authors |
PKU n age (years) gender |
Controls n age (years) gender |
Results |
|---|---|---|---|
| Australia (Allen et al 1994) | n=24 9 ± 2 17 M, 7 F |
n=55 9 ± 2 34 M, 21 F |
Spine BMD lower in PKU than control subjects (0.619 ± 0.100 g/cm2 in PKU, 0.710 ± 0.097 g/cm2 in controls, p<0.05). Spine BMD still lower in PKU subjects after adjustment for height (0.637 ± 0.075 g/cm2 in PKU, 0.692 ± 0.062 g/cm2 in controls, p=0.003) |
| United States (Hillman et al 1996) | n=11 11 ± 4 5 M, 6 F |
n=11 11 ± 4 5 M, 6 F |
Spine BMD lower in PKU than control subjects (0.610 ± 0.150 g/cm2 in PKU, 0.720 ± 0.240 g/cm2 in controls, p=0.04) |
| Egypt (Koura et al 2011) | n=32 9 ± 4 19 M, 13 F |
42 “age sex matched controls” | Spine BMD similar in PKU and control subjects (0.500 ± 0.030 g/cm2 vs 0.600 ± 0.020 g/cm2, p “NS”) but spine Z-score lower in but hose with PKU (−0.4 ± 0.12 vs. 0.1 ± 0.11, p=0.01). |
Figure 2. Spine Bone Mineral Density in Subjects with and without PKU.

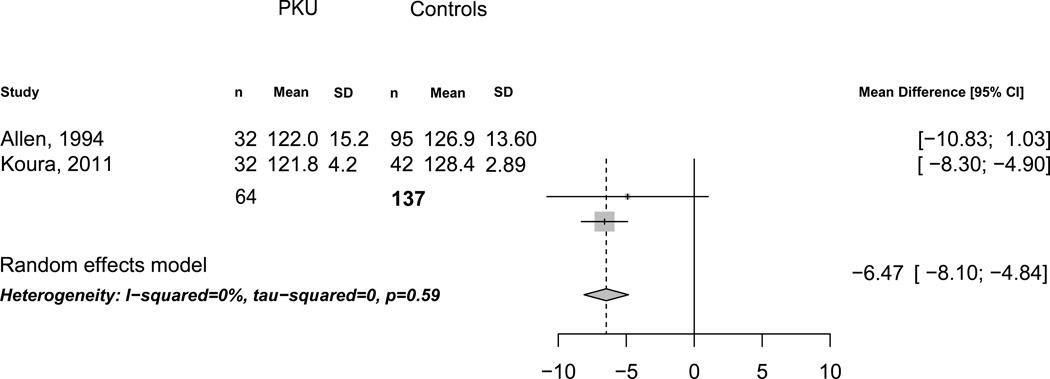
Reduced body size is a potential confounder when measuring BMD. Two-dimensional measurements of the skeleton via DXA can underestimate BMD in shorter individuals and overestimate BMD in tall individuals (Zhang et al 2012), since DXA measurements do not fully account for body size. All three BMD studies reported weight in PKU and control subjects, but only two studies reported height. Of note, weight was lower by 4.53 kg (95% CI, −5.58 to −3.48) in PKU compared to control subjects (n=3 studies, I2=0%). Additionally, height was lower by 6.47 cm (95% CI, −8.10 to −4.84) in PKU compared to control subjects (n=2 studies, I2=0%, Figure 3 on line supplemental material). Similar findings were also found for weight and height Z-scores (data not shown).
Figure 3.

Height in Subjects with and without PKU
Only one study adjusted BMD data for height. In that study (Allen et al 1994), spine BMD was still lower after controlling for the shorter height of PKU subjects, compared to controls (0.637 ± 0.075 g/cm2 in PKU, 0.692 ± 0.062 g/cm2 in controls, p=0.003).
Fractures in PKU
Six studies (Carson et al 1990; Greeves et al 1997; Perez-Duenas et al 2002; Modan-Moses et al 2007; Porta et al 2008; Porta et al 2011) reported fracture rates in 263 PKU subjects (Table 2). Only one study (Greeves et al 1997) reported fractures in a group of controls. All fractures were based on a clinical diagnosis; no studies used spine radiographs to detect asymptomatic vertebral fractures. Three of six studies reported the circumstances leading to fractures (e.g. high trauma).
