Abstract
The covalent attachment of β-D-N-acetylglucosamine monosaccharides (O-GlcNAc) to serine/threonine residues of nuclear and cytoplasmic proteins is a major regulatory mechanism in cell physiology. Aberrant O-GlcNAc modification of signaling proteins, metabolic enzymes, and transcriptional and epigenetic regulators has been implicated in cancer. Relentless growth of cancer cells requires metabolic reprogramming that is intertwined with changes in the epigenetic landscape. This review highlights the emerging role of protein O-GlcNAcylation at the interface of cancer metabolism and epigenetics.
1. Introduction
In the United States, it has been estimated that half of all men and one third of all women will suffer from cancer during their lifetime. The transition of normal cells to cancer cells is marked by a series of genetic and epigenetic changes associated with chromosomal instability, oncogene activation, tumor suppressor functions, gene silencing, and DNA repair deficiency. Epigenetic reprogramming, including alterations in DNA methylation and histone modifications, drive tumorigenesis by altering chromosomal structure and gene expression [11, 31, 39, 52]. Epigenetic DNA modifications such as global hypomethylation and tumor suppressor specific hypermethylation in CpG rich regions have been observed in multiple types of cancer cells [98]. Gene specific alterations in histone modifications, loss of histone H4 acetylation and trimethylation has frequently been observed in cancer cells [9, 11, 31].
Among the most distinguished hallmarks of cancer, metabolic rewiring is characterized by increased glucose uptake and aerobic glycolysis to facilitate rapid cell growth and proliferation [116, 117]. Metabolic rewiring is closely associated with epigenetic reprogramming, which can be influenced by environmental factors, such as diet [65] and genetic defects in metabolic enzymes [2, 7, 24, 29, 57, 58, 89, 111]. Mounting evidence has shown that epigenetics can contribute to reprogramming of cancer metabolism by modulating gene expression [20, 56, 120, 123].
O-GlcNAcylation is a posttranslational covalent modification by O-linkage of a β-N-acetyglucosamine (O-GlcNAc) moiety at serine or threonine residues of proteins [40, 41, 110]. Similar to other posttranslational modifications such as phosphorylation and acetylation, O-GlcNAc can modify a wide spectrum of intracellular proteins, including signaling proteins, transcription factors, metabolic enzymes, and histones, through which it regulates crucial cellular processes, such as signal transduction, transcription, translation, and protein degradation [34, 40, 41, 122-125, 128]. Cellular O-GlcNAc levels are linked to both physiological and disease conditions. A growing body of evidence reveals its relevance to diabetes, cancer, neurodegenerative disease, and cardiovascular disease [22, 26, 30, 94, 126]. As reviewed elegantly elsewhere [32], aberrant O-GlcNAcylation has been observed in a wide range of cancer types, and a regulatory role of O-GlcNAcylation in cancer has begun to be uncovered (Table 1).
Table 1.
Studies related to O-GlcNAc modification in cancer
| Cancer type | Remarks | Ref. |
|---|---|---|
| Colorectal | Aberrant O-GlcNAcylation | [88] |
| Pancreatic | Excessive O-GlcNAcylation is anti-apoptotic | [69] |
| Ovarian | O-GlcNAcylation, cell migration and changes in E-Catherin level are correlated |
[50] |
| Prostate | OGT regulates stability of c-Myc | [47] |
| Breast | O-GlcNAcylated cofilin promotes cell invasion | [45] |
| Breast | Proteomics of O-GlcNAcylated proteins | [18] |
| Pancreatic | Triptolide induces cell death via O-GlcNAcylation of transcription factor Sp1 |
[8] |
| Hepatocellular | O-GlcNAcylation is linked with tumor recurrence | [133] |
| Bladder | Urinary content of OGT/OGA mRNAs helps predicting bladder cancer |
[93] |
| Cholangiocarcinoma | OGT Overexpression and aggressiveness are correlated |
[87] |
| Prostate | Role of OGT in invasion, angiogenesis, and metastasis |
[68] |
| Endometrial | Clinicpathologic conditions are correlated with OGT and OGA mRNA expression |
[64] |
| Breast | Gene expression of O-GlcNAc cycling enzymes | [61] |
| Liver | Crosstalk between HSP27 O-GlcNAcylation and phosphorylation |
[38] |
| Lung and colon | O-GlcNAcylation regulates malignancy | [74] |
|
Chronic
Lymphocytic leukemia |
Chronic lymphocytic leukemia is characterized by aberrant O-GlcNAcylation |
[103] |
