Abstract
BACKGROUND CONTEXT
Lumbar discectomies are common surgical interventions that treat radiculopathy by removing herniated and loose intervertebral disc (IVD) tissues. However, remaining IVD tissue can continue to degenerate resulting in long-term clinical problems. Little information is available on the effects of discectomy on IVD biology. Currently no treatments exist that can suspend or reverse the degeneration of the remaining IVD.
PURPOSE
To improve knowledge how discectomy procedures influence IVD physiology and to assess the potential of growth-factor treatment as an augmentation during surgery
STUDY DESIGN
To determine effects of discectomy on IVDs with and without TGFβ3 augmentation using bovine IVD organ culture.
METHODS
This study determined effects of discectomy with and without TGFβ3 injection using 1, 6, and 19 days organ culture experiments. Treated IVDs were injected with 0.2μg TGFβ3 in 20μl PBS+BSA into several locations of the discectomy site. Cell viability, gene expression, nitric-oxide release, IVD height, aggrecan degradation, and proteoglycan content were determined.
RESULTS
Discectomy significantly increased cell death, aggrecan degradation and nitric-oxide release in healthy IVDs. TGFβ3 injection treatment prevented or mitigated those effects for the 19 days culture period.
CONCLUSIONS
Discectomy procedures induced cell death, catabolism and nitric-oxide production in healthy IVDs, and we conclude that post-discectomy degeneration is likely to be associated with cell death and matrix degradation. TGFβ3 injection augmented discectomy procedures by acting to protect IVD tissues by maintaining cell viability, limiting matrix degradation and suppressing nitric-oxide. We conclude that discectomy procedures can be improved with injectable therapies at the time of surgery although further in vivo and human studies are required.
Introduction
Lumbar intervertebral disc (IVD) herniation is a common spine disorder with a lifetime occurrence as high as 40% [1]. While the majority of lumbar IVD herniations improve over time or with non-operative therapy, a proportion of patients require surgical intervention [2,3], and some patients may develop a recurrent herniation requiring additional intervention [4]. The U.S. Medicare system spends an estimated $300 million annually on lumbar discectomies [5] and while clinical studies have demonstrated benefits with surgical intervention [2,6,7], the long-term sequelae are unclear and may present with additional clinical problems [8-10]. Costs of managing post-discectomy low back pain were estimated with $4,934 per surgery [10] and therefore are a significant healthcare burden. The development of cost effective strategies to prevent or reduce the severity of post-discectomy degeneration may dramatically improve outcomes and reduce healthcare costs. There remains little information on the effects of discectomy on the biology of the remaining IVD or on strategies to augment discectomy procedures to limit post-discectomy degeneration.
While removal of pathologic IVD fragments during discectomy alleviates radicular symptoms, the remaining tissue and enlarged hole in the annulus fibrosus (AF) may promote or accelerate degenerative changes resulting in long-term clinical problems [8,9,11]. Imaging studies suggest that degenerative changes such as loss of IVD height, facet joint arthritis and endplate changes are likely to occur within months following discectomy [12] and these changes are significantly associated with functional disability and low back pain [8-10]. The concept of accelerated degeneration following injury is also supported by in vivo studies where experimentally induced annular puncture leads to significant changes in the biomechanical properties of IVDs [13-15] resulting in decreased glycosaminoglycan content and increased expression of catabolic and inflammatory mediators [16,17]. It is obvious that the puncture of a healthy IVD creates a different situation from discectomy where the IVD is herniated and often degenerated. Yet, deeper knowledge is required to understand the effects of discectomy with its profound impact on the remaining IVD tissue and to investigate opportunities to develop biological treatments to improve outcomes after discectomy.
Injection of growth factors has been shown to have dose dependent effects on improving both the structural and biomechanical properties of IVDs including reversing IVD degeneration [18,19] in animal models. Intradiscal injections of osteogenic protein-1 in an annular puncture animal model partly restored IVD height, increased proteoglycan content and was correlated with improved elastic and viscous moduli of the IVD [20]. Several studies demonstrated that TGFβ increases proteoglycan synthesis and expression of extracellular matrix (ECM) genes in the IVD. TGFβ3 has the capability to maintain the phenotype of IVD cells in organ culture [21] and endogenous TGFβ activity limits pro-inflammatory cytokine expression, suggesting an important role in maintaining the IVD homeostasis [19,20,22-28]. Masuda & An (2006) suggested that identifying biological agents that can modify both symptoms and IVD structure would be highly desirable for the treatment of IVD degeneration.
Intervention during discectomy surgery would allow an immediate treatment that could inhibit catabolic responses and slow or arrest progressive degeneration. While some studies exist about the effectiveness of growth factor treatment for vertebral body fusion and IVD implants [29-31]; to our knowledge nothing is known about the effect of growth factor injection during discectomy. Our broad aim is to improve knowledge of how discectomy procedures influence IVD physiology and assess the potential of growth factor injection at the time of surgery, and we specifically evaluate the effects of TGFβ3 injection into the discectomy site during surgery using a bovine organ culture model. This study assessed the effects of discectomy with and without TGFβ3 augmentation cell viability, structural, and protein measurements.
