Abstract
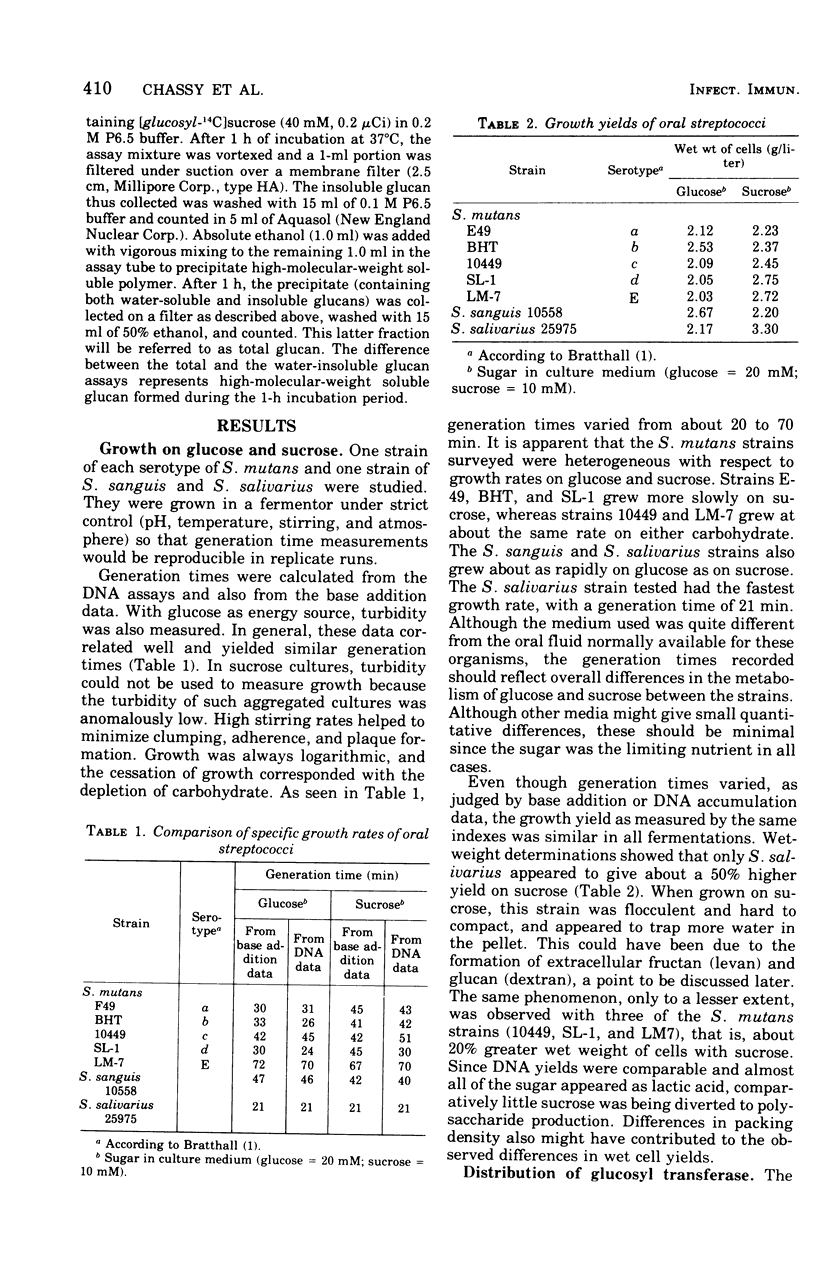
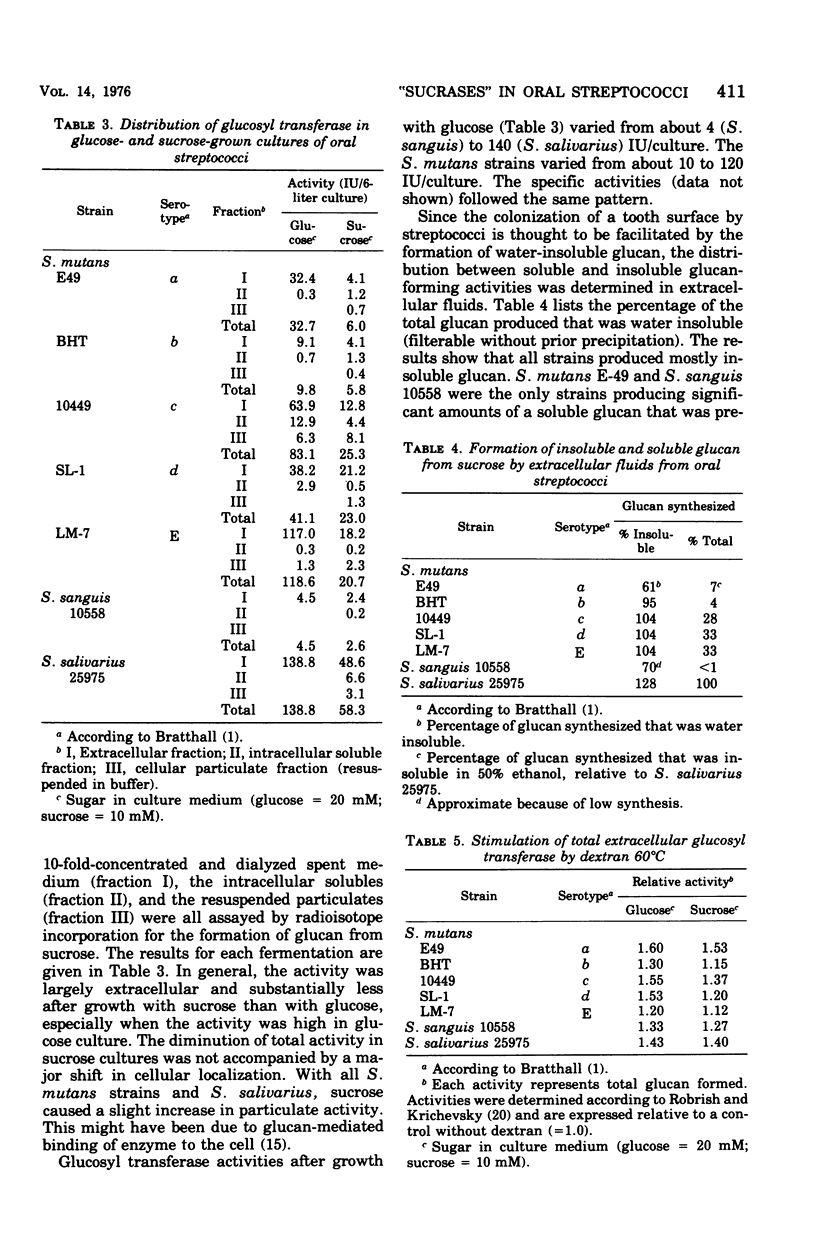
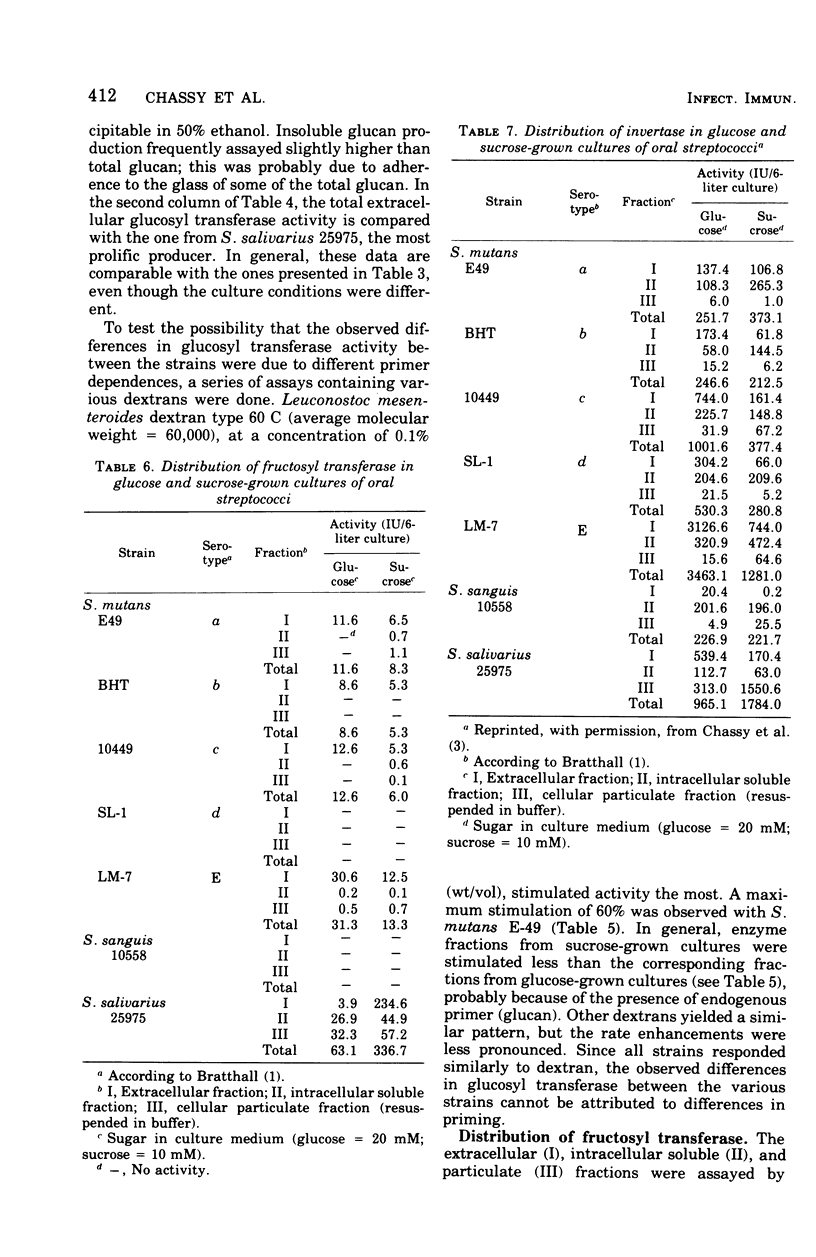
Specific growth rates, growth yields, and the level and cellular distribution of three sucrose-metabolizing enzyme activities were determined for seven oral streptococci (Streptococcus mutans strains E49, BHT, 10449, SL-1, and LM-7, S. sanguis 10558, and S. salivarius 25975). Cultures were grown in a fermentor at pH 6 with either 20 mM glucose or 10 mM sucrose.Generation times varied between 21 and 70 min. Whereas some strains grew 10 to 50% more slowly with sucrose than with glucose, others did not. Growth was always logarithmic, and the growth yields were similar. Glcosyl transferase (EC 2.4.1.5) was largely extracellular; in sucrose cultures it was appreciably lower, but no major shift to a cell-associated form was found. In glucose cultures, the activity varied between 4 and 140 IU per 6-liter culture. The glucan formed was mostly or exclusively water insoluble. Glcosyl transferase was stimulated weakly (60% or less) by various dextrans. Fructosyl transferase (EC 2.4.1.10) was primarily extracellular (except in glucose cultures of S. salivarius) and varied between 0 and 