| Thyroid | OGA enzyme activity is increased in thyroid cancer |
[63] |
| Breast | OGT regulates oncogenesis through FoxM1 | [14] |
| Erwing sarcoma | O-GlcNAc regulates transcriptional activity of transcription factor FLI1 |
[6] |
| Uterus | O-GlcNAc containing epitope H expressed in smooth muscle cell tumors |
[101] |
| Breast | O-GlcNAc-containing epitope H are localized in the nucleus of epithelial cells |
[42] |
| Lymphoma | Role of O-GlcNAc modification and subcellular distribution of transcription factor Sp1 |
[27] |
| Lymphoma | c-Myc is O-GlcNAcylated at Thr 83, a mutational hot spot in lymphoma |
[21] |
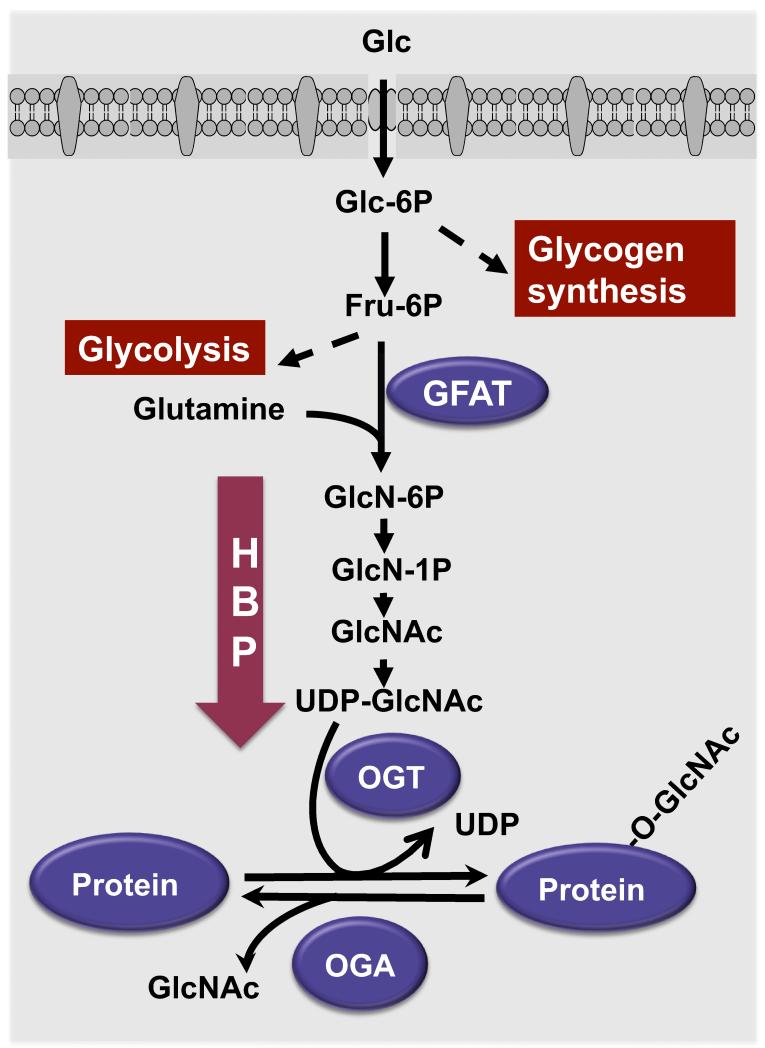
Yet unlike the cycling of phosphorylation, which involves 428 serine/threonine kinase and ~40 phosphatases [4, 76], the cycling of O-GlcNAcylation depends merely on two opposing enzymes: O-linked β-N-acetylglucosamine transferase (OGT) catalyzes the addition of the sugar moiety to the protein and O-GlcNAcase (OGA, NCOAT, or MGEA5) catalyzes the sugar removal. O-GlcNAc modification dynamically responds to environmental and physiological cues, among which nutrient availability is vital. Cellular O-GlcNAcylation levels can fluctuate in response to the availability of nutrients such as glucose, free fatty acid, uridine, and glutamine, endowing this modification with the unique property as a nutrient sensor [13, 32, 40, 67, 118, 127]. The addition of the O-GlcNAc moiety requires the high-energy molecule UDP-GlcNAc, as the donor substrate. UDP-GlcNAc is a major end product of hexosamine biosynthesis pathway (HBP), which is fed by nutrient flux into the cell. In this regard, the cellular O-GlcNAcylation level is believed to reflect on systemic metabolic status (Figure 1).
Figure 1. Hexosamine biosynthetic pathway targets protein O-GlcNAc modification.
Glucose (Glc) uptaken by cells is mainly used in glycogen synthesis and glycolysis pathways. 2~5% of glucose fluxes into hexosamine pathway through the conversion of fructose-6-phosphate (Fru-6P) to glucosamine-6-phosphate (GlcN-6P) by a rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase (GFAT). Subsequent acetylation and uridylation of GlcN-6P produce UDP-GlcNAc as a substrate for protein glycosylation. O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) catalyze the addition and removal of O-GlcNAc on proteins, respectively.
The role of O-GlcNAc modification in epigenetics has emerged as a topic of interest. OGT and OGA can target histones and enzymes involved in epigenetic modifications, which could potentially influence gene expression. O-GlcNAc can serve as the link between nutrient availability and epigenetics, as epigenetic modifications also require nutrient derived metabolites as substrates. In this review, we summarize the current understanding of the role of O-GlcNAc at the interface of cancer metabolism and epigenetics.
2. Protein O-GlcNAcylation in cancer metabolism
2.1 O-GlcNAcylation of signaling proteins
In analogy to phosphorylation, O-GlcNAcylation is a regulatory mechanism that modifies cellular proteins at serine and threonine residues in response to stress, hormones and nutrients. Crosstalk between O-GlcNAcylation and phosphorylation has been implicated in regulation of signal transduction in cancer [44, 107]. Direct O-GlcNAcylation of kinases and phosphatases may contribute to cancer phenotypes. Akt Ser 473 undergoes both phosphorylation and O-GlcNAcylation in murine pancreatic cells, and the balance between O-GlcNAcylation and phosphorylation determines cell survival or apoptosis [54]. In thyroid anaplastic cancer cells, down-regulation of OGA activity increases cell proliferation partially depending on the IGF-1-Akt1-GSK3 -cyclin D1 pathway [62]. OGT targets several mitotic kinases and inhibits cyclin-dependent kinase 1 (CDK1) activity by increasing phosphorylation, suggesting a vital role for OGT in cell division [115]. Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays an important role in various cancers, such as prostate cancer, liver cancer and neuroblastomas [72, 73, 91]. This kinase has been implicated as a link between metabolic state and apoptosis [82]. Moreover, acute hyperglycaemia triggers O-GlcNAcylation and autonomous activation of CaMKII in cardiomyocytes, pointing to the role of CaMKII O-GlcNAcylation as a metabolic sensor [30]. Further studies are expected to provide direct evidence that O-GlcNAcylation of kinases and/or phosphatases regulates cancer metabolism.