Material and Methods
Tissue harvest and culture of IVDs
Skeletally mature bovine tails were obtained from a local abattoir and four caudal IVDs were prepared from each tail. Vertebrae were cut proximal and distal to vertebral endplates with a histological band saw (Exakt 310, Exakt, Norderstedt, Germany) and IVD dimensions (height, width, weight) were measured. Blood clots and bone debris were removed by flushing the endplates with water using an orthopaedic irrigation system for debridement (Inter Pulse®, Stryker). IVDs were then rinsed in ethanol, 1xPBS containing 3% penicillin/streptomycin and 1.5% fungizone, and PBS and assigned to following groups: d0-control, cultured control (control), discectomy (injured), and discectomy+TGFβ3 (treated). To exclude IVD level dependent differences in cell metabolism rates [32], the different groups were randomly distributed among the harvested caudal levels.
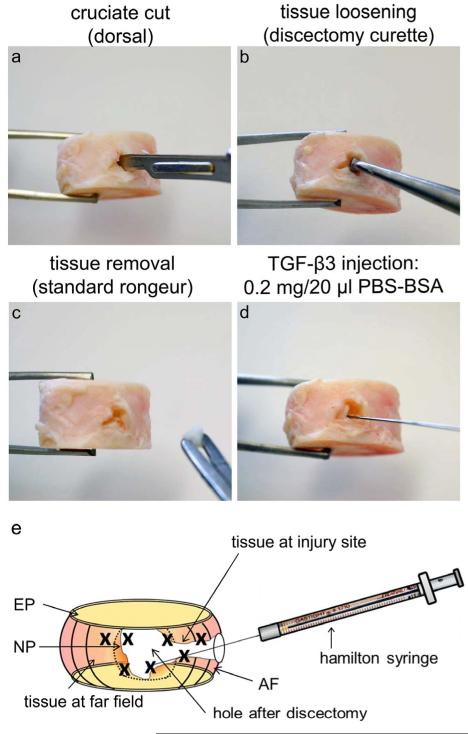
Discectomies were created on the dorsal side by performing a cruciate cut with a #15 scalpel to the center of the IVD. Tissue was loosened and removed using a discectomy curette and a standard IVD rongeur (Figure 1). Depending on size (diameter=23.36±2.59 mm, volume=5.15±1.2 cm3), an avg. of 18.8±11.8μg tissue/IVD was removed. After discectomy, 0.2μg TGFβ3 in 20μl PBS+BSA were injected into the injury site of treated IVDs with a high precision syringe (Hamilton, Reno, NV; Figure 1d-e). IVDs were loaded in bioreactors and cultured for 1, 6 or 19 days under diurnal loading (8h/16h=0.1MPa/0.2MPa) in standard high glucose DMEM containing 0.2% Primocin, 1% penicillin/streptomycin, and 0.2% ascorbic acid. Media was changed every 3-4 days and collected for further analyses. After culture, the endplates were removed and AF and NP were separated with a biopsy punch (Ø 8 mm). Only very limited amounts of NP were available after discectomy and therefore excluded for further analyses. Areas of treated and injured AF were divided into 2 categories: far field (injured & treated) and injury site (injured is & treated is; Figure 1e) and divided for cell viability, PCR, protein extraction and proteoglycan content analyses. For the 1d diffusion study, whole IVDs were fixed in 10% paraformaldehyde and embedded in plastic (Table 1).
Figure 1. Discectomy and injection procedures.
Images and schematic of (a-c) discectomy and (d-e) TGFβ3 injection procedures. (a) A cruciate-style discectomy was performed using a #15 scalpel blade followed by (b) loosening of NP tissue with a discectomy curette and (c) removal of loose tissue with a standard rongeur. A total of 20μl TGFβ3 solution was injected into multiple locations of the remaining NP, inner and outer AF tissue surrounding the discectomy space using a (d) high precision Hamilton syringe (25g needle), (e) as shown schematically. The discectomy is created at the dorsal side of the IVD and TGFβ3 is injected into multiple sites within the defect; note that the ‘x’ marks indicate the multiple injection sites where treatment occurred.
Table 1. Study design.