337 IU/culture. In S. salivarius, the extracellular fructosyl transferase was induced by sucrose. In all S. Mutans cultures, the total fructosyl transferase activity was lower after growth with sucrose. All strains had extra- and intracellular invertase (EC 3.2.1.26) activity. Total levels varied between 210 and 3,500 IU/culture. Less extracellular activity was present in sucrose cultures. Only S. salivarius had appreciable activity in the cellular particulate fraction. Invertase activity was significantly higher than the combined glucosyl and fructosyl transferase activities in all cultures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Carlsson J., Johansson T. Sugar and the production of bacteria in the human mouth. Caries Res. 1973;7(4):273–282. doi: 10.1159/000259851. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Bielawski R. M., Beall J. R., Porter E. V., Krichevsky M. I., Donkersloot J. A. Extracellular invertase in Streptococcus mutans and Streptococcus salivarius. Life Sci. 1974 Sep 15;15(6):1173–1180. doi: 10.1016/s0024-3205(74)80013-5. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Robrish S. A., Krichevsky M. I. Fluorometric determination of deoxyribonucleic acid in bacteria with ethidium bromide. Appl Microbiol. 1972 Aug;24(2):179–183. doi: 10.1128/am.24.2.179-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Jordan H. V. Bacteriological aspects of experimental dental caries. Ann N Y Acad Sci. 1965 Sep 30;131(2):905–912. doi: 10.1111/j.1749-6632.1965.tb34856.x. [DOI] [PubMed] [Google Scholar]
- Krasse B., Carlsson J. Various types of streptococci and experimental caries in hamsters. Arch Oral Biol. 1970 Jan;15(1):25–32. doi: 10.1016/0003-9969(70)90142-1. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Krichevsky M. I. Acid production from glucose and sucrose by growing cultures of caries-conducive streptococci. J Dent Res. 1972 May-Jun;51(3):734–739. doi: 10.1177/00220345720510030801. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Gawronski T. H., Schachtele C. F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect Immun. 1973 Dec;8(6):1009–1016. doi: 10.1128/iai.8.6.1009-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Wood W. I., Krichevsky M. I. Linear growth kinetics of plaque-forming streptococci in the presence of sucrose. J Gen Microbiol. 1969 Sep;58(1):125–133. doi: 10.1099/00221287-58-1-125. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