2.2 O-GlcNAcylation of metabolic enzymes
Cancer cells exhibit increased HBP flux and O-GlcNAcylation of multiple metabolic enzymes [18, 70]. The O-GlcNAc moiety has been detected on a majority of glycolytic enzymes. phosphofructokinase-1 (PFK1) catalyzes the first committed step unique to the glycolytic pathway. O-GlcNAcylation at Ser 529 inhibits PFK1 activity, thereby rerouting glucose flux through the pentose phosphate pathway (PPP) to increase biosynthetic precursors for cell growth. The mechanism of inhibition by O-GlcNAc is possibly due to shielding the substrate-binding site and dampening oligomerization of PFK1 [125]. Pyruvate kinase catalyzes the last committed step in glycolysis. O-GlcNAcylated pyruvate kinase muscle isozyme 2 (PKM2) is present in breast cancer but not the normal tissues; however, whether O-GlcNAcylation of PKM2 plays a regulatory role remains unknown [18]. Additionally, proteomic analysis reveals the presence of O-GlcNAc moieties on many enzymes involved in amino acid and nucleotide metabolism, such as, phosphoglycerate dehydrogenase (PHGDH), argininosuccinate synthetase (ASS), and thymidylate synthase (TYMS) [80]. Therefore, it is very likely that O-GlcNAcylation is involved in reprogramming the entire metabolic network in cancer cells.
2.3 O-GlcNAcylation of transcription factors
A growing number of transcription factors involved in cancer have been shown to harbor O-GlcNAc. This modification regulates transcription factors through multiple mechanisms, including protein-protein interaction, protein stability, transcriptional activity, DNA-binding activity, nucleo-cytoplasmic shuttling, and transcription factor expression [85]. The oncoprotein c-Myc is regulated by reciprocal glycosylation and phosphorylation at Thr 58 [20, 21, 53]. OGT increases the stability of c-Myc proteins and, on the other hand, c-Myc can drive global O-GlcNAc modification and act as a potential upstream regulator of OGT target genes [47] [77]. Consistently, the expression of c-Myc and OGT is tightly correlated in human prostate cancer samples [47]. NF-κB is O-GlcNAcylated at Thr 322 and Thr 352, and Thr 352 O-GlcNAcylation is especially critical for releasing NF-κB from I B inhibition and allowing nuclear translocation [121]. In pancreatic cancer cells, hyper-O-GlcNAcylation of NF-κB promotes activating phosphorylation at Ser 536, nuclear translocation, and transcriptional activity [69]. Furthermore, the activation of the IKK-NF-κB pathway by loss of tumor suppresser p53 increases aerobic glycolysis [55]. In MCF7 cells, serum-stimulated cell cycle entry is associated with progressive OGT binding and O-GlcNAcylation of β-catenin [83]. The expression of β-catenin in colon cancer cells is also correlated with HBP flux and O-GlcNAcylation [84]. Hence, O-GlcNAcylation of transcription factors may be an important layer of regulation of cancer cell growth, proliferation, and metabolism.
3. Protein O-GlcNAcylation in epigenetics
3.1 The epigenetic code
Genetic and epigenetic regulation is essential for life. Cancer arises from a combination of changes to the genome and the epigenome [10]. An epigenome is defined as the complete set of DNA methylation and posttranslational modifications of histone proteins [5, 12]. These covalent modifications alter chromatin structure and regulate gene expression [9, 59]. Histones can be posttranslationally modified by phosphorylation, acetylation, succinylation, malonylation, methylation, and ubiquitination [5, 59, 90, 119, 130]. Lysine acetylation of histones by histone acetyltransferases (HATs) generally correlates with increased transcriptional activity, whereas deacetylation by histone deacetylases (HDACs) is frequently involved in gene silencing in many human cancer types [92]. Histone lysine methylation is implicated in a wide range of processes such as tissue-specific gene expression, maintenance of genome stability, stem cell self-renewal and lineage commitment. Molecular consequences of histone lysine methylation at different sites vary widely. For example, H3K4 methylation is a signature for transcriptionally active genes, whereas H3K9 methylation is generally associated with repressed genes [105]. H3K27 trimethylation is typical of silent chromatin, but also present at the promoters of poised developmental genes in stem cells. Depending on the context and degree, H3K36 methylation regulates different molecular events such as transcriptional activation, suppression of aberrant transcription during transcriptional elongation, and alternative splicing [114]. Globally, H3K79 methylation is associated with actively transcribing genes, and is implicated in transcriptional regulation, DNA damage response, and cell cycle control [81]. Additionally, H2B monoubiquitination facilitates H3K4 and H3K79 methylation [104]. Monoubiquitination of histone proteins, primarily H2A and H2B, is linked to gene silencing and activation. These histone modifications are essential for fundamental biological processes and disease conditions, such as cancer [10, 51].
Recent studies reveal that histones also bear O-GlcNAcylation. Intriguingly, OGA is a bifunctional enzyme harboring O-GlcNAc cleavage activity as well as HAT activity, implying an intrinsic relationship between histone O-GlcNAcylation and acetylation. OGA HAT activity has been implicated in orexin neurogenesis [43]. However, we are just beginning to understand the function of histone O-GlcNAcylation as part of the epigenetic code.
3.2 O-GlcNAcylation of histones
Recent reports indicate a regulatory role for histone O-GlcNAcylation in mitosis, chromatin dynamics and gene expression [33, 96, 97]. Analysis of histone proteins in HeLa cells has shown that histones H2A, H2B, and H4 are dynamically O-GlcNAcylated, which depends on the phase of cell cycle and cellular stress conditions [97]. Sakabe et al. reported that both OGT catalytic activity and O-GlcNAc levels on histones (particularly H3) are reduced during early mitosis and are gradually increased during late mitosis to G1 phase [96]. Zhang et al. described that histone O-GlcNAcylation is reduced in S phase and that H3 O-GlcNAcylation persists through late G2 and mitosis [130]. Forced expression of OGT alters a variety of histone modifications, such as H3K9 acetylation, S10 phosphorylation, and R17 and K27 methylation, indicating that O-GlcNAc signaling might regulate chromatin dynamics by affecting other histone marks [96, 97]. It was also reported that H2B O-GlcNAcylation at S112 is sensitive to glucose and facilitates adjacent K120-monoubiquitination that is associated with transcriptionally active loci [34]. Crosstalk between histone O-GlcNAcylation and phosphorylation may be important for epigenetic regulation. O-GlcNAcylation of histone H3 at T32 is inversely correlated with phosphorylation at S10, S28, and S32 during cell cycle progression, further indicating a role for histone O-GlcNAcylation in cell cycle regulation [33, 130]. Aurora B kinase and protein phosphatase 1 (PP1) mutually regulate H3 phosphorylation at S10, S28 and S32 [23, 36, 78]. Also, It is known that aurora B and PP1 are in a transient complex with OGT and OGA during mitosis [107, 108]. Therefore, it is possible that aurora B, PP1, OGT and OGA cooperatively regulate chromatin dynamics, gene expression and cell division by controlling histone phosphorylation and O-GlcNAcylation [19, 107].