Discectomy studies included 1 day, 6 day and 19 day investigations of gene expression, transport, cell viability and model characterization. Groups included d0 = day 0 control; Control = intact control without intervention; Injured = discectomy, Treated = 0.2μg TGFβ3 in 20μl PBS-BSA. Group, sample size and dependent variable measurements are given.
| Study | Group | Sample size |
Dependent variables |
|---|---|---|---|
|
Discectomy
1 day |
d0 | 4 | PCR |
| Control | 4 | ||
| Injured | 4 | ||
| Treated | 4 | ||
| Dextran | 2 | Diffusion | |
|
Discectomy
6 days |
d0 | 6 | cell viability PCR GAG IVD height |
| Control | 6 | ||
| Injured | 6 | ||
| Treated | 6 | ||
|
Discectomy
19 days |
d0 | 9 | Cell viability PCR Western blot NO release in media GAG IVD height |
| Control | 9 | ||
| Injured | 9 | ||
| Treated | 9 |
Cell viability
Cell viability was determined by calceinAM and ethidium-homodimer. Samples were incubated in serum free media supplemented with 5 μmol/l calceinAM and 1 μmol/l ethidium-homodimer for 2 hours at 37°C. Samples were then washed in PBS and visualized on a confocal laser scanning microscope (Leica SP5 DMI). Data quantification was performed with ImageJ software (http://rsb.info.nih.gov/ij/) [33] and normalized to d0 (n=6/group at d6, d19 and d0 controls)
Nitric oxide (NO) release into the media
NO released into the media over the 19d culture was measured using standard Griess reagent assay according to manufacturer’s instructions (n=7/group; Promega) and normalized to the IVD weight after culture.
Dextran diffusion
Dextran uptake and localization after 1d culture was determined by fluorescence and second-harmonic-generation. Dextran of a comparable molecular weight to TGFβ3 (dextran=25.4 kDa; TGFβ3=20.4 kDa; both positively charged) was used to simulate TGFβ3 injection in 20μl PBS+0.1%BSA were injected into the injury site. Samples were fixed in Z-fix fixative and embedded in methacrylate resin and thick sections (~3mm) were cut (Leica SP1600) and imaged with a multiphoton microscope (3μm/slice x ~75 slices; dimension: 1.242μm/px; Olympus FluoView FV1000MPE; n=2).
Height measurement
IVD height was measured with a caliper at three regions of the IVD and the height of 3 areas was averaged. Measurements were performed before and after culture and the % IVD height loss was calculated (n=6/group [d6], n=8/group [d19]).
Gene expression
To assess gene expression, samples were flash-frozen in liquid N2, pulverized, and total RNA was extracted with TRI Reagent (Molecular Research Center), by using a modified TRIspin method [34]. Reverse transcription was performed using the SuperScript VILO cDNA Synthesis kit (Invitrogen). Expression was determined by qRT-PCR (ABI GeneAmp 7500, Applied Biosystems) using Taqman primers and normalized to 18S ribosomal RNA as endogenous control. Genes of interest were the inflammatory IL-1β and IL-6, and the anabolic and catabolic genes ACAN, COL1, COL2, and metallopeptidase inhibitor 1 (TIMP1), ADAMTS-5 and MMP-13 (n=4/group [1d]; n=6/group [d6] n=9/group [d19]). To account for the variability of the control samples the average CT was set to zero with a variation around the mean. This was done by subtracting the average of all CT controls from the individual control CT values. The negative and positive errors were determined by calculating the fold change of the sum or difference of average CT and the standard deviation of the CT, as described in Livak 2001 [35]. The CT values for the different groups were calculated within one experiment/animal by subtracting the corresponding control CT from the sample of interest CT. Fold changes for samples of interest were calculated as described for the controls.
Aggrecan degradation
Aggrecan degradation was determined by western blot as previously described [36]. Briefly: tissues were chopped into small pieces and lyophilized. Proteins and proteoglycans were extracted, precipitated in ethanol and air dried. The precipitate was digested with keratanase I (Seikagu) and chondroitinase ABC (Seikagu). Samples were separated by SDS/page under reducing conditions (10% polyacrylamide gels), transferred to nitrocellulose membranes (BIORAD) and incubated overnight at 4°C with antibodies directed against intact aggrecan (G1 domain), and cleavage sites for aggrecanase (NITEGE) and MMPs (DIPES) [37]. Aggrecan and its degradation fragments were visualized by chemiluminescence (n=9/group [d19]).
Proteoglycan content
Proteoglycan content in papain digested tissue and in culture media was measured with the dimethylmethylene blue dye assay (Sigma-Aldrich; n=5) [38].
Statistics
One way ANOVAs were performed with subsequent post hoc testing (Bonferroni’s Multiple Comparison Test; GraphPad Prism5). For statistical analyses, p-value p<0.05 was considered significant.