Table 2.
Fractures in Subjects with PKU
| Author, Country |
PKU Subjects age (years), gender |
Controls1 age (years), gender |
Main Findings |
|---|---|---|---|
| Ireland (Carson et al 1990) | n=11 Range 19–34, 5 M, 6 F |
none | Fractures in 6 of 11 subjects (56%) including one with a thoracic compression fracture |
| Ireland (Greeves et al 1997) | n=85 12 (0.3–34) 44 M, 41 F |
n=98 13 (0.1–31) 50 M, 48 F |
Fractures in 21 of 85 subjects (25%) and 18 of 98 controls (18%); fracture rate 2.6 fold higher (95% CI 1.1, 6.1) after age 8 in subjects compared to controls |
| Israel (Modan-Moses et al 2007) | n=31 25 ± 5 13 M, 18 F |
none | Fractures in 4 of 31 subjects (13%), all associated with significant trauma |
| Spain (Perez-Duenas et al 2002) | n=28 10–33, 11 M, 17 F |
none | Five fractures in 2 of 28 patients (7%); circumstances not reported |
| Porta-2008 Italy | n=30 15 ± 6 13 M, 17 F |
none | Fractures in 5 of 30 subjects (17%), all associated with significant trauma |
| Porta-2011 Italy | n=78 12 ± 7 35 M, 43 F |
none | Fractures in 15 of 78 subjects (19%), all associated with significant trauma |
Among the six studies, 53 of 263 subjects with PKU experienced clinical fractures, suggesting a fracture rate of 20% in children with PKU. In the single study (Greeves et al 1997) using a control arm, clinical fractures occurred in 21 of 85 PKU subjects (25%) and 18 of 98 healthy sibling controls (18%). In that study, the fracture rate was 2.6 fold higher (95% CI, 1.1 to 6.1) after age 8 in subjects with PKU, compared to healthy sibling controls. Authors suggested that increased consumption of phenylalanine might account for the higher rate of fractures after age 8, as historically children with PKU were permitted to liberalize their diet after this age.
Relationship between Bone Mineral Density and Serum Phenylalanine
Twelve studies (McMurry et al 1992; Allen et al 1994; Hillman et al 1996; Al-Qadreh et al 1998; Barat et al 2002; Perez-Duenas et al 2002; Millet et al 2005; Modan-Moses et al 2007; Adamczyk et al 2010; Lage et al 2010; Koura et al 2011; de Groot et al 2012) in 412 PKU patients evaluated correlations between BMD and phenylalanine levels (Table 3). Three of 12 studies (McMurry et al 1992; Al-Qadreh et al 1998; Adamczyk et al 2010) in 119 of 412 (29%) PKU patients reported an inverse association between phenylalanine and BMD. One study reported the correlation coefficient and p-value for the observed association. The remaining nine studies in 293 PKU patients found no association between phenylalanine levels and BMD. Among the nine studies, only one reported the correlation coefficient and p-value for study data.
Table 3.
Correlations between Serum Phenylalanine and Bone Mineral Density in Subjects with PKU
| Country Authors |
n Age (years) |
Frequency & Method of Measuring Phenylalanine |
Correlation between Phenylalanine and Bone Mineral Density |
|---|---|---|---|
| Poland (Adamczyk et al 2010) | n=45 14 ± 5 |
Neither frequency or method was reported | Phenylalanine negatively correlated with spine BMD, 1 R 2 & p value not reported |
| Australia (Allen et al 1994) | n=32 9 ± 2 |
Once, high performance liquid chromatography | No correlation between phenylalanine and spine BMD, R & p value not reported |
| Greece (Al-Qadreh et al 1998) | n=48 9 ± 4 |
Monthly × 12 months, method not reported | Mean phenylalanine level negatively correlated with right forearm BMD by single photon absorptiometry (r = −0.49, p<0.007) |
| France (Barat et al 2002) | n=13 median 12, range 5–21 |
Age 1 month to time of BMD test, method not reported | phenylalanine levels similar in subjects with spine Z-score < −1 (n=8) and Z-score ≥ −1 (n=5), R & p value not reported |
| Netherlands (de Groot et al 2012) | n=53 17 ± 9 |
Every 2–4 weeks for one year, high performance liquid chromatography | No correlation between phenylalanine and spine Z-score R=0.034, p=0.199 (n=50) |
| United States (Hillman et al 1996) | n=11 11 ± 4 |
3 recent measures, method not reported | No correlation between phenylalanine and spine BMD after correction for age, R & p value not reported |
| Egypt (Koura et al 2011) | n=32 9 ± 5 |
monthly × 2; enzyme linked immunoassay | No correlation between phenylalanine and spine BMD, R & p value not reported |
| Spain (Lage et al 2010) | range 6–42, n=47 |
Once, fluorometric assay | No correlation between phenylalanine and spine Z-score, R & p value not reported |
