Abstract
Background & Aims
Little is known about differences in rates of fibrosis progression between patients with nonalcoholic fatty liver (NAFL) vs nonalcoholic steatohepatitis (NASH). We conducted a systematic review and meta-analysis of all studies that assessed paired liver biopsies to estimate the rates of fibrosis progression in patients with NAFLD including NAFL and NASH.
Methods
Through a systematic search of multiple databases and author contact, up to June 2013, we identified studies of adults with NAFLD that collected paired liver biopsies at least 1 year apart. From these, we calculated a pooled-weighted annual fibrosis progression rate (number of stages changed between the 2 biopsy samples) with 95% confidence intervals (CI), and identified clinical risk factors associated with progression.
Results
We identified 11 cohort studies including 411 patients with biopsy-proven NAFLD (150 with NAFL and 261 with NASH). At baseline, the distribution of fibrosis for stages 0, 1, 2, 3, and 4 was 35.8%, 32.5%, 16.7%, 9.3%, and 5.7%, respectively. Over 2145.5 person-years of follow up, 33.6% had fibrosis progression, 43.1% had stable fibrosis, and 22.3% had improvement in fibrosis stage. The annual fibrosis progression rate in patients with NAFL who had stage 0 fibrosis at baseline was 0.07 stages (95% CI, 0.02–0.11 stages), compared with 0.14 stages in patients with NASH (95% CI, 0.07-0.21 stages). These findings correspond to 1 stage of progression over14.3 years for patients with NAFL (95% CI, 9.1–50.0 years) and 7.1 years for patients with NASH (95% CI, 4.8–14.3 years).
Conclusions
Based a meta-analysis of studies of paired liver biopsy studies, liver fibrosis progresses in patients with NAFL and NASH.
Keywords: Fibrosis, cirrhosis, nonalcoholic steatohepatitis, fatty liver, natural history
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States.1-6 It is defined as presence of hepatic steatosis in at least 5% of hepatocytes on liver biopsy examination in individuals who consume little or no alcohol, after exclusion of other causes of liver disease.2 NAFLD can be broadly classified into two subtypes; nonalcoholic fatty liver (NAFL), which is generally considered to be benign with negligible risk of progression to advanced fibrosis and liver-related mortality, and nonalcoholic steatohepatitis (NASH), which is generally considered to be progressive with substantial risk of progression to advanced fibrosis, and liver-related mortality.
Several longitudinal cohort studies have provided novel insight into the natural history of liver disease in patients with NAFLD.5, 7-10 Despite these advances, the fibrosis progression rate in NAFLD remains to be quantified and is poorly understood.9 Observational studies with paired liver biopsies in patients with NAFLD, although prone to ascertainment and selection bias, offer some of the best available natural history data on rate and risk factors associated with progressive fibrosis. Based on a pooled analysis of 10 studies including 221 patients with NASH alone, Argo and colleagues had estimated that 37.6% of patients have progressive fibrosis over 5.3 years; the mean rate of progression for the entire cohort was 0.03 stages/year.11 However, in this study, patients with NAFL were excluded. Though previous studies have suggested that NAFL may be benign, and does not lead to progressive fibrosis,12 emerging data suggest that fibrosis progression may be seen in not only in NASH but also in NAFL.13
There are limited data on the differences in the fibrosis progression rate in patients with NAFL versus NASH. Therefore, we aimed to perform a systematic review and meta-analysis of studies in patients with biopsy-proven NAFL and NASH who underwent paired liver biopsies at least 1 year apart and quantify differences in fibrosis progression in patients with NAFL versus NASH.
METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the process followed a priori established protocol.14
Selection Criteria
Studies included in the meta-analysis met the following inclusion criteria: (a) cohort studies and placebo-controlled RCTs in (b) adult patients (>18 years of age) with histological diagnosis of NAFLD at any stage of baseline fibrosis, (c) repeat liver biopsy performed at least 1 year apart, and (d) contained sufficient information to allow estimation of FPR by each baseline fibrosis stage.
We excluded the following studies: (a) the diagnosis of NAFLD and/or degree of fibrosis (either baseline or during follow-up) was established using non-invasive means; (b) participants in the active arm of a clinical trial, i.e., patients randomized to potentially disease-modifying active intervention for NAFLD; (c) cross-sectional studies; (d) studies in which the time difference between paired biopsies was <12 months; and (e) studies in which there was insufficient data to allow estimation of FPR (i.e., insufficient data on person-years of follow-up, or mean/median duration of follow-up/time between 2 biopsies, or only contained information on mean change in fibrosis stage for the entire cohort). In case of multiple studies from the same cohort, we included data from the most recent comprehensive report.
Search Strategy
We conducted a comprehensive search of multiple electronic databases from 1985 to June 2013 in adults with no language restrictions. The databases included: Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search strategy was designed and conducted by an experienced medical librarian (LJP) with input from the study's investigators (SS, RL), using controlled vocabulary supplemented with keywords, for cohort studies and placebo-controlled RCTs of NAFLD. The details of the search strategy are included in the Supplementary Appendix. The title and abstract of studies identified in the search were reviewed by two authors independently (SS, AMA) to exclude studies that did not address the research question of interest, based on pre-specified inclusion and exclusion criteria (see above). The full text of the remaining articles was examined to determine whether it contained relevant information. Next, the bibliographies of the selected articles and review articles on the topic were manually searched for additional studies. Third, a manual search of conference proceedings of major gastroenterology and hepatology conferences (The Liver Meeting, organized by the American Association for the Study of the Liver; The International Liver Congress, organized by the European Association for the Study of the Liver; Digestive Diseases Week, organized in conjunction with the American Gastroenterological Association) between 2008-2012 was conducted to identify additional studies published only in the abstract form. Supplementary figure 1 shows the schematic diagram of study selection.