Results
Cell viability after discectomy
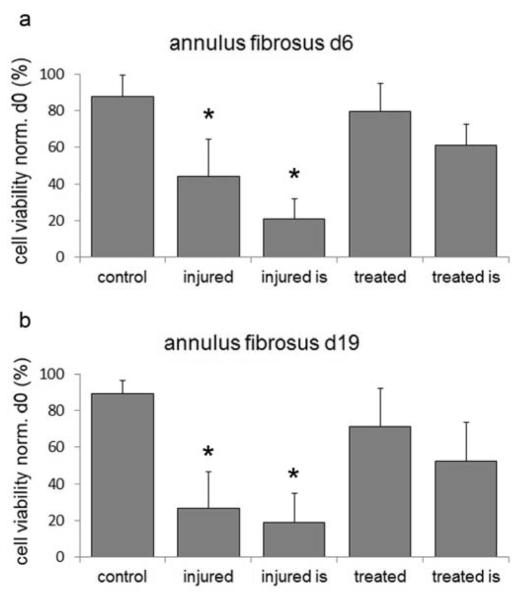
To determine the effect of TGFβ3 after discectomy, 0.2μg TGFβ3 was injected directly into the surrounding tissue of the IVD space after discectomy (Figure 1e). In control IVDs cell viability was maintained for up to 19 day culture (d6=87.9±11.5%; d19=89.3±7.1% Figure 2 a,b). Discectomy led to a significant decrease of cell viability in the far-site (d6=43.9±20.4%; d19=26.5±19.9%; p<0.01; Figure 2 a,b) and was even lower adjacent to the injury-site (d6=20.7±16%; d19=18.9±15.8%; p<0.01; Figure 2a,b). TGFβ3 treatment maintained cell viability during culture in the far field-site (d6=79.4±15.4%; d19=71.1±20.9%; Figure 3b) and mitigated the extent of cell death adjacent to injury-site (d6=61.3±11.5%; d19=52.3±21.3%). Discectomy sites in injured and treated IVDs were clearly visible after 19 days (Figure 3).
Figure 2. Cell viability analyses after discectomy.
Cell viability at (a) day 6 and (b) day 19 of control, injured and treated AF as measured in the tissue at the injury site (is) and in the far field; injured vs. control: *=p<0.01.
Figure 3. Representative cell viability images after 19 day culture.
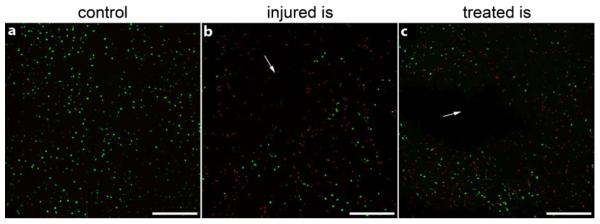
Images of (a) control, (b) injured and (c) treated IVDs using calcein AM and ethidium homodymer to identify live (green) and dead (red) cells, respectively, as visualized on confocal microscopy. High cell viability was observed in all control and intact IVDs while dead cells were observed at the injury site (is), as denoted by arrows. Scale bar = 200 μm.
Dextran diffusion
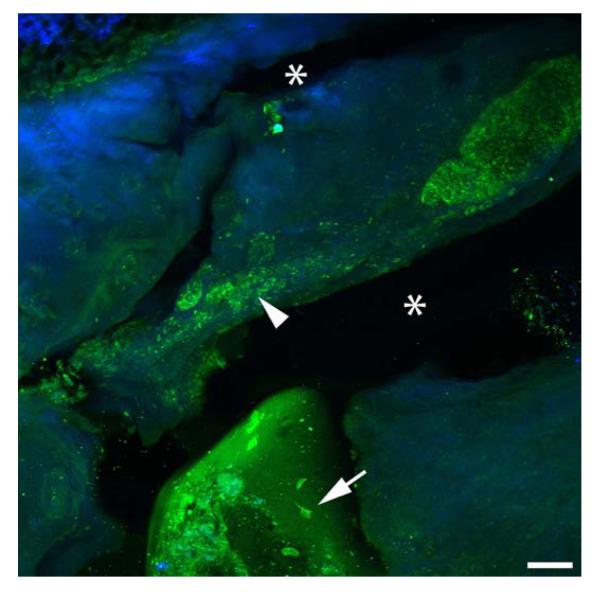
To assess if the injected solution remains within the IVD, a total of 20μl dextran was injected into several locations adjacent to the discectomy. To simulate TGFβ3 diffusion under best possible condition we chose dextran of the same charge and with similar molecular weights (positively charged; molecular weights: dextran=25.4 kDa; TGFβ3=20.4 kDa). The FITC labeled dextran remained within the tissue within areas close to the injection site and appeared to be partly taken up by the cells. Second-Harmonic-Generation-microscopy analyses visualized the collagen rich matrix and demonstrated ruptures of the inner AF due to discectomy and dye injection (Figure 4).
Figure 4. Dextran injection after discectomy.