3.3 O-GlcNAcylation of chromatin regulators
Oncogenic transformation frequently involves global DNA hypomethylation, gene promoter hypermethylation and aberrant histone posttranslational modifications. Evidence is emerging that OGT can affect local and global chromatin states by interacting with various enzymes responsible for DNA methylation and histone modifications [131].
The Polycomb group (PcG) proteins regulate patterning of body segments by silencing Hox genes during Drosophila development [86]. Among the earliest evidence that OGT is involved in epigenetic regulation, two groups reported that Drosophila OGT is encoded by a PcG gene known as super sex combs (sxc) [35, 106]. Sxc/Ogt glycosylates another member of PcG proteins, Polyhomeotic, to facilitate its binding to target sites [35, 106]. In Drosophila and mammals, PcG proteins assemble into two Polycomb repressive complexes (PRC1 and PRC2), which play critical roles in stem cell fate determination, embryonic development, and cancer [3, 100]. PRC1 mediates H2A monoubiquitylation that interferes with transcriptional elongation, whereas PRC2 is responsible for H3K27 di- and tri-methylation, known as repressive epigenetic marks. PRC2 integrity is essential for OGT protein stability and cellular O-GlcNAc distribution in mouse embryonic stem cells, suggesting a link between O-GlcNAcylation and Polycomb repression in mammals [79]. Human homologues of Drosophila additional sex combs, ASXL1 and AXL2, are frequently mutated in myeloid malignancies [1].
Genome-wide mapping reveals that histone methylation reliably discriminate the genes that are expressed, poised for expression, or stably repressed [75]. Mixed lineage leukemia 5 (MLL5) is a SET domain-containing methyltransferase that mediates H3K4 methylation amenable to transcriptional activation [132]. Host cell factor C1 (HCF-1) is a regulator of cell cycle that is subject to the proteolytic maturation catalyzed by OGT [15, 66]. HCF-1 can recruit MLL5 to E2F1-responsive promoters to induce H3K4 trimethylation, transcriptional activation, and cell cycle progression [132].
In addition to H3K4 methyltransferases, HCF-1 interacts with a variety of histone-modifying enzymes, such as the H3K9 demethylase LSD1, histone acetyltransferase, and mSin3/histone deacetylase (HDAC) complexes [60]. Strikingly, approximately 50% of nuclear OGT proteins are associated with HCF-1, suggesting a functional link between O-GlcNAcylation and distinct histone modifications through this abundant nuclear complex [15, 25]. Recently, we observed that the OGT/HCF-1 complex recruits the ubiquitin carboxyl-terminal hydrolase BAP1 (BRCA associated protein 1) to deubiquitinate and stabilize PGC1α, a key metabolic regulator [95]. BAP1 is also a component of the Polycomb repressive deubiquitinase complex to deubiquitinate histone H2A [99]. BAP1 acts as a tumor suppressor and mutations in BAP1 have been observed in multiple cancer types such as melanoma, leukemia, lung, ovarian, breast and renal cancer [16, 113]. Therefore, O-GlcNAcylation may play a major role in epigenetic regulation by co-opting histone phosphorylation, methylation, acetylation and ubiquitination.
3.4 OGT and DNA methylation
DNA methylation at the 5-carbon position of cytosine (5mC) is an epigenetic mechanism that is important for embryo development, stem cell differentiation, tissue-specific gene expression, X-chromosome inactivation and oncogenic transformation [46]. This biochemical reaction is catalyzed by DNA methyltransferases (DMNTs) [17, 46]. Conversely, active DNA demethylation is initiated by a group of Fe(II)/2-oxoglutarate-dependent dioxygenases known as ten-eleven translocation (TET) proteins. TET proteins hydrolyze 5mC to produce 5hmC (5-hyxroxymethyl cytosine) [37, 109]. Furthermore, TET proteins can convert 5mC to 5-formylcytocine (5fC) and 5-carboxylcytosine (5-CaC) in mouse embryonic stem cells and mouse organs [48]. Several recent studies elegantly illustrate physical and functional interactions between TET proteins and OGT [19, 71, 102, 112, 129]. One study reported that TET2 and TET3 bind to OGT, in which OGT does not seem to affect the function of TET proteins, but TET proteins facilitate the association of OGT with chromatin and O-GlcNAcylation of histone H2B at S112 [19]. Notably, S112 O-GlcNAcylation promotes K120 monoubiquitination of H2B and transcriptional activation [34]. Therefore, it can be speculated that TET2 facilitates gene transcription through DNA demethylation and OGT recruitment at transcriptionally active promoters. In embryonic stem cells, OGT preferentially associates with TET1 across the genome in close proximity of CpG-rich transcriptional start sites [112]. In 293T cells, TET2/3 and OGT co-localize at active promoters and promote the binding of H3K4 methyltransferase SET1/COMPASS complex to chromatin [28]. These studies suggest that OGT and TET proteins act in concert to regulate transcription.