Data Abstraction
Data on the following study- and patient-related characteristics, as well as histological classification and risk factors associated with progressive fibrosis were abstracted onto a standardized form: (a) study characteristics – primary author, time period of study/year of publication, country of the population studied; (b) patient characteristics – total number of patients with NAFLD who underwent paired biopsies, demographic and clinical characteristics at time of first biopsy (age, sex, body mass index or obesity, presence of diabetes, hypertension, metabolic syndrome) and baseline laboratory characteristics (AST, ALT, AST/ALT ratio, platelet count, ferritin, and measure of insulin resistance, using Homeostasis Model of Assessment - Insulin Resistance, i.e., HOMA-IR), and treatment undertaken by participants, including lifestyle changes and potentially disease-modifying therapy (vitamin E, thiazolidinediones, metformin); (c) histological characteristics – proportion of patients with NASH and NAFL separately, baseline and follow-up fibrosis stage for individual patients in the study, time interval between paired biopsies to allow calculation of FPR (person-years, mean or median follow-up of cohort, time difference between paired biopsies), attrition rate, criteria used for diagnosis and staging of fibrosis, proportion of patients with progression of fibrosis, stable fibrosis stage and regression of fibrosis on repeat biopsy; and (d) risk factors at baseline associated with progressive and non-progressive fibrosis (in univariate analysis of individual studies).
Quality Assessment
The quality of included studies was assessed using a modified scale derived from the Newcastle-Ottawa scale.15 This quality score consisted of 8 questions: representative of the average adult in the community (1 point for unselected participants in population-based or multicenter studies, 0.5 points for unselected participants in single-center hospital-based study; 0 points for participants in an RCT); large cohort size (1 point if cohort size >50 patients with baseline and follow-up biopsies, 0.5 points if cohort size between 25-50 patients, 0 points if cohort size of <25 patients); histological confirmation of NAFLD (1 point if standardized assessment of NAFLD with information on both NASH and NAFL separately, 0.5 points if standardized assessment of NAFLD, without distinction between NASH or NAFL, 0 points if reviewed only non-standardized classification of NAFLD); histological assessment of fibrosis progression (1 point if assessed using Brunt classification or NASH-CRN, 0.5 point if assessed using Ishak classification, 0 point if assessed using non-standardized classification); adequate follow-up of cohort for outcome to occur (1 point if mean follow-up of entire cohort >5 years, 0.5 points if cohort follow-up between 3-5 years, 0 points if mean follow-up of cohort <3 years); attrition rate (1 point if >80% of cohort followed-up, 0.5 points if 50-80% cohort followed-up, 0 points if >50% lost to follow-up); clear information on potential disease-modifying therapy adopted by participants (1 point if adequate information on lifestyle modification as well as medication use, 0.5 points if incomplete information, 0 points if no information on adoption of disease-modifying therapy); reported risk factors associated with progression of fibrosis (1 point if factors studied in multivariate models, 0.5 points if factors reported in only univariate models, 0 points if factors associated with fibrosis progression not reported). A score of ≥6, 3-5 and ≤2 was considered suggestive of high-, medium-and low-quality study.
Outcomes Assessed
Primary Outcome
The primary outcome measure was estimation of fibrosis progression rate (FPR) in, (i) the entire cohort of patients with NAFLD, and (ii) separately in patients with biopsy-proven NAFL and NASH, with baseline stage 0 fibrosis.
Estimation of FPR
As previously reported by Ghany et al,16 FPR was calculated as number of stages migrated in paired biopsy specimens, over the time difference between the two biopsy samples. In this systematic review, we estimated the average FPR for each baseline fibrosis stage (calculated as the difference in fibrosis stage between first and last biopsy divided by the time between biopsies in years) in patients with NAFL and NASH, with special focus on patients with baseline stage 0 fibrosis, as well as clinical risk factors associated with progression of fibrosis.
Sensitivity Analyses
A priori hypotheses to assess stability of FPR and sources of heterogeneity included location of study (Western population v. Asian population) and study quality. Due to inherent differences in participants in an observational study and RCT, these two groups of studies were analyzed separately.
Co-variate Assessment
In order to identify risk factors associated with progressive fibrosis in patients with NAFLD, we performed a meta-analysis of differences in baseline clinical (age, sex, presence of diabetes/hypertension/obesity/metabolic syndrome), laboratory (mean AST, ALT, AST:ALT ratio, ferritin, platelet count and HOMA-IR) and biopsy features (grade of inflammation and steatosis) by comparing patients who showed fibrosis progression and those who did not.
Statistical Analysis
We used the random effects model described by DerSimonian and Laird to calculate summary FPR and 95% CI for each stage of baseline fibrosis, using binomial distribution.17 We assessed heterogeneity between study-specific estimates using 2 methods.18 First, the Cochran's Q statistical test for heterogeneity, which tests the null hypothesis that all studies in a meta-analysis have the same underlying magnitude of effect, was measured. Because this test is underpowered to detect moderate degrees of heterogeneity, a p-value of < 0.10 was considered suggestive of significant heterogeneity. Second, when heterogeneity was present, in order to estimate what proportion of total variances across studies was due to heterogeneity rather than chance, I2 statistic was calculated. In this, a value of >50% was suggestive of substantial heterogeneity.19 Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to study characteristics as described above. Publication bias was ascertained, qualitatively, by visual inspection of Funnel plot,20 and quantitatively, by Egger's regression test.21 For all tests (except for heterogeneity and publication bias), a probability level <0.05 was considered statistically significant. All analyses were performed using STATA version 12 (StataCorp).
RESULTS
From a total of 1994 unique studies identified using the pre-specified systematic search strategy, 11 observational studies were included in this analysis.7, 8, 12, 13, 22-28 Six studies were excluded due to lack of sufficient information to calculate FPR despite the availability of paired liver biopsies. We were able to obtain additional information (which was not available in the published manuscript) required to calculate FPR by presence of baseline NASH and NAFL for three studies through contact with study authors.8, 26, 28
Only two RCTs provided sufficient data to estimate FPR by baseline fibrosis stage, obtained by contacting study's lead author.29, 30 Two RCTs provided data on overall FPR but not by baseline stage.31, 32 Two RCTs reported the proportion of placebo-treated patients who progressed or remained stable, but no additional information to estimate FPR.33, 34 Overall, these RCTs reported on 186 patients treated with placebo (158 with paired biopsies), of whom 38 (24.0%) had worsening fibrosis, 84 (53.2%) remained stable and 36 (22.8%) had improvement in fibrosis stage on follow-up biopsy (Supplementary Table 1 and 2); due to limited number of studies with short duration of follow-up, an accurate estimate of FPR could not be obtained. Eight placebo-controlled RCTs with paired biopsies performed 12-months apart provided data only on mean change in fibrosis stage. Hence, RCTs were excluded from further analysis.