| United States (McMurry et al 1992) | Range 1–25, n=26 |
Mean level over past six months, agar diffusion microbial assay | Mean phenylalanine levels over prior six months <1200 µmol/L associated with higher radius BMC, R & p value not reported |
| Spain (Millet et al 2005) | 18 (range 4– 38), n=46 |
Day of bone density test, levels for past 12 months, ion exchange chromotography | No correlation between phenylalanine level on day of bone density test and spine Z-score, No correlation between prior year phenylalanine levels and spine Z-score, R & p value not reported |
| Israel (Modan-Moses et al 2007) | 25±5 n=31 |
Once, bacterial inhibition assay and fluorometric assay | No correlation between phenylalanine and spine Z-score, R & p value not reported |
| Spain (Perez-Duenas et al 2002) | 10–33, n=28 |
Monthly × 12 months, ion exchange chromotography | No correlation between mean phenylalanine levels and spine BMD, R & p value not reported |
BMD indicates bone mineral density;
R indicates the correlation coefficient
Since only two of twelve studies reported correlation coefficients and p-values, a meta-analysis of all 12 studies was not possible. In summary, 75% of studies representing 71% of studied subjects reported no association phenylalanine and BMD.
Assessment of publication bias
We generated funnel plots, or scatterplots of standard error (vertical axis) versus mean difference between subjects and controls (horizontal axis), to detect potential publication bias. Plots suggested low publication bias with respect to spine BMD, height, weight and height Z-score. The one exception was the funnel plot for weight Z-score, which did suggest a significant publication bias. However, only two studies reported subjects’ weight Z-score (Allen et al 1994; Koura et al 2011) and the larger study was the one that appeared biased, emphasizing the need for more data on weight Z-score in PKU patients.
DISCUSSION
We performed a systematic review of literature describing spine BMD and fracture rates in patients with PKU. Meta-analysis confirmed that spine BMD was significantly lower in individuals with PKU, compared to age and gender matched controls. However, only one study corrected for the reduced height of PKU subjects. Although authors still found that BMD was lower in PKU subjects, experts acknowledge that measurement of BMD in short individuals can underestimate BMD. Future studies of BMD in PKU subjects should correct measurements for height, using published equations developed for this purpose (Zhang et al 2012).
We identified six studies reporting fractures in subjects with PKU. Together these studies suggest a clinical fracture rate of ~20%, but regrettably only one study also determined fracture rates in healthy controls. Compared to healthy sibling controls, children with PKU had more fractures, particularly after age 8 when historically, intake of phenylalanine was liberalized. The study therefore suggested that higher phenylalanine levels might be the cause of low BMD in patients with PKU. However, scrutiny of 12 studies representing 412 individuals with PKU detected no consistent relationship between high phenylalanine levels and lower BMD.
It is noteworthy that the study reporting the highest percent of PKU patients with fractures (Carson et al 1990) recruited individuals who were likely born in the 1960’s. By contrast, the other fracture studies (Table 2) included children born in the 1980’s and 1990’s. Since lifelong strict dietary adherence was recommended by the year 2000 for all PKU patients (2001), suggesting that high phe levels might be responsible for low BMD and higher fracture rates. However, we found no consistent association between phe and BMD, regardless of the decade of birth (Table 3). We therefore suspect that the higher fracture rate in the Carson study might be due to the older age of the participants, leading to greater chances to experience events contributing to fractures.
To our knowledge, this is the first systematic review and meta-analysis on BMD and fractures in subjects with PKU. Our study has several strengths. We limited our analysis to measures of spine BMD, as the International Society for Clinical Densitometry (Gordon et al 2008) states spine BMD is one of two reliable indicators of skeletal status in children. Two authors reviewed all abstracts to determine eligibility for inclusion of each study and performed duplicate data extraction; disagreements were resolved by consensus.