FITC labeled dextran was injected as a surrogate to visualize TGFβ3 drug distribution and visualized with a multiphoton microscope to label the injected agent and Second Harmonic Generation imaging to visualize the dense collagenous network. After 20 hours organ culture, the injected dextran remained within the tissue close to the injections sites (arrow head) and appeared to be partly taken up by the cells (arrow). Defects in the tissue associated with discectomy and/or injection are visible in the collagen rich tissue (*); scale bar = 100 μm; dextran = green; collagen = blue
Aggrecan degradation
Aggrecan is the main proteoglycan in the IVD and is prone to degradation by both aggrecanase and MMPs. The presence of intact aggrecan and aggrecan fragments due to cleavage was determined by western blot analysis using antibodies directed against intact aggrecan (G1 domain), and cleavage sites for aggrecanase (NITEGE) and MMPs (DIPES). While the band intensity of intact aggrecan indicated high amounts of aggrecan in all conditions (Figure 5); cleavage products were mostly observed in injured inner AF, with the highest intensity for MMP fragments (Figure 5). In contrast, control and treated inner AF samples contained only faint bands of MMP cleavage products and fragments of aggrecanase cleavage were absent (Figure 5).
Figure 5. Aggrecan degradation due to discectomy after 19 day culture.
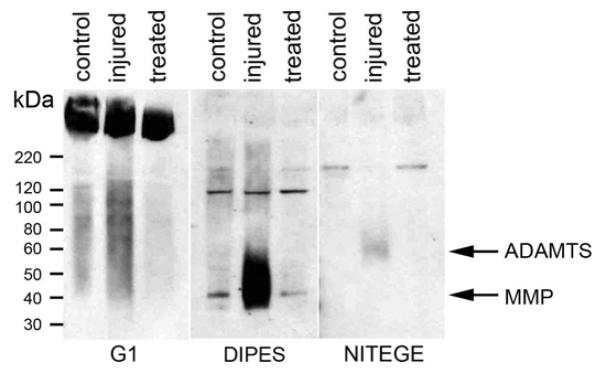
Aggrecan degradation products were analyzed in inner AF extracts of control, injured and treated IVDs by SDS page and western blotting using antibodies recognizing the aggrecan G1 domain (left) and the cleavage products of MMPs (DIPES, center) and aggrecanase (NITEGE, right). Arrows mark bands representing degradation products due to cleavage at sites for aggrecanase (ADAMTS) and matrixmetalloproteinases (MMP).
NO release
To determine cell stress, the amount of NO released to the media was determined throughout the 19 day culture. No difference in total NO release was detected between control and treated IVDs (p=0.336; Figure 6). In contrast, untreated discectomy lead to a significant increase of total NO release in injured IVDs (p<0.005). During the first week of culture, treated and injured IVDs released twice as much NO compared to control IVDs. With progressing culture, NO release increased slightly in the control group, whereas it decreased in treated IVDs. NO concentration in the media converged for the treated and control groups at 15 days but further increased in injured IVDs (Figure 6).
Figure 6. Total NO released to media due to discectomy.
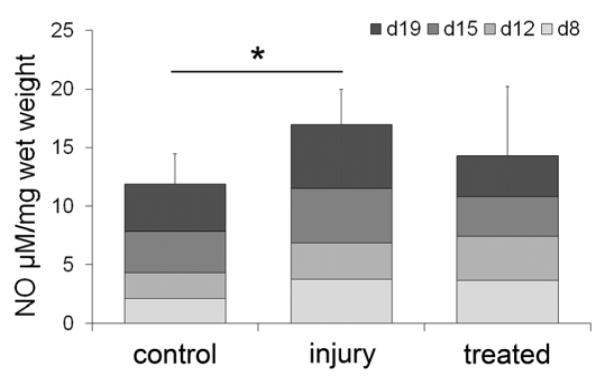
NO released to the media was measured using the Griess reaction at media changes at day 8 (d8), day 12 (d12), day 15 (d15) and day 19 (d19) and summed (injured vs. control: *=p<0.05).
IVD height
Over time IVD height decreased in all groups with loss in IVD height being highest in injured IVDs (22±4%) and lowest in treated IVDs (treated=18±4%; p=0.06 injured vs. treated; Figure 7). No differences were observed between control (21±9%) and injured or treated IVDs (p=0.79; p=0.43)
Figure 7. IVD height loss after 19 day culture normalized to control.
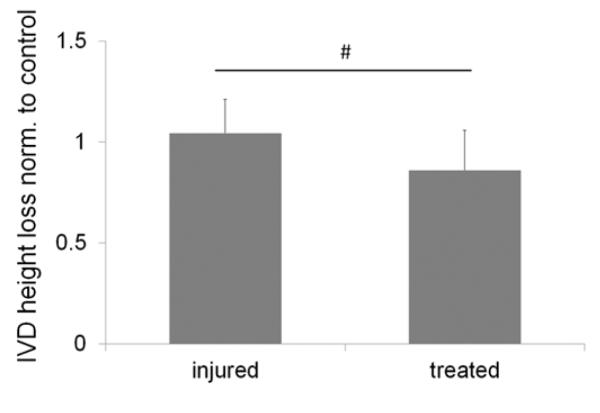
Treated IVDs lost less IVD height than injured IVDs (injured vs. treated: # p=0.06).
GAG content in tissue and media, and gene expression
No differences between control, injured and treated IVDs were observed at any time point (results not shown).