Characteristics and Quality of Included Studies
Baseline Characteristics
Table 1 describes the characteristics of the included studies. We identified 11 observational studies including 411 patients with biopsy-proven NAFLD (150 with NAFL and 261 with NASH). Overall, 366 patients had sufficient information for FPR estimation by baseline stage. Among the 11 studies included in this meta-analyses, 8 were derived from Western population 7, 8, 12, 13, 22-24, 27 and 3 were derived from Asian population. 25, 26, 28. Nine of the 11 studies utilized Brunt's classification for fibrosis staging suggesting good generalizability across studies.35 Supplementary Table 3 lists the definition of NAFLD, NAFL and NASH in the studies.
Table 1.
Details of included observational studies (Please note that study by Harrison et al was not included in the overall analysis since it did not provide information on FPR based on baseline stage)
| Author, year of publication (ref) | Location; setting | Period of recruitment | Total no. of patients with paired biopsies | Criterion for histological staging | Duration of follow-up – total p-y; mean (SD); range | Change in fibrosis scores | Quality Score (Max, 8)b | ||
|---|---|---|---|---|---|---|---|---|---|
| Worse, n (%) | Stable, n (%) | Improved, n (%) | |||||||
| Adams 20057 | Rochester, MN; Hospital-based | 1980-2003 | 103 •NAFL – 7 •NASH – 96 |
Brunt | NR; 3.2 (3.0); 0.7-21 | 38 (37%) | 35 (34%) | 30 (29%) | 5 |
| Argo 200922 | Charlottesville, VA | 1997-1999 | 5 •NAFL – 0 •NASH – 5 |
Brunt; NASH CRN | 22 p-y; 4.4; 3.0-6.4 | 3 (60%) | 0 | 2 (40%) | 5 |
| Ekstedt 20068 | Linkoping, Sweden | 1988-1993; F/U in 2003 | 70 •NAFL – 36 •NASH – 34 |
Brunt | 1202 p-y; 13.8 (1.2); 10.3-16.3 | 29 (41%) | 30 (43%) | 11 (16%) | 6.5 |
| Evans 200223 | Glasgow, UK | 1985-1994; F/U 1999 | 7 •NAFL – 0 •NASH – 7 |
Brunt | 57.2 p-y; 8.2; 5.5-11.9 | 4 (57%) | 3 (43%) | 0 | 3.5 |
| Fassio 200424 | Buenos Aires, Argentina | 1986-2002 | 22 •NAFL – 0 •NASH – 22 |
Brunt, Ishak | NR; 5.3 (2.7); 3.0-14.3 | 7 (32%) | 11 (50%) | 4 (18%) | 5.5 |
| Hamaguchi 201025 | Ishikawa, Japan | 1997-2008 | 39 •NAFL – 22 •NASH – 17 |
Brunt | NR; 2.4; 1.0-8.5 | 11 (28%) | 16 (41%) | 12 (31%) | 4.5 |
| Harrison 200350 | San Antonio, TX | 1985-2001 | 22 •NAFL – 0 •NASH – 22 |
Brunt | NR; 5.7; 1.4-15.7 | 7 (32%) | 11 (50%) | 4 (18%) | 4.5 |
| Hui 200526 | Hong Kong | 1996-2004 | 17 •NAFL – 3 •NASH – 14 |
Brunt | 97.8 p-y; 6.1; 3.8-8.0 | 9 (53%) | 8 (47%) | 0 | 4.5 |
| Pais 201313 | Paris, France | 1998-2009 | 70 • NAFL – 25a • NASH – 45 |
Kleiner and Brunt | 102.7 p-y; 3.7 (2.1); NR | 20 (29%) | 30 (42%) | 20 (29%) | 5 |
| Ratziu 200027 | Paris, France | 1988-1999 | 14 •NAFL – 10 •NASH – 4 |
Metavir | 73 p-y; NR; 1.5-15.0 | 2 (14%) | 8 (57%) | 4 (29%) | 1.5 |
| Teli 199512 | Newcastle, UK | 1978-1985 | 12 •NAFL – 12 •NASH – 0 |
NR | NR; 11.6; 7.6-16 | 1 (8%) | 11 (92%) | 0 | 1.5 |
| Wong 201028 | Hong Kong | 2006-2009 | 52 •NAFL – 35 •NASH – 17 |
Kleiner and Brunt | NR; 3.0; NR | 14 (27%) | 25 (48%) | 13 (25%) | 7 |
Fibrosis progression only reported for subset with NAFL
Details of quality assessment of individual studies reported in supplementary table 2
[Abbreviations: CRN-clinical research network, max.-maximum; NAFL-non-alcoholic fatty liver, NASH-non-alcoholic steatohepatitis, NR-not reported, p-y-person-years]
Table 2 describes the baseline characteristics of the patients in the included studies. The mean age of participants at the time of initial biopsy ranged from 44 to 55 years; 53.0% of patients were males. The mean BMI ranged from 27.4 to 37.7 kg/m2, and almost half of the patients (49.9%) were diabetics.
Table 2.