Our study also has a number of limitations. Only one study measured whole body BMD, precluding meta-analysis of this additional measure of skeletal health. Fractures were based on clinical diagnosis. No studies used spine imaging to detect silent vertebral fractures, the hallmark of skeletal fragility. Several studies assessing relationships between phenylalanine levels and BMD relied on a single phenylalanine level. However, two of the three studies reporting an inverse correlation between phenylalanine levels and BMD used multiple phenylalanine levels collected over 6–12 months. Finally, we found no publications linking BMD values to subsequent risks of fracture.
Additional well-designed, long-term prospective research studies are recommended in individuals with PKU, to evaluate skeletal health. We recommend that such studies measure spine and total body BMD and bone mineral content (BMC), and control for smaller body size and Tanner stage when analyzing data. Measurement of BMD and BMC in age, gender and racially matched control subjects is mandatory. Whether individuals with PKU are able to accrue similar peak bone mass by 30 years of age, compared to healthy controls, is uncertain. Of course, the long-term consequences of achieving lower peak BMD would include higher risk of osteoporotic fragility fracture in later life, but fracture data in adults with PKU is lacking. We therefore recommend that prospective clinical studies of skeletal health in PKU collect data on BMD and subsequent clinical and asymptomatic vertebral fractures, including the circumstances in which fractures occurred. Finally, the etiology of low BMD in children with PKU is unknown; existing data does not support the widely held notion that increased phenylalanine levels cause lower BMD.
CONCLUSION
In summary, limited data suggest that children with PKU have lower spine BMD compared to healthy children. However, existing data do not clarify whether lower spine BMD results in a higher rate of clinical or morphometric vertebral fracture in childhood or later life. Additionally, the cause of low BMD in PKU is unknown.
Acknowledgments
Funding
KEH received support from NIH (R01 AG028739, NIA and ODS) during the conduct of this study. DMN received support from FDA (R01 FD003711) and the United States Department of Agriculture Hatch grant WISO 1517 during the conduct of this study. No agency funded the current project.
Footnotes
Conflicts of Interest:
Karen Hansen is a consultant to Takeda Pharmaceuticals and Deltanoid Pharmaceuticals.
Denise Ney is a co-inventor on US Patent 8,604,168 B2, Glycomacropeptide Medical Foods for Nutritional Management of Phenylketonuria and other Metabolic Disorders, held by the Wisconsin Alumni Research Foundation and licensed to Cambrooke Therapeutics, LLC.
Informed Consent:
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Animal Rights:
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Contributions of individual authors:
KEH: Planning, literature search, data extraction, statistical analysis, manuscript writing
DN: Planning, data extraction, data synthesis, manuscript revision
Contributor Information
Karen E. Hansen, Department of Medicine, Division of Rheumatology, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Denise Ney, College of Agricultural & Life Sciences, Department of Nutritional Sciences, ney@nutrisci.wisc.edu.
REFERENCES
- National Institutes of Health Consensus Development Conference Statement: phenylketonuria: screening and management, October 16–18, 2000. Pediatrics. 2001;108(4):972–982. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- Adamczyk P, Morawiec-Knysak A, Pludowski P, et al. Bone metabolism and the muscle-bone relationship in children, adolescents and young adults with phenylketonuria. J Bone Miner Metab. 2010;29(2):236–244. doi: 10.1007/s00774-010-0216-x. [DOI] [PubMed] [Google Scholar]
- Al-Qadreh A, Schulpis KH, Athanasopoulou H, et al. Bone mineral status in children with phenylketonuria under treatment. Acta Paediatr. 1998;87(11):1162–1166. doi: 10.1080/080352598750031158. [DOI] [PubMed] [Google Scholar]
- Allen JR, Humphries IR, Waters DL, et al. Decreased bone mineral density in children with phenylketonuria. Am J Clin Nutr. 1994;59(2):419–422. doi: 10.1093/ajcn/59.2.419. [DOI] [PubMed] [Google Scholar]