Discussion
This study investigated the effects of discectomy on IVD properties and evaluated if TGFβ3 injection during discectomy could augment surgical procedures. Our results indicated that discectomy procedures induced IVD height loss, GAG degradation, NO production and substantial cell death in AF tissue adjacent to the discectomy site and on the adjacent sides of the IVD. TGFβ3 injection at the time of discectomy inhibited several of these changes with improved cell viability, reduced aggrecan degradation and reduced cellular stress. Results should be contextualized for the human condition, since we expect discectomy procedures on healthy bovine IVDs to result in greater degenerative changes than would be observed on human IVDs that are typically herniated, degenerated, or otherwise eliciting a painful response. Nevertheless, the degenerative changes found from discectomy and improvements with injected TGFβ3 are compelling and highly suggestive that biologic injection or other augmentation at the time of discectomy offers potential to improve current discectomy procedures.
The most likely cause of IVD height loss was due to progressive breakdown of the ECM, known to be associated with IVD degeneration [39]. In this study western blots for aggrecan and its fragments suggest increased aggrecan breakdown by MMPs and ADAMTS in the inner AF of injured IVDs while injection of TGFβ3 during discectomy prevented aggrecan breakdown and helped to maintain the ECM structure. In addition to our findings, others have previously demonstrated the anabolic potential of TGFβ on ECM homeostasis [25-28]. TGFβ isoforms in combination with dexamethasone could inhibit the expression of catabolic genes such as ADAMTS-5 or members of the MMP family [23,27].
TGFβ can promote survival of IVD cells through inhibition of apoptosis [40] and maintaining cell viability [41-43] and it is likely that the catabolic shift of injured IVDs after discectomy also resulted from the significant loss of cell viability due to injury, as recent studies suggested that IVD degeneration could be induced through sudden cell death or senescence [44]. The increased cell death in injured IVDs is further reflected by elevated levels of NO, as extended production of NO is known to be pro-apoptotic through activation of proteases and the alteration of apoptosis related protein expression [45].
The major ECM degradation process is mediated by MMPs and ADAMTS’ which are activated by NO, low pH and proteases [46-48]. While gene expression analyses did not show any differences between control, injured and treated IVDs, the observed increase in aggrecan degradation after discectomy suggests activation of MMPs and ADAMTS’ in injured IVDs. TGFβ3 treatment likely counteracted this process as aggrecan degradation and NO release were comparable to controls, and this may offer an explanation for sustained effects of TGFβ3 on the IVD metabolism after 19 days.
To evaluate the distribution of TGFβ3 injection within the IVD and to assess possible uptake by cells we injected FITC labeled dextran into the discectomy space as a surrogate for TGFβ3. Local staining close to the injection sites indicated dextran diffused throughout the AF tissue and was likely taken up by cells in the region (Figure 4).
Furthermore, treatment with 20μl TGFβ3 injection into discectomy IVDs limited aggrecan degradation, maintained IVD height, and prevented cell death adjacent to and away from the discectomy site. Taken together, we infer that our multiple TGFβ3 injection strategy into IVDs with discectomy distributed the growth factor throughout the remaining IVD tissue and was taken up by cells. We note that injection of high volumes can result in back flow of the injected solution out of the IVD and put the AF at risk of delamination [49], therefore we limited our injection volumes to 20μl.
From a clinical perspective, it would be appealing if injection of a single growth factor had the capacity to ‘reprogram’ cells to prevent apoptosis and degeneration; however, it is also plausible that degenerative changes were delayed. Future animal model and clinical studies are required to investigate how long such a treatment would persist in vivo and in the human condition. We considered the 0.2μg TGFβ dose to be optimal since it produced the largest up-regulation of genes involved in matrix remodeling in preliminary experiments comparing doses from 0.02 to 2 μg. Protein validation reinforced this as an effective choice with detectable inhibition of aggrecan degradation. However, additional studies are required to determine dose on human IVDs and cells.
Although all IVDs isolated for this study were healthy, effects of growth factor in cases of IVD herniation and degeneration are most relevant clinically. IVD herniation in adult humans is mostly seen as a result of IVD degeneration although IVD herniation in young patients and athletes mostly follows trauma in non-degenerated IVDs [50-54]. We expect that the changes for pre-existing degeneration of most discectomy patients would be less than the changes from discectomy observed in these healthy bovine IVDs used in our model since degenerated human IVDs contain fewer cells that are exposed to chronic inflammatory conditions. In addition most patients do not get immediate surgery for IVD herniation and often undergo surgery weeks to months after sustaining a lumbar IVD herniation. It is therefore likely that the dose of growth factors and efficacy would vary depending on the cause of IVD herniation and state of degeneration. The presented explant system of bovine IVDs with endplates provides a potential platform for screening of different inflammatory [55] and dosage conditions. However, longer term in vivo animal studies, human IVD studies, and clinical trials are required to fully test this concept that discectomy procedures can be improved with growth factor injections. It is possible that other growth factors, such as BMPs and GDF-5, which have been implicated as potential therapeutic agents in the IVD [19,56,57] could also be successful augmentations to discectomy procedures yet this remains a speculation requiring further testing.