Conclusions
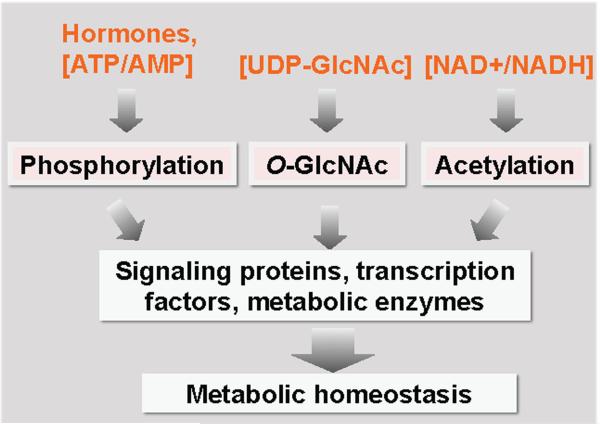
Posttranslational modifications are a major toolbox in cell physiology. Availability of metabolites, such as UDP-GlcNAc, acetyl-CoA and ATP, is essential for O-GlcNAcylation, acetylation and phosphorylation, respectively. Combinatorial changes in different posttranslational modifications, referred to as the “PTM code”, dictate protein activity and ultimately influence metabolic homeostasis (Figure 2). Cancer cells appear to alter HBP flux and O-GlcNAcylation to reprogram metabolism in favor of rapid growth. An exciting new area is to understand how O-GlcNAc orchestrates metabolic pathways for biosynthesis by interplaying with other posttranslational modifications on a wide variety of signaling proteins and metabolic enzymes.
Figure 2. Nutritional and hormonal regulation of metabolism through the “PTM code”.
External hormonal and nutritional cues modulate intracellular fluctuation of ATP/AMP, UDP-GlcNAc, Acetyl-CoA and NAD+ levels. These metabolites influence phosphorylation, O-GlcNAcylation, and acetylation of a wide variety of intracellular proteins such as signaling proteins, metabolic enzymes, transcriptional factors/cofactors and histones. Combinatorial changes in these posttranslational modifications may constitute the “PTM code” that integrates environmental cues to regulate metabolic homeostasis. Alteration of the “PTM code” by carcinogens may be central to metabolic and epigenetic reprogramming in cancer.
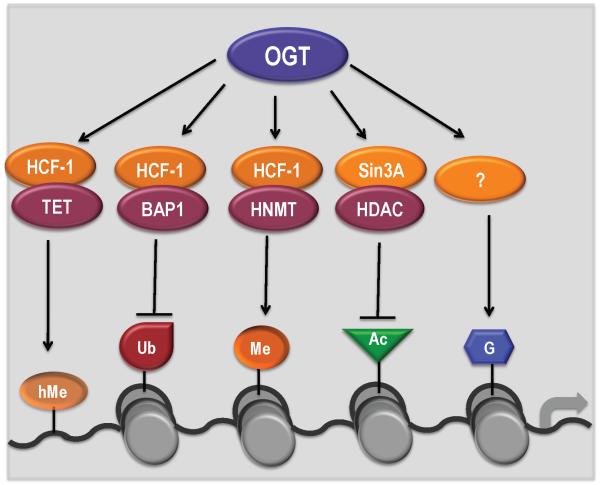
O-GlcNAcylation is a recently identified addition to the epigenetic code. OGT can O-GlcNAcylate histones H2A, H2B, and H4 at specific residues; however, the molecular determinants of site specificity and the functional consequences of histone O-GlcNAcylation are largely unknown (Figure 3). OGT interacts with an assortment of protein complexes involved in phosphorylation, ubiquitination, methylation, and acetylation of histone proteins, but the functional link between these modifications remains to be determined (Figure 3). Another enzyme essential for O-GlcNAc cycling is OGA, which harbors HAT and O-GlcNAcase activities. Whether both activities are involved in gene regulation and how they cooperate warrant careful investigation. Association with both DNA methylation and histone modifications suggests a role for OGT in integrating transcriptional and epigenetic regulation. Further studies are required to decipher overall biological information encoded therein. This might provide an epigenetic explanation for the impact of aberrant O-GlcNAcylation on tumorigenesis. The epigenome is susceptible to metabolic disturbance such as diet, which is well known to affect cancer [40, 49]. Therefore, O-GlcNAc signaling may play a central role in integrating metabolic and epigenetic reprogramming in cancer. A better understanding of O-GlcNAc signaling in cancer initiation, progression and metastasis would help to identify new targets that can be used for diagnosis, prevention, and treatment of human cancer.
Figure 3. OGT is associated with multiple epigenetic modifications.