Baseline characteristics of patients included in the respective studies (Please note that study by Harrison et al was not included in the overall analysis since it did not provide information on FPR based on baseline stage)
| Author | Age, mean (S.D.) | Sex (as % males), n | BMI, mean (S.D.) | Obesity (as % total) | Diabetes (as % total), n | AST, mean (S.D.) | Potential disease-modifying therapy | Non-pharmacological interventions | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin, n (%) | TZDs, n | Vitamin E, n (%) | ||||||||
| Adams 20057 | 45 (11) | 37 (38) | - | 67 | 42 (43) | 75 (50) | 1 (0.9%) | 0 | 0 | NR |
| Argo 200922 | NR | NR | 37.7 (6.5) | NR | 60 (3) | 94 (39) - ALT | 2 (40%) | 1 (20%) | 0 | Physical activity, High intensity– 40%; Moderate intensity –40%; Low intensity – 20% |
| Ekstedt 20068 | 51 (13) | 67 (47) | 28.3 (3.8) | 29 | 85 (60) | 45 (23) | NR | NR | ||
| Evans 200223 | 49 (10) | 43 (3) | 32.4 (5.0) | 57 | 43 (3) | 38 (18) | NR | NR | ||
| Fassio 200424 | 45 (13) | 41 (9) | 29.8 (24.0-38.2)a | 45 | 36 (8) | 73 (41-129)a - ALT | No specific disease-modifying pharmacologic therapy | Nutritional counseling | ||
| Hamaguchi 201025 | 47 (20-79)a | 56 (22) | 27.8 (22.5-44.4)a | - | 77 (30) | 40 (11-106)a | 4 (10%) | 1 (2.6%) | 0 | Diet/insulin for diabetics |
| Harrison 200350 | 51 (33-64)a | 59 | 33.8 (26.5-48.6)a | 77 | 41 | 74.5 (13-158)a | NR | NR | ||
| Hui 200526 | 42 (3) | 65 (11) | 29.3 (23-35.5)a | 35 | 24 (4) | 104 (32-193)a - ALT | 3 (18%) | 0 | 0 | NR |
| Pais 201313 | 52 (10) | NR | 29 (3.6) | NR | 35 (25) | 85 (43) - ALT | NR | 0 | NR | |
| Ratziu 200027 | 49 (20-79) | 34 (8) | 29.1 (25.1-45.7)a | NR | 16 (2) | NR | NR | NR | ||
| Teli 199512 | 55 (26-79)a | 55 (6) | NR | 30 | 10 (1) | 35 (26) | NR | NR | ||
| Wong 201028 | 44 (9) | 65 (34) | 27.4 (3.7) | NR | 50 (26) | 61 (37-100)a - ALT | 16 (31%) | 0 | 0 | Baseline dietician visit; advised to exercise 3x/wk |
median (range)[Abbreviations: ALT-alanine aminotransferase, AST-aspartate aminotransferase, BMI-body mass index, NR-not reported, SD-standard deviation, TZDs-thiazolidinediones]
Entire Cohort Changes in Fibrosis and Duration of Follow-up
At baseline, the distribution of fibrosis for stage 0, 1, 2, 3, and 4 was 35.8%, 32.5%, 16.7%, 9.3% and 5.7%, respectively. Over 2145.5 person-years of follow-up, 33.6% (n=138) had progression (increase by at least one fibrosis stage as compared to baseline), 43.1% (n=177) remained stable and 22.3% (n=96) had improvement (decrease by at least one fibrosis stage as compared to baseline) in fibrosis stage.
Quality Assessment
Detailed quality assessment of the studies included in the meta-analyses is provided in Supplementary Table 4. Most of the included studies were at moderate risk of bias, with two studies being at low risk of bias.8, 28 None of the included studies were population-based, and only one observational study was multicenter.8 Three studies were restricted only to patients with NASH;22-24 two studies included only patients with NAFL.12, 13 Of the remaining six studies, four provided sufficient data (either in primary manuscript or through contact with authors) to allow estimation of FPR for patients with NASH and NAFL separately;8, 25, 26, 28 we were not able to obtain this information on two studies.7, 27 Five studies provided information on use of potential disease-modifying pharmacological therapy and recommendations offered for lifestyle changes;22, 24, 25, 28, 29 however, the extent to which these recommendations were adopted could not be reliably ascertained.
Rate of Fibrosis Progression in Patients with NAFL versus NASH
Fibrosis Progression Rate in the Entire Cohort of NAFLD Patients
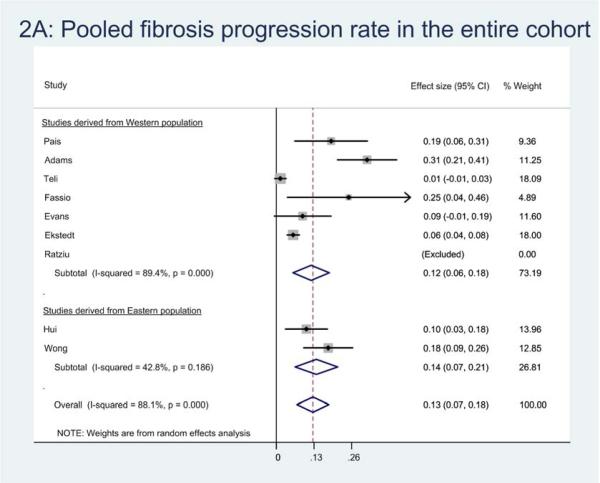
Eleven studies provided sufficient information to estimate FPR in 366 patients with NAFLD. Of these, 132 (36.1%) patients developed progressive fibrosis, 158 (46.2%) patients remained stable, and 76 (20.8%) patients had improvement in fibrosis. On meta-analysis, the overall annual FPR in patients with NAFLD with baseline stage 0 fibrosis (131 patients) was 0.13 stages (95% CI, 0.07-0.18), corresponding to an average progression by 1 stage over 7.7 (95% CI, 5.5-14.8) years (Figure 1A).
