Abstract
Objective
To assess the association between in utero exposure to either diethylstilbestrol (DES) or an oral contraceptive in pregnancy and offspring obesity.
Design and Methods
Using data from the Collaborative Perinatal Project (1959–1974), a multicenter prospective study of pregnant women and their offspring, we examined overweight or obesity among 34,419 children with height and weight data at age 7 years. We used generalized linear models to estimate the adjusted odds ratio (aOR) for overweight or obesity (≥85th percentile) or obesity (≥95th percentile) in the offspring according to exposure during different months of pregnancy.
Results
Oral contraceptive use during pregnancy was positively associated with offspring overweight or obesity and obesity. The magnitude of association was strongest in the first 2 months of pregnancy for obesity (aOR 2.0, 95% CI: 1.1, 3.7). DES use was also associated with offspring overweight or obesity and obesity, with the association being strongest for exposure beginning between months 3–5 (e.g., for exposure beginning in months 3–4, the aOR for obesity was 2.8, 95% CI: 1.3, 6.3).
Conclusions
Pharmacologic sex hormone use in pregnancy may be associated with childhood obesity. Whether contemporary, lower-dose oral contraceptive formulations are similarly associated with increased risk of childhood obesity is unclear.
Keywords: Obesogens, Ethinyl estradiol or mestranol, Diethylstilbestrol, Fetal origins of disease
Introduction
The possibility that agents with weak estrogenicity are obesogenic has received considerable attention in the epidemiologic literature1, 2. Experiments on animals have shown a positive association between perinatal exposure to diethylstilbestrol (DES) and exogenous 17-β estradiol and offspring development of overweight or obesity3–6. One human study identified a positive association between DES exposure in utero and subsequent development of obesity among adult women. Women exposed in utero, at ≥ 15 weeks of gestation, were at highest risk7. There have been no published studies evaluating this association in children.
Oral contraceptives were first introduced in 1960 and generally consist of a synthetic estrogen (primarily ethinyl estradiol in contemporary formulations, mestranol at the time oral contraceptives were first introduced) and a progestin component. Since the introduction of oral contraceptives, there have been many different, less potent formulations developed to help minimize the risk for adverse effects8.
DES is no longer in use, but was commonly prescribed in the 1950’s and 1960’s to pregnant women considered at risk of pregnancy complications -- complications such as threatened miscarriage, as evidenced by vaginal bleeding, or having a history of fetal loss. Once a woman initiated use of DES, she typically used it throughout the remainder of pregnancy9. DES exposure in in vitro and animal models has resulted in a dose-respondent increase in stem cell differentiation into preadipocytes and adipocytes4, 10–12. In humans, mesenchymal stem cell differentiation into preadipocytes and, subsequently, adipocytes occurs after the first trimester of pregnancy, between gestational weeks 14 – 2213. Studies using cohorts exposed in utero to DES and first generation formulations of oral contraceptives offer the opportunity to study the association given a higher dose exposure and to evaluate possible developmentally sensitive periods of exposure.
In the present study we assessed whether exposure to pharmacologic sources of estrogenic agents, including oral contraceptives in early pregnancy (ethinyl estradiol or mestranol) and diethylstilbestrol (DES) during pregnancy, was associated with early childhood overweight or obesity or obesity only. Few children are exposed to pharmacologic sex hormones in early pregnancy. Large cohort studies offer the opportunity to study exposures that are infrequent, while maintaining sufficient power for adjustment for possible confounders and assessment of possible effect modification. The present study used data from the Collaborative Perinatal Project (1959–1974).
Methods and procedures
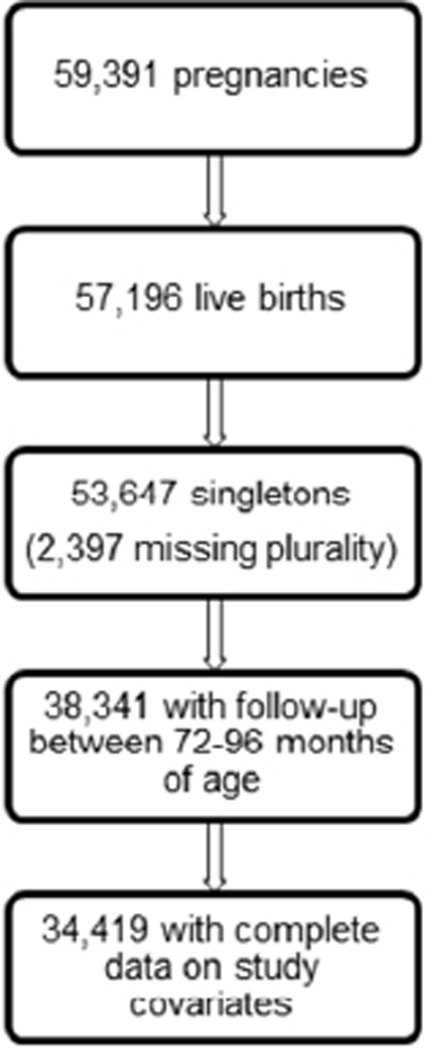
The Collaborative Perinatal Project was a prospective pregnancy cohort study of 58,760 pregnancies in 48,197 women enrolled from 1959 to 1966. Women were enrolled at 12 U.S. academic medical centers during pregnancy and were followed through delivery, and their children were followed up to 7 or 8 years of age. Details of the data collection methods and study design have been described previously14. In the present study we assessed the association between use of oral contraceptives in early pregnancy (months 1–4) or use of DES throughout pregnancy (months 1–9) and offspring overweight or obesity (≥85th percentile) or obesity (≥95th percentile) at approximately age 7. We included all pregnancies resulting in a live, singleton birth (n=55,740), with complete data on study covariates and follow-up between 6 and 8 years of age (72–96 months). This resulted in a study population of 34,419 pregnancies (fig. 1) among 29,161 women. Thus, of the pregnancies that met our inclusion criteria, 38% were lost to follow-up.