Conclusion
After discectomy, the remaining IVD tissue can undergo accelerated degenerative changes resulting in long-term clinical problems [8,9]. This study provides direct evidence for such degeneration involving increased cell death, matrix breakdown, and NO production. TGFβ3 injection during discectomy prevented the development of inflammatory and catabolic responses and this study suggests that even a single dose of TGFβ3 treatment can prevent cell death and limit matrix catabolism for 19 days. Whether or not this single injection is sufficient to ‘reprogram’ degenerated cells to a new phenotype with increased anabolism and remodeling rates requires additional studies. We conclude that biologic injection at the time of surgery offers potential to improve the current treatments which are focused on reducing pain by augmenting them with simultaneous treatments to slow progressive degeneration, and the current study indicates that such procedures warrant further investigation.
Acknowledgements
Funded by NIH grants R01AR051146 & R01AR057397 & AO Research Fund (F-09-10I) of the AO Foundation. Confocal and multi-photon laser scanning microscopy was performed at the ISMMS Microscopy Shared Resource Facility. Multi-photon microscopy was supported with funding from NIH Shared Instrumentation Grant (1 S10 RR0 26639-01). We thank M. Likhitpanichkul, D.F. Laudier, C.C. Guterl, and P. Nasser for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Amer Acad Orthop Surg. 2008:247. http://www.boneandjointburden.org/ [Google Scholar]
- 2.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008;33:2789–2800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 1994;19:1207–1212. doi: 10.1097/00007632-199405310-00002. discussion 1213. [DOI] [PubMed] [Google Scholar]
- 4.Guterl CC, See EY, Blanquer SB, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenfeld AJ, Weiner BK. Treatment of lumbar disc herniation: Evidence-based practice. Int J Gen Med. 2010;3:209–214. doi: 10.2147/ijgm.s12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine (Phila Pa 1976) 2001;26:1179–1187. doi: 10.1097/00007632-200105150-00017. [DOI] [PubMed] [Google Scholar]
- 7.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30:927–935. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 8.Loupasis GA, Stamos K, Katonis PG, Sapkas G, Korres DS, Hartofilakidis G. Seven- to 20-year outcome of lumbar discectomy. Spine (Phila Pa 1976) 1999;24:2313–2317. doi: 10.1097/00007632-199911150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hanley EN, Jr., Shapiro DE. The development of low-back pain after excision of a lumbar disc. J Bone Joint Surg Am. 1989;71:719–721. [PubMed] [Google Scholar]
- 10.Parker SL, Xu R, McGirt MJ, Witham TF, Long DM, Bydon A. Long-term back pain after a single-level discectomy for radiculopathy: incidence and health care cost analysis. J Neurosurg Spine. 2010;12:178–182. doi: 10.3171/2009.9.SPINE09410. [DOI] [PubMed] [Google Scholar]
- 11.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariconda M, Galasso O, Attingenti P, Federico G, Milano C. Frequency and clinical meaning of long-term degenerative changes after lumbar discectomy visualized on imaging tests. Eur Spine J. 2010;19:136–143. doi: 10.1007/s00586-009-1201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalek AJ, Iatridis JC. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. Spine J. 2012;12:425–432. doi: 10.1016/j.spinee.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 15.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 16.Chan DD, Khan SN, Ye X, et al. Mechanical deformation and glycosaminoglycan content changes in a rabbit annular puncture disc degeneration model. Spine (Phila Pa 1976) 2011;36:1438–1445. doi: 10.1097/BRS.0b013e3181f8be52. [DOI] [PubMed] [Google Scholar]
- 17.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 18.Masuda K, An HS. Growth factors and the intervertebral disc. Spine J. 2004;4:330S–340S. doi: 10.1016/j.spinee.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976) 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Masuda K, Kim JG, et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692–703. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Risbud MV, Di Martino A, Guttapalli A, et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine (Phila Pa 1976) 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Ohba T, Ando T, et al. Endogenous TGF-beta activity limits TSLP expression in the intervertebral disc tissue by suppressing NF-kappaB activation. J Orthop Res. 2013;31:1144–1149. doi: 10.1002/jor.22337. [DOI] [PubMed] [Google Scholar]
- 23.Abbott RD, Purmessur D, Monsey RD, Iatridis JC. Regenerative potential of TGFbeta3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J Orthop Res. 2012;30:482–488. doi: 10.1002/jor.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maerz T, Herkowitz H, Baker K. Molecular and genetic advances in the regeneration of the intervertebral disc. Surg Neurol Int. 2013;4:S94–S105. doi: 10.4103/2152-7806.109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-beta1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976) 2013;38:E49–58. doi: 10.1097/BRS.0b013e31827aa896. [DOI] [PubMed] [Google Scholar]