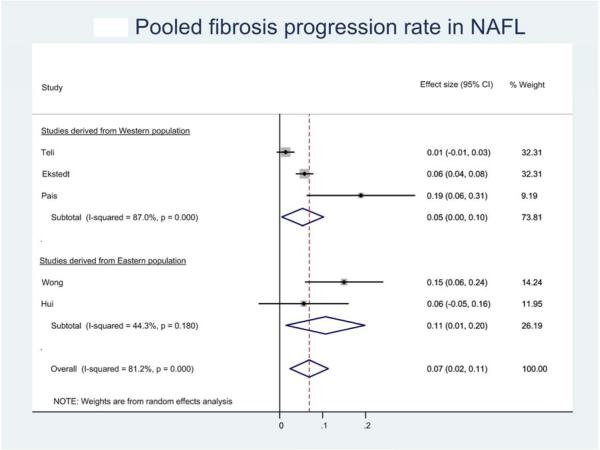
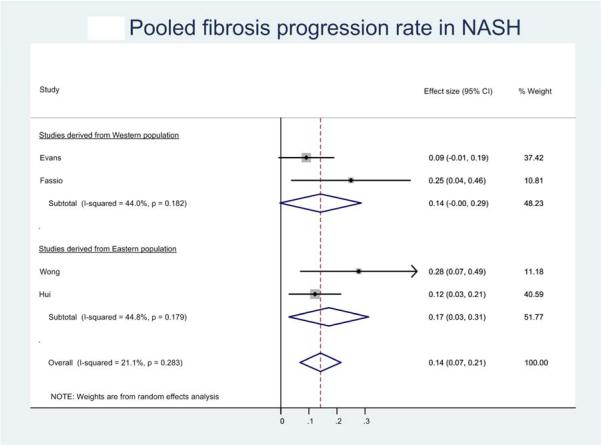
Figure 1.
Pooled fibrosis progression rate in patients with (a) nonalcoholic fatty liver disease, (b) nonalcoholic fatty liver and (c) nonalcoholic steatohepatitis, and baseline stage 0 fibrosis. Effect size represents the annual rate of progression of fibrosis stage.
Fibrosis Progression Rate in NAFL
Six studies provided sufficient information to estimate FPR in 133 patients with NAFL.8, 12, 13, 25, 26, 28 Of these, 52 (39.1%) patients developed progressive fibrosis, 70 (52.6%) patients remained stable and 11 (8.3%) patients had improvement in fibrosis. On meta-analysis, the overall annual FPR in patients with NAFL with baseline stage 0 fibrosis (81 patients) was 0.07 stages (95% CI, 0.02-0.11), corresponding to an average progression by 1 stage over 14.3 (95% CI, 9.1-50.0) years (Figure 1B).
Fibrosis Progression Rate in NASH
Seven studies provided sufficient information to estimate FPR in 116 patients with NASH.8, 22-26, 28 Overall, 40 (34.5%) patients developed progressive fibrosis, 45 (38.8%) patients remained stable and 31 (26.7%) had improvement in fibrosis. On meta-analysis of patients with NASH with baseline F0 fibrosis (21 patients), the annual FPR was estimated at 0.14 stages (95% CI, 0.07-0.21), corresponding to an average 7.1 (95% CI, 4.8-14.3) years as time taken to progress by 1 stage (Figure 1C).
Table 3 depicts the overall FPR by baseline stage for patients with NAFLD, NAFL and NASH, and demonstrates generalizability across studies. Supplementary Table 5 depicts the baseline and follow-up fibrosis stage for each individual study.
Table 3.
Overall fibrosis progression rate by baseline fibrosis stage in patients with NAFLD, NAFL alone and NASH alone. Please note that if the lower limit of 95% CI for fibrosis progression rate was negative (i.e., the lower limit suggested there could be net regression of fibrosis stage), then time taken to progress to fibrosis by one stage was not calculated.
| Final fibrosis Stage | Total stages of fibrosis progressed | Person-years of follow-up | Fibrosis Progression Rate (95% CI) | Time taken to progress by 1 stage (95% CI) | ||||||
| Non-Alcoholic Fatty Liver Disease (NAFLD) (11 studies) | ||||||||||
| 0 | 1 | 2 | 3 | 4 | ||||||
| Baseline fibrosis stage | 0 (131) | 79 | 28 | 13 | 7 | 4 | +91 | 968 | 0.13 (0.07-0.18) | 7.7 (5.5-14.8) |
| 1 (119) | 26 | 44 | 32 | 15 | 2 | +43 | 628.4 | 0.10 (0.04-0.16) | 10.0 (6.2-25.0) | |
| 2 (61) | 9 | 17 | 14 | 13 | 8 | −6 | 331.8 | NA | - | |
| 3 (34) | 2 | 5 | 10 | 7 | 10 | −16 | 153.4 | NA | - | |
| 4 (21) | 0 | 0 | 1 | 6 | 14 | −8 | 63.8 | NA | - | |
| Overall (366) | +104 | 2145.4 | NA | - | ||||||
| Stage 0 plus Stage 1 fibrosis (250) | +134 | 1596.4 | 0.12 (0.07-0.16) | 8.3 (6.2-14.3) | ||||||
| Non-Acoholic Fatty Liver alone (NAFL) (6 studies) | ||||||||||
| 0 | 1 | 2 | 3 | 4 | ||||||
| Baseline fibrosis stage | 0 (81) | 52 | 16 | 8 | 4 | 1 | +48 | 751.3 | 0.07 (0.02-0.11) | 14.3 (9.1-50.0) |
| 1 (39) | 6 | 13 | 14 | 6 | 0 | +20 | 112.6 | 0.15 (−0.09-40) | NA | |
| 2 (13) | 2 | 3 | 5 | 2 | 1 | −3 | 40.7 | NA | - | |
| 3 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | - | |
| 4 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | - | |
| Overall (133) | +75 | 904.6 | NA | - | ||||||
| Stage 0 plus Stage 1 fibrosis (120) | +68 | 863.9 | 0.09 (0.04-0.14) | 11.1 (7.1-25.0) | ||||||
| Non-Alcoholic Steatohepatitis (NASH) (7 studies) | ||||||||||
| 0 | 1 | 2 | 3 | 4 | ||||||
| Baseline fibrosis stage | 0 (21) | 10 | 7 | 2 | 1 | 1 | +18 | 115.5 | 0.14 (0.07-0.21) | 7.1 (4.8-14.3) |
| 1 (49) | 9 | 25 | 9 | 5 | 1 | +13 | 396.6 | 0.08 (−0.01-0.17) | NA | |
| 2 (25) | 3 | 10 | 4 | 4 | 4 | −4 | 222.3 | NA | ||
| 3 (16) | 0 | 4 | 4 | 2 | 6 | −6 | 95.8 | NA | ||
| 4 (5) | 0 | 0 | 0 | 1 | 4 | −1 | 12.6 | NA | ||
| Overall (116) | +20 | 842.8 | NA | |||||||
| Stage 0 plus Stage 1 fibrosis (70) | +31 | 512.1 | 0.10 (0.03-0.17) | 10.0 (5.9-33.3) | ||||||
Subgroup and Sensitivity Analyses
As expected, heterogeneity was observed in the FPR in patients with NAFLD and NAFL; however, NASH patients had less heterogeneity in FPR. (I2 for NAFLD, NAFL and NASH = 88%, 81% and 21%, respectively). On sub-group analysis, there was no significant difference in FPR in Western and Asian patients with NAFLD (Supplementary Table 6). Likewise, there were no significant differences in FPR based on study quality. To assess whether any one study had a dominant effect on the meta-analytic FPR, each study was excluded and its effect on the main summary estimate and I2 test for heterogeneity was evaluated; no study markedly affected the overall FPR or degree of heterogeneity. Since substantial heterogeneity was observed in the overall analysis, evaluation for publication bias using funnel plot was unremarkable.36
Explanation for Heterogeneity in NAFLD (Rapid Progressors and Slow Progressors)