OGT interacts with the HCF-1/TET complex that mediates DNA demethylation, the HCF-1/BAP1 complex that mediates histone deubiquitination, the HCF-1/HNMT complex that mediates histone methylation, and the Sin3A/HDAC complex that mediates histone deacetylation. OGT directly modifies histones through unknown adaptor proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abdel-Wahab O, Dey A. The ASXL-BAP1 axis: new factors in myelopoiesis, cancer and epigenetics. Leukemia. 2013;27:10–15. doi: 10.1038/leu.2012.288. [DOI] [PubMed] [Google Scholar]
- [2].Adam J, Yang M, Bauerschmidt C, Kitagawa M, O’Flaherty L, Maheswaran P, Ozkan G, Sahgal N, Baban D, Kato K, Saito K, Iino K, Igarashi K, Stratford M, Pugh C, Tennant DA, Ludwig C, Davies B, Ratcliffe PJ, El-Bahrawy M, Ashrafian H, Soga T, Pollard PJ. A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell reports. 2013;3:1440–1448. doi: 10.1016/j.celrep.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013;140:2525–2534. doi: 10.1242/dev.091553. [DOI] [PubMed] [Google Scholar]
- [4].Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein Tyrosine Phosphatases in the Human Genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- [5].F. American Association for Cancer Research Human Epigenome Task N.o.E.S.A.B. European Union, Moving AHEAD with an international human epigenome project. Nature. 2008;454:711–715. doi: 10.1038/454711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bachmaier R, Aryee DN, Jug G, Kauer M, Kreppel M, Lee KA, Kovar H. O-GlcNAcylation is involved in the transcriptional activity of EWS-FLI1 in Ewing’s sarcoma. Oncogene. 2009;28:1280–1284. doi: 10.1038/onc.2008.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta neuropathologica. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- [8].Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, Saluja AK. Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1. The Journal of biological chemistry. 2013;288:33927–33938. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [10].Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications, Nature reviews. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Developmental cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- [12].Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- [13].Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochimica et biophysica acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- [15].Capotosti F, Guernier S, Lammers F, Waridel P, Cai Y, Jin J, Conaway JW, Conaway RC, Herr W. O-GlcNAc transferase catalyzes site-specific proteolysis of HCF-1. Cell. 2011;144:376–388. doi: 10.1016/j.cell.2010.12.030. [DOI] [PubMed] [Google Scholar]
- [16].Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer, Nature reviews. Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chahwan R, Wontakal SN, Roa S. Crosstalk between genetic and epigenetic information through cytosine deamination. Trends in genetics : TIG. 2010;26:443–448. doi: 10.1016/j.tig.2010.07.005. [DOI] [PubMed] [Google Scholar]
- [18].Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013;13:2088–2099. doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- [19].Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493:561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chou TY, Dang CV, Hart GW. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci U S A. 1995;92:4417–4421. doi: 10.1073/pnas.92.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. The Journal of biological chemistry. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- [22].Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: Roles in insulin resistance and glucose toxicity. American Journal of Physiology - Endocrinology and Metabolism. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Molecular and cellular biology. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Daou S, Mashtalir N, Hammond-Martel I, Pak H, Yu H, Sui G, Vogel JL, Kristie TM, Affar el B. Crosstalk between O-GlcNAcylation and proteolytic cleavage regulates the host cell factor-1 maturation pathway. Proc Natl Acad Sci U S A. 2011;108:2747–2752. doi: 10.1073/pnas.1013822108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked β-N-acetylglucosamine: A novel effector of cardiomyocyte metabolism and function. Journal of Molecular and Cellular Cardiology. 2012;52:538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dauphinee SM, Ma M, Too CK. Role of O-linked beta-N-acetylglucosamine modification in the subcellular distribution of alpha4 phosphoprotein and Sp1 in rat lymphoma cells. Journal of cellular biochemistry. 2005;96:579–588. doi: 10.1002/jcb.20508. [DOI] [PubMed] [Google Scholar]
- [28].Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, Shih AH, Levine RL, Bernard O, Mercher T, Solary E, Urh M, Daniels DL, Fuks F. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. The EMBO journal. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ducray F, Marie Y, Sanson M. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360:2248–2249. [PubMed] [Google Scholar]
- [30].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps, Nature reviews. Genetics. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- [32].Fardini Y, Dehennaut V, Lefebvre T, Issad T. O-GlcNAcylation: A New Cancer Hallmark? Frontiers in endocrinology. 2013;4:99. doi: 10.3389/fendo.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fong JJ, Nguyen BL, Bridger R, Medrano EE, Wells L, Pan S, Sifers RN. beta-N-Acetylglucosamine (O-GlcNAc) is a novel regulator of mitosis-specific phosphorylations on histone H3. The Journal of biological chemistry. 2012;287:12195–12203. doi: 10.1074/jbc.M111.315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, Imai Y, Kim J, He HH, Igarashi K, Kanno J, Ohtake F, Kitagawa H, Roeder RG, Brown M, Kato S. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–96. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- [36].Goto H, Yasui Y, Nigg EA, Inagaki M. Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes to cells : devoted to molecular & cellular mechanisms. 2002;7:11–17. doi: 10.1046/j.1356-9597.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- [37].Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guo K, Gan L, Zhang S, Cui FJ, Cun W, Li Y, Kang NX, Gao MD, Liu KY. Translocation of HSP27 into liver cancer cell nucleus may be associated with phosphorylation and O-GlcNAc glycosylation. Oncology reports. 2012;28:494–500. doi: 10.3892/or.2012.1844. [DOI] [PubMed] [Google Scholar]
- [39].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [40].Hanover JA, Krause MW, Love DC. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nature Reviews Molecular Cell Biology. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- [41].Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross Talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. 2011. pp. 825–858. in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Havaki S, Voloudakis-Baltatzis I, Goutas N, Arvanitis LD, Vassilaros SD, Arvanitis DL, E C. Kittas, Marinos, Nuclear localization of cytokeratin 8 and the O-linked N-acetylglucosamine-containing epitope H in epithelial cells of infiltrating ductal breast carcinomas: a combination of immunogold and EDTA regressive staining methods. Ultrastructural pathology. 2006;30:177–186. doi: 10.1080/01913120600689806. [DOI] [PubMed] [Google Scholar]