- 26.Sohn P, Cox M, Chen D, Serra R. Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc. BMC Dev Biol. 2010;10:29. doi: 10.1186/1471-213X-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585:1209–1215. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson JP, Oegema TR, Jr., Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976) 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine (Phila Pa 1976) 2006;31:775–781. doi: 10.1097/01.brs.0000206357.88287.5a. [DOI] [PubMed] [Google Scholar]
- 30.Michielsen J, Sys J, Rigaux A, Bertrand C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J Bone Joint Surg Am. 2013;95:873–880. doi: 10.2106/JBJS.L.00137. [DOI] [PubMed] [Google Scholar]
- 31.Frenkel MB, Cahill KS, Javahary RJ, Zacur G, Green BA, Levi AD. Fusion rates in multilevel, instrumented anterior cervical fusion for degenerative disease with and without the use of bone morphogenetic protein. J Neurosurg Spine. 2013;18:269–273. doi: 10.3171/2012.12.SPINE12607. [DOI] [PubMed] [Google Scholar]
- 32.Wiseman MA, Birch HL, Akmal M, Goodship AE. Segmental variation in the in vitro cell metabolism of nucleus pulposus cells isolated from a series of bovine caudal intervertebral discs. Spine (Phila Pa 1976) 2005;30:505–511. doi: 10.1097/01.brs.0000154615.22311.66. [DOI] [PubMed] [Google Scholar]
- 33.Gantenbein-Ritter B, Potier E, Zeiter S, van der Werf M, Sprecher CM, Ito K. Accuracy of three techniques to determine cell viability in 3D tissues or scaffolds. Tissue Eng Part C Methods. 2008;14:353–358. doi: 10.1089/ten.tec.2008.0313. [DOI] [PubMed] [Google Scholar]
- 34.Junger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34:1264–1271. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 2011;30:145–153. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sztrolovics R, White RJ, Roughley PJ, Mort JS. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002;362:465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 39.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 40.Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 41.Gruber HE, Chow Y, Hoelscher GL, et al. Micromass culture of human anulus cells: morphology and extracellular matrix production. Spine (Phila Pa 1976) 2010;35:1033–1038. doi: 10.1097/BRS.0b013e3181bc3e04. [DOI] [PubMed] [Google Scholar]
- 42.Hegewald AA, Zouhair S, Endres M, et al. Towards biological anulus repair: TGF-beta3, FGF-2 and human serum support matrix formation by human anulus fibrosus cells. Tissue Cell. 2013;45:68–76. doi: 10.1016/j.tice.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Ruan D, Zhang C. Effects of TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus cells in medium with different serum concentrations. J Orthop Surg Res. 2006;1:9. doi: 10.1186/1749-799X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18:777–785. doi: 10.1007/s10495-013-0839-1. [DOI] [PubMed] [Google Scholar]
- 45.Brune B, Schneiderhan N. Nitric oxide evoked p53-accumulation and apoptosis. Toxicol Lett. 2003;139:119–123. doi: 10.1016/s0378-4274(02)00426-5. [DOI] [PubMed] [Google Scholar]
- 46.Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 47.Chen Lc Fau - Noelken ME, Noelken Me Fau - Nagase H, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1) Biochemistry. 1993 Oct 5;32(39):10289–10295. doi: 10.1021/bi00090a003. [DOI] [PubMed] [Google Scholar]
- 48.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine (Phila Pa 1976) 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 50.Parisini P, Di Silvestre M, Greggi T, Miglietta A, Paderni S. Lumbar disc excision in children and adolescents. Spine (Phila Pa 1976) 2001;26:1997–2000. doi: 10.1097/00007632-200109150-00011. [DOI] [PubMed] [Google Scholar]
- 51.Cahill J, Frost G, Solanki GA. Paediatric lumbar disc herniation in the very young: a case-based update. Childs Nerv Syst. 2011;27:687–691. doi: 10.1007/s00381-010-1369-6. [DOI] [PubMed] [Google Scholar]
- 52.Kruse D, Lemmen B. Spine injuries in the sport of gymnastics. Curr Sports Med Rep. 2009;8:20–28. doi: 10.1249/JSR.0b013e3181967ca6. [DOI] [PubMed] [Google Scholar]
- 53.Standaert CJ. Low back pain in the adolescent athlete. Phys Med Rehabil Clin N Am. 2008;19:287–304. ix. doi: 10.1016/j.pmr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence JP, Greene HS, Grauer JN. Back pain in athletes. J Am Acad Orthop Surg. 2006;14:726–735. doi: 10.5435/00124635-200612000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFalpha in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433:151–156. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei A, Williams LA, Bhargav D, et al. BMP13 prevents the effects of annular injury in an ovine model. Int J Biol Sci. 2009;5:388–396. doi: 10.7150/ijbs.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]