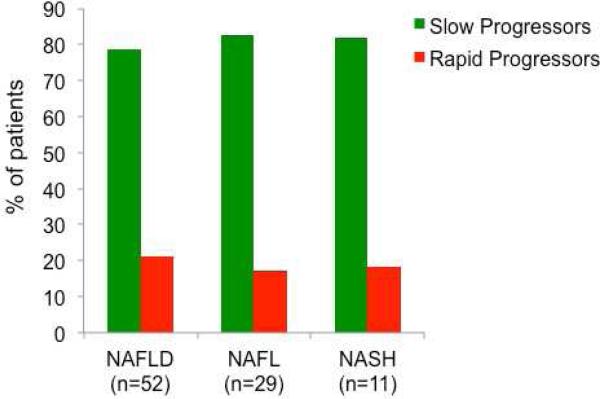
In this study, we observed two subsets of patients with NAFLD stratified by the rate of fibrosis progression; rapid progressors and slow progressors. Rapid progressors included a small subset of patients with NAFLD (with baseline stage 0 fibrosis) who developed rapid progression to advanced fibrosis (stage 3 or 4 fibrosis). Of the 52 patients with NAFLD with stage 0 fibrosis at baseline who showed progression in their fibrosis stage at the follow-up liver biopsy, 11 (21.2%) progressed to stage 3 or 4 fibrosis over an mean (±SD) follow-up of 5.9 (±3.7) years (Figure 2). The vast majority of patients with NAFLD showed slow progression in their fibrosis stage at the follow-up liver biopsy usually only by 1 or 2 stages.
Figure 2.
Differential rate of fibrosis progression in patients with non-alcoholic fatty liver disease (NAFLD), nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), among patients with progressive fibrosis. Rapid progressors refers to a subset of patients with baseline stage 0 fibrosis who developed rapid progression to advanced fibrosis (stage 3 or 4 fibrosis); slow progressors refers to patients with baseline stage 0 fibrosis who had slow progression by 1-2 stages on follow-up biopsy.
Of the 29 patients with NAFL with baseline stage 0 fibrosis who developed progressive fibrosis, 5 (17.2%) were rapid progressors. Similarly, of the 11 patients with NASH with baseline stage 0 fibrosis who developed progressive fibrosis, 2 (18.2%) were rapid progressors,.
Risk Factors Associated with Progressive Fibrosis
Ten studies reported baseline clinical, laboratory and/or histological features among patients with and without progressive fibrosis on paired biopsies. Meta-analysis of these clinical and laboratory risk factors for progressive fibrosis could be performed for 7 studies (Supplementary Table 7). The presence of hypertension (OR, 1.94; 95% CI, 1.00-3.74) and low AST: ALT ratio at the time of baseline biopsy was associated with development of progressive fibrosis (Table 4). Data on histological variables associated with risk of fibrosis progression was not amenable to quantitative synthesis. Two 7, 8 (out of 4 studies) 7, 8, 24, 26 observed that patients with higher steatosis grade were more likely to develop progressive fibrosis; no association was found between baseline severity of necroinflammation and risk of progressive fibrosis.7, 8, 24, 26
Table 4.
Meta-analysis of clinical variables comparing patients with progressive fibrosis v. nonprogressive fibrosis (reference).
| Risk Factor | No. of studies | OR (or weighted difference in means) | 95% CI | p-value | I2 (%) |
|---|---|---|---|---|---|
| Categorical variables | |||||
| Sex (Males v. females) | 7 | 0.75 | 0.44-1.28 | 0.29 | 2.2 |
| Diabetes (yes v. no) | 7 | 1.05 | 0.64-1.72 | 0.86 | 0.0 |
| Hypertension (yes v. no) | 5 | 1.94 | 1.00-3.74 | 0.05 | 0.0 |
| Metabolic syndrome (yes v. no) | 3 | 0.63 | 0.32-1.25 | 0.19 | 0.0 |
| Continuous variables | |||||
| Age (years) | 7 | 0.71 | −2.37 to 3.80 | 0.65 | 68.5 |
| BMI (kg/m2) | 5 | 1.41 | −0.28 to 3.10 | 0.10 | 45.3 |
| AST (U/L) | 4 | 8.09 | −1.84 to 18.02 | 0.11 | 46.0 |
| ALT (U/L) | 7 | 7.60 | −6.97 to 22.17 | 0.31 | 73.3 |
| AST:ALT ratio | 4 | −0.08 | −0.16 to 0.00 | 0.06 | 0.0 |
| Platelet count (x109/L) | 3 | −2.72 | −56.82 to 51.38 | 0.92 | 83.1 |
| Ferritin (μg/L) | 3 | −77.15 | −368.6 to 214.3 | 0.60 | 76.8 |
| HOMA-IR | 4 | 0.11 | −1.05 to 1.27 | 0.86 | 96.8 |
[Abbreviations: ALT-alanine aminotransferase, AST-aspartate aminotransferase, BMI-body mass index, CI-confidence intervals, HOMA-IR-homeostatic model assessment for insulin resistance, I2-inconsistency index (low value indicates low heterogeneity), OR-odds ratio]
DISCUSSION
Based upon evidence-derived from this systematic review and meta-analysis of paired liver biopsy studies, we demonstrate that both patients with NAFL and NASH may develop progressive liver fibrosis. The annual fibrosis progression rate (FPR) in patients with NAFL versus NASH, with baseline stage 0 fibrosis, was 0.07 stages versus 0.14 stages, respectively, corresponding to an average progression by 1 stage over 14.3 versus 7.1 years, respectively. Among patients who developed progressive liver fibrosis on follow-up liver biopsy, we identified two potentially distinct subsets and classified them into rapid progressors (progression from stage 0 to bridging fibrosis or cirrhosis) and slow progressors (progression from stage 0 to stage 1 or 2 fibrosis). One in five patients who developed interval fibrosis progression could be classified a rapid progressor, whereas the rest of the patients were slow progressors; however, in the absence of information on individual patient data and small sample size, more detailed assessment on this differential rate of fibrosis progression was not feasible.