- [43].Hayakawa K, Hirosawa M, Tabei Y, Arai D, Tanaka S, Murakami N, Yagi S, Shiota K. Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. The Journal of biological chemistry. 2013;288:17099–17110. doi: 10.1074/jbc.M113.455899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS letters. 2010;584:2526–2538. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- [45].Huang X, Pan Q, Sun D, Chen W, Shen A, Huang M, Ding J, Geng M. O-GlcNAcylation of cofilin promotes breast cancer cell invasion. The Journal of biological chemistry. 2013;288:36418–36425. doi: 10.1074/jbc.M113.495713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A. 2011;108:3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer research. 2013;73:5277–5287. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- [48].Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- [50].Jin FZ, Yu C, Zhao DZ, Wu MJ, Yang Z. A correlation between altered O-GlcNAcylation, migration and with changes in E-cadherin levels in ovarian cancer cells. Experimental cell research. 2013;319:1482–1490. doi: 10.1016/j.yexcr.2013.03.013. [DOI] [PubMed] [Google Scholar]
- [51].Johnsen SA. The enigmatic role of H2Bub1 in cancer. FEBS letters. 2012;586:1592–1601. doi: 10.1016/j.febslet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- [52].Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kamemura K, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Progress in nucleic acid research and molecular biology. 2003;73:107–136. doi: 10.1016/s0079-6603(03)01004-3. [DOI] [PubMed] [Google Scholar]
- [54].Kang ES, Han D, Park J, Kwak TK, Oh MA, Lee SA, Choi S, Park ZY, Kim Y, Lee JW. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Experimental cell research. 2008;314:2238–2248. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- [55].Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nature cell biology. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- [56].Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 2009;106:3431–3436. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kerr DA, Lopez HU, Deshpande V, Hornicek FJ, Duan Z, Zhang Y, Rosenberg AE, Borger DR, Nielsen GP. Molecular distinction of chondrosarcoma from chondroblastic osteosarcoma through IDH1/2 mutations. The American journal of surgical pathology. 2013;37:787–795. doi: 10.1097/PAS.0b013e31827ab703. [DOI] [PubMed] [Google Scholar]
- [58].King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- [59].Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [60].Kristie TM, Liang Y, Vogel JL. Control of alpha-herpesvirus IE gene expression by HCF-1 coupled chromatin modification activities. Biochimica et biophysica acta. 2010;1799:257–265. doi: 10.1016/j.bbagrm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Krzeslak A, Forma E, Bernaciak M, Romanowicz H, Brys M. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clinical and experimental medicine. 2012;12:61–65. doi: 10.1007/s10238-011-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Krzeslak A, Jozwiak P, Lipinska A. Down-regulation of beta-N-acetyl-D-glucosaminidase increases Akt1 activity in thyroid anaplastic cancer cells. Oncology reports. 2011;26:743–749. doi: 10.3892/or.2011.1333. [DOI] [PubMed] [Google Scholar]
- [63].Krzeslak A, Pomorski L, Lipinska A. Elevation of nucleocytoplasmic beta-N-acetylglucosaminidase (O-GlcNAcase) activity in thyroid cancers. International journal of molecular medicine. 2010;25:643–648. doi: 10.3892/ijmm_00000387. [DOI] [PubMed] [Google Scholar]
- [64].Krzeslak A, Wojcik-Krowiranda K, Forma E, Bienkiewicz A, Brys M. Expression of genes encoding for enzymes associated with O-GlcNAcylation in endometrial carcinomas: clinicopathologic correlations. Ginekologia polska. 2012;83:22–26. [PubMed] [Google Scholar]
- [65].Landecker H. Food as exposure: Nutritional epigenetics and the new metabolism. Biosocieties. 2011;6:167–194. doi: 10.1057/biosoc.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lazarus MB, Jiang J, Kapuria V, Bhuiyan T, Janetzko J, Zandberg WF, Vocadlo DJ, Herr W, Walker S. HCF-1 is cleaved in the active site of O-GlcNAc transferase. Science. 2013;342:1235–1239. doi: 10.1126/science.1243990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. The Journal of biological chemistry. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. The Journal of biological chemistry. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ma Z, Vosseller K. O-GlcNAc in cancer biology. Amino acids. 2013;45:719–733. doi: 10.1007/s00726-013-1543-8. [DOI] [PubMed] [Google Scholar]
- [71].Mariappa D, Pathak S, van Aalten DM. A sweet TET-a-tete-synergy of TET proteins and O-GlcNAc transferase in transcription. The EMBO journal. 2013;32:612–613. doi: 10.1038/emboj.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McGinnis KM, Wang KK, Gnegy ME. Calcium/calmodulin-dependent protein kinase inhibition potentiates thapsigargin-mediated cell death in SH-SY5Y human neuroblastoma cells. Neuroscience letters. 2001;301:99–102. doi: 10.1016/s0304-3940(01)01629-9. [DOI] [PubMed] [Google Scholar]
- [73].Meng Z, Li T, Ma X, Wang X, Van Ness C, Gan Y, Zhou H, Tang J, Lou G, Wang Y, Wu J, Yen Y, Xu R, Huang W. Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca(2)(+)/calmodulin-dependent protein kinase II. Molecular cancer therapeutics. 2013;12:2067–2077. doi: 10.1158/1535-7163.MCT-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochimica et biophysica acta. 2011;1812:514–519. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [75].Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Moorhead GBG, Trinkle-Mulcahy L, Ulke-Lemée A. Emerging roles of nuclear protein phosphatases. Nature Reviews Molecular Cell Biology. 2007;8:234–244. doi: 10.1038/nrm2126. [DOI] [PubMed] [Google Scholar]
- [77].Morrish F, Isern N, Sadilek M, Jeffrey M, Hockenbery DM. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28:2485–2491. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Murnion ME, Adams RR, Callister DM, Allis CD, Earnshaw WC, Swedlow JR. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. The Journal of biological chemistry. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- [79].Myers SA, Panning B, Burlingame AL. Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2011;108:9490–9495. doi: 10.1073/pnas.1019289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, Zhao Y. Global identification of O-GlcNAc-modified proteins. Analytical chemistry. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- [81].Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes & development. 2011;25:1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Olivier-Van Stichelen S, Drougat L, Dehennaut V, El Yazidi-Belkoura I, Guinez C, Mir AM, Michalski JC, Vercoutter-Edouart AS, Lefebvre T. Serum-stimulated cell cycle entry promotes ncOGT synthesis required for cyclin D expression. Oncogenesis. 2012;1:e36. doi: 10.1038/oncsis.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Olivier-Van Stichelen S, Guinez C, Mir AM, Perez-Cervera Y, Liu C, Michalski JC, Lefebvre T. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of beta-catenin and cell proliferation. American journal of physiology. Endocrinology and metabolism. 2012;302:E417–424. doi: 10.1152/ajpendo.00390.2011. [DOI] [PubMed] [Google Scholar]