- Allen JR, Humphries IR, Waters DL, et al. Decreased bone mineral density in children with phenylketonuria. The American journal of clinical nutrition. 1994;59(2):419–422. doi: 10.1093/ajcn/59.2.419. [DOI] [PubMed] [Google Scholar]
- Barat P, Barthe N, Redonnet-Vernhet I, et al. The impact of the control of serum phenylalanine levels on osteopenia in patients with phenylketonuria. Eur J Pediatr. 2002;161(12):687–688. doi: 10.1007/s00431-002-1091-9. [DOI] [PubMed] [Google Scholar]
- Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953;265(6790):812–813. doi: 10.1016/s0140-6736(53)90473-5. [DOI] [PubMed] [Google Scholar]
- Carson DJ, Greeves LG, Sweeney LE, et al. Osteopenia and phenylketonuria. Pediatric radiology. 1990;20(8):598–599. doi: 10.1007/BF02129064. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, van Rijn M, et al. Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients. Mol Genet Metab. 2012;105(4):566–570. doi: 10.1016/j.ymgme.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Fölling A. Über Ausscheidung von Phenylbrenztraubensäure in den Harn als Stoffwechselanomalie in Verbindung mit Imbezillität. Hoppe-Seyler´s Zeitschrift für physiologische Chemie. 1934;227(1–4):169–181. [Google Scholar]
- Gordon CM, Bachrach LK, Carpenter TO, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Greeves LG, Carson DJ, Magee A, et al. Fractures and phenylketonuria. Acta Paediatr. 1997;86(3):242–244. doi: 10.1111/j.1651-2227.1997.tb08882.x. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman L, Schlotzhauer C, Lee D, et al. Decreased bone mineralization in children with phenylketonuria under treatment. European journal of pediatrics. 1996;155(Suppl 1):S148–S152. doi: 10.1007/pl00014234. [DOI] [PubMed] [Google Scholar]
- Koura HM, Abdallah Ismail N, Kamel AF, et al. A long-term study of bone mineral density in patients with phenylketonuria under diet therapy. Arch Med Sci. 2011;7(3):493–500. doi: 10.5114/aoms.2011.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage S, Bueno M, Andrade F, et al. Fatty acid profile in patients with phenylketonuria and its relationship with bone mineral density. Journal of inherited metabolic disease. 2010;33(Suppl 3):363–371. doi: 10.1007/s10545-010-9189-0. [DOI] [PubMed] [Google Scholar]
- McMurry MP, Chan GM, Leonard CO, et al. Bone mineral status in children with phenylketonuria--relationship to nutritional intake and phenylalanine control. Am J Clin Nutr. 1992;55(5):997–1004. doi: 10.1093/ajcn/55.5.997. [DOI] [PubMed] [Google Scholar]
- Millet P, Vilaseca MA, Valls C, et al. Is deoxypyridinoline a good resorption marker to detect osteopenia in phenylketonuria? Clin Biochem. 2005;38(12):1127–1132. doi: 10.1016/j.clinbiochem.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Modan-Moses D, Vered I, Schwartz G, et al. Peak bone mass in patients with phenylketonuria. J Inherit Metab Dis. 2007;30(2):202–208. doi: 10.1007/s10545-007-0462-9. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Perez-Duenas B, Cambra FJ, Vilaseca MA, et al. New approach to osteopenia in phenylketonuric patients. Acta Paediatr. 2002;91(8):899–904. doi: 10.1080/080352502760148603. [DOI] [PubMed] [Google Scholar]
- Porta F, Mussa A, Zanin A, et al. Impact of metabolic control on bone quality in phenylketonuria and mild hyperphenylalaninemia. Journal of pediatric gastroenterology and nutrition. 2011;52(3):345–350. doi: 10.1097/MPG.0b013e3182093b32. [DOI] [PubMed] [Google Scholar]
- Porta F, Spada M, Lala R, et al. Phalangeal quantitative ultrasound in children with phenylketonuria: a pilot study. Ultrasound Med Biol. 2008;34(7):1049–1052. doi: 10.1016/j.ultrasmedbio.2007.12.013. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing.R. F. f. S. Austria: Computing.Vienna; 2008. [Google Scholar]
- Vockley J, Andersson HC, Antshel KM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genetics in medicine : official journal of the American College of Medical Genetics. 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- Zhang F, Whyte MP, Wenkert D. Dual-energy X-ray absorptiometry interpretation: a simple equation for height correction in preteenage children. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2012;15(3):267–274. doi: 10.1016/j.jocd.2012.01.004. [DOI] [PubMed] [Google Scholar]