Through a systematic review of the published literature, performed with additional data obtained through contact with authors, we were able to estimate the rate of progression of fibrosis in patients with NAFL and NASH with baseline stage 0 fibrosis, separately. Although previously under-appreciated, we observed that patients with NAFL can develop progressive fibrosis, albeit at a slower rate than patients with NASH. We were not able to identify specific risk factors for development of progressive fibrosis in these patients with NAFL. It is possible that these may represent a subset of patients with NAFL who have mild lobular inflammation, without hepatocyte ballooning or fibrosis, not fulfilling criteria for definite steatohepatitis, but having persistent inflammation, which in turn may lead to progression to definite NASH in due course of time. Pais and colleagues observed that among 16 patients with NAFL with mild lobular inflammation at baseline, 13 patients progressed to typical NASH or bridging fibrosis; on the other hand, of the 5 patients with simple steatosis alone, only 1 progressed to NASH.13 Our observations on FPR were seemingly different than that reported by Argo et al, in their meta-analysis of 10 studies in patients with NASH.11 They estimated an overall FPR of 0.03 stages/year (standard deviation, 0.53 stages/year). In their analysis, they had included a heterogenous cohort of patients with NASH at any stage of fibrosis at baseline, and estimated an overall fibrosis progression, regardless of baseline fibrosis stage. By combining patients at all stages of fibrosis to estimate a single FPR, their analysis was more likely to be affected by selection bias. Individual patient data on how patients moved across stages of fibrosis at baseline and follow-up was not available in their analysis.
Progression of fibrosis in patients with NAFLD represents a complex interplay of genetic factors, extrinsic environmental and intrinsic microbial factors.37-39 The natural history of NAFLD is potentially modifiable through diet and lifestyle changes, and hence, is not a universally progressive condition as was confirmed in our study.2, 40 Previous studies have shown that age and diabetes are two of the major drivers of fibrosis progression. 41-45 In this meta-analysis, age and diabetes did not reach statistical significance, but hypertension and AST:ALT ratio were more closely associated with fibrosis progression. It is plausible that AST:ALT ratio is perhaps a very sensitive discriminative marker of fibrosis progression early on in the natural history of NAFLD. Interestingly, we observed that among a subset of patients who progress, a small proportion of patients may progress rapidly to advanced fibrosis. This may have accounted for the considerable heterogeneity observed in our analysis. While it is possible that this may be due to a longer time interval between serial biopsies in these patients (we were not able to ascertain this in the absence of individual patient data) along with the inherent limitations of sampling variability of a liver biopsy, it is unlikely to be the only reason. It is probable that these groups of patients have inherent genetic or modifiable environmental factors putting them at risk for rapid progression, as has been observed in other chronic liver diseases by independent groups.46-49 This finding underscores the need for further research in identifying the pathogenetic factors that determine the differential rate of fibrosis progression between rapid and slow progressors, and efforts aimed at identifying this subset of patients would allow appropriate clinical follow-up and enrollment in clinical trials of disease-modifying pharmacological agents. Due to limited data on potential rapid and slow progressors in the included studies, a systematic evaluation of such factors was not possible in the current analysis.
Strengths and Limitations
The strengths of this review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria; (b) estimation of FPR in patients with NASH and NAFL separately; (c) estimation of FPR by baseline stage of fibrosis to overcome the limitation of selection bias; (d) separate analysis of observational studies and placebo-controlled RCTs; (e) rigorous evaluation of study quality; and (e) qualitative and quantitative assessment of clinical risk factors associated with development of progressive fibrosis.
Several limitations of the included studies and consequently, the meta-analysis merit further discussion. First, the studies were at high-risk for selection bias. The high attrition rate, due to lack of patient consent or physician recommendation for a follow-up liver biopsy, may have influenced the study results. However, in the absence of accurate non-invasive biomarkers of NASH and fibrosis, paired liver biopsy studies offer the best insight into the natural history of this condition. Second, we were unable to estimate FPR for patients with intermediate and advanced fibrosis at baseline. Clinical indications for repeat biopsy in patients with NAFLD are not well defined. It is conceivable that more health-conscious and informed patients, especially those with baseline intermediate or advanced fibrosis, may have opted for a repeat biopsy after they followed recommendations for a healthy lifestyle, resulting in selection of patients who were slower to progress (or more likely to regress). It is also likely that patients who progressed from advanced fibrosis to decompensated cirrhosis were not advised repeat histological examination, potentially underestimating the rate of fibrosis progression. To overcome this limitation, we specifically estimated FPR in patients with stage 0 fibrosis. Third, the effect of lifestyle, including weight loss and physical activity and potential disease-modifying therapy, which would alter the rate of fibrosis progression, was not adequately captured in individual studies. Likewise, risk factors associated with progressive fibrosis were not consistently and uniformly reported in individual studies. Our assessment of risk factors was based on comparison of patients with progressive fibrosis and those without progressive fibrosis; ideally, a stratified analysis of FPR based on putative risk factors would have been ideal (eg, comparing FPR in diabetics v. non-diabetics, obese v. non-obese etc.) but such data were not available. Fourth, none of the studies were population-based, and majority of the studies were single site studies. There was no centralized reading of biopsies, resulting in variability in the diagnosis of NAFLD and increasing the possibility of misclassification bias. However, despite these limitations, majority of the studies utilized a common histologic scoring system that allows for generalizability of results, in single academic centers with expert gastrointestinal pathologists.