- [85].Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochimica et biophysica acta. 2010;1799:353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning, Nature reviews. Genetics. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- [87].Phoomak C, Silsirivanit A, Wongkham C, Sripa B, Puapairoj A, Wongkham S. Overexpression of O-GlcNAc-Transferase Associates with Aggressiveness of Mass-Forming Cholangiocarcinoma. Asian Pacific journal of cancer prevention : APJCP. 2012;13(Suppl):101–105. [PubMed] [Google Scholar]
- [88].Phueaouan T, Chaiyawat P, Netsirisawan P, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J, Champattanachai V. Aberrant O-GlcNAc-modified proteins expressed in primary colorectal cancer. Oncology reports. 2013;30:2929–2936. doi: 10.3892/or.2013.2794. [DOI] [PubMed] [Google Scholar]
- [89].Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Human pathology. 2012;43:1541–1551. doi: 10.1016/j.humpath.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [90].Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- [91].Rokhlin OW, Taghiyev AF, Bayer KU, Bumcrot D, Koteliansk VE, Glover RA, Cohen MB. Calcium/calmodulin-dependent kinase II plays an important role in prostate cancer cell survival. Cancer biology & therapy. 2007;6:732–742. doi: 10.4161/cbt.6.5.3975. [DOI] [PubMed] [Google Scholar]
- [92].Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Molecular oncology. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P, Lipinski M. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clinical laboratory. 2012;58:579–583. [PubMed] [Google Scholar]
- [94].Ruan H-B, Singh JP, Li M-D, Wu J, Yang X. Cracking the O-GlcNAc code in metabolism. Trends in Endocrinology & Metabolism. 2013;24:301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ruan HB, Han X, Li MD, Singh JP, Qian K, Azarhoush S, Zhao L, Bennett AM, Samuel VT, Wu J, Yates JR, 3rd, Yang X. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell metabolism. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. The Journal of biological chemistry. 2010;285:34460–34468. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sakabe K, Wang Z, Hart GW. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci U S A. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sandoval J, Esteller M. Cancer epigenomics: beyond genomics. doi: 10.1016/j.gde.2012.02.008. [DOI] [PubMed] [Google Scholar]
- [99].Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schwartz YB, Pirrotta V. A new world of Polycombs: unexpected partnerships and emerging functions, Nature reviews. Genetics. 2013;14:853–864. doi: 10.1038/nrg3603. [DOI] [PubMed] [Google Scholar]
- [101].Sgantzos MN, Galani V, Arvanitis LD, Charchanti A, Psathas P, Nakou M, Havaki S, Kallioras V, Marinos E, Vamvakopoulos NC, Kittas C. Expression of the O-linked N-acetylglucosamine containing epitope H in normal myometrium and uterine smooth muscle cell tumors. Pathology, research and practice. 2007;203:31–37. doi: 10.1016/j.prp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [102].Shi FT, Kim H, Lu W, He Q, Liu D, Goodell MA, Wan M, Songyang Z. Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. The Journal of biological chemistry. 2013;288:20776–20784. doi: 10.1074/jbc.M113.460386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–1598. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annual review of biochemistry. 2012;81:65–95. doi: 10.1146/annurev-biochem-051710-134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes & development. 2011;25:781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sinclair DA, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, Vocadlo DJ, Brock HW, Honda BM. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc Natl Acad Sci U S A. 2009;106:13427–13432. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Molecular biology of the cell. 2008;19:4130–4140. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. The Journal of biological chemistry. 1984;259:3308–3317. [PubMed] [Google Scholar]
- [111].Vandy FC, Sisk G, Berguer R. Synchronous carotid body and thoracic paraganglioma associated with a germline SDHC mutation. Journal of vascular surgery. 2011;53:805–807. doi: 10.1016/j.jvs.2010.09.064. [DOI] [PubMed] [Google Scholar]
- [112].Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, Pasini D. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Molecular cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- [113].Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer research. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3, Nature reviews. Molecular cell biology. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Science signaling. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- [117].Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [118].Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cellular and molecular life sciences : CMLS. 2003;60:222–228. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, Boeke JD, Zhao Y. Lysine succinylation and lysine malonylation in histones. Molecular & cellular proteomics : MCP. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nature cell biology. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- [121].Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- [123].Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc Transferase to Promoters by Corepressor mSin3A: Coupling Protein O-GlcNAcylation to Transcriptional Repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- [125].Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, Iii, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yuzwa SA, Vocadlo DJ. O-GlcNAc modification and the tauopathies: Insights from chemical biology. Current Alzheimer Research. 2009;6:451–454. doi: 10.2174/156720509789207967. [DOI] [PubMed] [Google Scholar]
- [127].Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochimica et Biophysica Acta (BBA) - General Subjects. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- [128].Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc Modification Is an Endogenous Inhibitor of the Proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- [129].Zhang Q, Liu X, Gao W, Li P, Hou J, Li J, Wong J. Differential Regulation of the Ten-Eleven Translocation (TET) Family of Dioxygenases by O-Linked beta-N-Acetylglucosamine Transferase (OGT) The Journal of biological chemistry. 2014;289:5986–5996. doi: 10.1074/jbc.M113.524140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang S, Roche K, Nasheuer HP, Lowndes NF. Modification of histones by sugar beta-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated. The Journal of biological chemistry. 2011;286:37483–37495. doi: 10.1074/jbc.M111.284885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes & development. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- [132].Zhou P, Wang Z, Yuan X, Zhou C, Liu L, Wan X, Zhang F, Ding X, Wang C, Xiong S, Wang Z, Yuan J, Li Q, Zhang Y. Mixed lineage leukemia 5 (MLL5) protein regulates cell cycle progression and E2F1-responsive gene expression via association with host cell factor-1 (HCF-1) The Journal of biological chemistry. 2013;288:17532–17543. doi: 10.1074/jbc.M112.439729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Medical oncology. 2012;29:985–993. doi: 10.1007/s12032-011-9912-1. [DOI] [PubMed] [Google Scholar]