It can be conservatively estimated that over 40% of patients with NAFLD will have progressive fibrosis. Although previously under-recognized, patients with NAFL without fibrosis, may also progress to advanced fibrosis, at a rate of one stage over 14.3 years. In the absence of disease-modifying pharmacological therapy and limited uptake of healthy lifestyle changes, and assuming (a conservative) linear progression of fibrosis, a vast proportion of young obese adults with NAFLD today, may develop advanced fibrosis over the next three decades. This has serious implications for liver-related morbidity and mortality, and highlights a major unmet need for disease-modifying therapy in patients with NASH.
Implications for Clinical Practice and Future Research
In this systematic review and meta-analysis of paired liver biopsy studies in patients with NAFLD, contrary to conventional paradigm, we found that both patients with NAFL and NASH develop progressive hepatic fibrosis, progressing by 1 fibrosis stage (from baseline stage 0 fibrosis) over 14.3 and 7.1 years, respectively. A small subset of these patients may develop rapidly progressive hepatic fibrosis. Based upon this systematic review, assuming that 80-100 million Americans are afflicted with NAFLD in the United States, a significant number of patients with NAFLD are at risk for progressive liver disease than previously appreciated. Effective therapies are needed to halt progression of fibrosis in NAFLD.
Supplementary Material
Acknowledgements
We sincerely thank the following investigators for kindly sharing additional data from their cohort, which enabled this meta-analysis to be undertaken: Manel Abdelmalek, M.D., M.P.H. (Division of Gastroenterology/Hepatology, Duke University Medical Center, Durham, North Carolina); Henry Chan, M.D. (Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong SAR, China); Stergio Kechagias, M.D., Ph.D. (Division of Gastroenterology & Hepatology, Linköping University, Sweden); Carmela Loguercio, M.D. (Department F. Magrassi e A. Lanzara, Second University of Naples, Napoli, Italy); Wade Shields, D.O. (Division of Gastroenterology and Hepatology, Naval Medical Center, San Diego, CA); Vincent Wong, M.B.Ch.B, M.D. (Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong SAR, China).
This work is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and P30CA23100-28, and K23DK090303 to Rohit Loomba, MD, MHSc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The other authors do not have any financial disclosures.
Author Contributions:
• Study concept and design: SS, RL
• Acquisition of data: SS, AMA, LJP
• Analysis and interpretation of data: SS, AMA, ZW, MHM, RL
• Drafting of the manuscript: SS
• Critical revision of the manuscript for important intellectual content: AMA, LJP, ZW, MHM, RL
• Approval of the final manuscript: SS, AMA, LJP, ZW, MHM, RL
• Study supervision: RL
REFERENCES
- 1.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Paredes AH, Torres DM, Harrison SA. Nonalcoholic fatty liver disease. Clin Liver Dis. 2012;16:397–419. doi: 10.1016/j.cld.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–73. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 6.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Loomba R, Wesley R, Pucino F, et al. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–8. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 11.Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–9. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–9. [PubMed] [Google Scholar]
- 13.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–6. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012 [Google Scholar]
- 16.Ghany MG, Kleiner DE, Alter H, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argo CK, Iezzoni JC, Al-Osaimi AM, et al. Thiazolidinediones for the treatment in NASH: sustained benefit after drug discontinuation? J Clin Gastroenterol. 2009;43:565–8. doi: 10.1097/MCG.0b013e31818f4fc2. [DOI] [PubMed] [Google Scholar]
- 23.Evans CD, Oien KA, MacSween RN, et al. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? J Clin Pathol. 2002;55:689–92. doi: 10.1136/jcp.55.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fassio E, Alvarez E, Dominguez N, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–6. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 25.Hamaguchi E, Takamura T, Sakurai M, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33:284–6. doi: 10.2337/dc09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui AY, Wong VW, Chan HL, et al. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Aliment Pharmacol Ther. 2005;21:407–13. doi: 10.1111/j.1365-2036.2005.02334.x. [DOI] [PubMed] [Google Scholar]
- 27.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 28.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 29.Abdelmalek MF, Sanderson SO, Angulo P, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818–26. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 30.Shields WW, Thompson KE, Grice GA, et al. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–63. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 32.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–86. [PubMed] [Google Scholar]
- 33.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratziu V, Charlotte F, Bernhardt C, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–53. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 35.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 36.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 37.Wree A, Broderick L, Canbay A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–36. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 38.Loomba R, Rao F, Zhang L, et al. Genetic covariance between gamma-glutamyl transpeptidase and fatty liver risk factors: role of beta2-adrenergic receptor genetic variation in twins. Gastroenterology. 2010;139:836–45. doi: 10.1053/j.gastro.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stinton L, Loomba R. Commentary: dissecting the PNPLA3 association with liver fat and stiffness, and interaction with diet. Aliment Pharmacol Ther. 2014;39:894–5. doi: 10.1111/apt.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–51. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 44.Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–7. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 45.Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644–54. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, Shiffman ML, Cheung RC, et al. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006;130:1679–87. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 47.Paris AJ, Snapir Z, Christopherson CD, et al. A polymorphism that delays fibrosis in hepatitis C promotes alternative splicing of AZIN1, reducing fibrogenesis. Hepatology. 2011;54:2198–207. doi: 10.1002/hep.24608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patin E, Kutalik Z, Guergnon J, et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143:1244–52. doi: 10.1053/j.gastro.2012.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poynard T, Ratziu V, Charlotte F, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–9. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 50.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–7. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.