Figure 1.
Study population obtained from the prospective, Collaborative Perinatal Project pregnancy cohort (1959–1974)
Use of an oral contraceptive or DES in early pregnancy was ascertained, at each prenatal visit, by maternal self-report as collected by study personnel. Most (83%) women were enrolled within the first 2 trimesters of pregnancy. The mean number of prenatal visits was 8.9 (sd: 4.0). Women reported the type of formulation used as well as the number of days the drug was taken. Women also reported whether they were using contraception at the time of conception and the type of contraception being used. The first month of pregnancy began with the first day of the last menstrual period. Of the 34,419 pregnancies studied, 196 (0.6%) were exposed to an oral contraceptive and 131 (0.4%) were exposed to DES. For oral contraceptive use, most of the women who were exposed were exposed within the first eight weeks of pregnancy (159 of the 196).
Once DES use was initiated, women generally continued use through the remainder of their pregnancy. Most women using DES began use within the first four months of pregnancy (n=103). An additional 28 women began using DES in months 5–9. In our primary analysis, we characterized DES exposure by the trimester in which use began. Next, given the literature suggesting increased adipogenesis in weeks 14–2213 and an increased susceptibility for obesity in adult women exposed to DES in utero ≥ 15 weeks of gestation7, we evaluated exposure according to the month of first use. Data for DES use, however, were generally too sparse to characterize exposure for each month. We therefore also present the results for DES exposure based on 2-month, overlapping increments. The overlapping periods of exposure is an approach used to create a smoothed representation of change in association over time and provides a more stable estimation of association when the number of exposed cases is small, the exposure is time varying and there is interest in how the association may vary over time, yet the periods of exposure are correlated15–17. We also characterized the month of initiation of use as a continuous measure to increase power in evaluating the association with timing. We used the same approach for OC use, characterizing use by trimester, by month, and by overlapping, 2-month increments, through the 4th month of pregnancy.
Overweight or obese or obese only status was ascertained from height and weight data collected by study personnel. Height was measured to the nearest 0.5 centimeters using a standardized backboard, and weight was recorded in pounds to the nearest 0.25 pounds or grams to the nearest 100 grams using scales calibrated semiannually18. The mean age at collection was 84 months (SD: 2.5, range 72–96). From height and weight measures we calculated body mass index (BMI) (kg/m2). Each child’s BMI was then expressed as a percentile, based on the Centers for Disease Control reference standards for age and sex19. Overweight or obese status was characterized as having a BMI of ≥85th percentile. Obesity was characterized as having a BMI of ≥95th percentile. These percentiles correspond to a BMI of ≥17.4 kg/m2 (overweight or obese) and ≥19.1 kg/m2 (obese only) for boys and ≥17.6 kg/m2 (overweight or obese) and ≥19.6 kg/m2 (obese only) for girls at age 7 years19. In the present study, 4,954 (14.4%) children were overweight or obese and 1,743 (5.1%) were obese. The mean BMI for boys was 16.0 kg/m2 (sd: 1.8) and for girls was 15.9 kg/m2 (sd: 2.1).
Covariate selection was informed by a directed acyclic graph16, constructed based on a review of the literature. Given the potential for differences in prescribing practices by study center and the observed differences in study population demographics by center, all multivariate models included adjustment by study center (12 centers). Additionally, multivariate models included adjustment for maternal age (<20, 20–34, ≥35)20, 21, race (Black, White, other)22, 23, education (<high school, high school diploma or equivalent, >high school diploma or equivalent)22, 23, prepregnancy BMI (continuous)24, 25, smoking during pregnancy22, 26 (any versus none)21, 25, and parity (0, 1, 2–3, ≥4)22, 27.
We used generalized linear models to estimate odds of overweight or obese or obese only for exposed versus unexposed pregnancies (logit link) and generalized estimating equations with an exchangeable working correlation matrix to estimate robust errors and account for correlation in the data resulting from sibling clusters28. For overlapping periods of exposure, each exposure period was modeled separately.
We explored the potential for confounding by indication for DES users, first by comparing the distribution of pregnancy-related events and complications among DES users and non-users and then by conducting a sensitivity analysis to assess the robustness of study results when adjusting on these additional factors associated with DES use.
In a supplementary analysis, we evaluated change in estimates with inclusion of additional model terms for birthweight, year of birth, and gestational diabetes. We also evaluated for possible effect modification by race, including an interaction term for race and OC or DES use. A priori, a p-value of <0.15 was considered to be evidence in support of possible interaction by race. All statistical analyses were conducted using SAS v9.3 (SAS Institute Inc., Cary, North Carolina). This study was approved by the Institutional Review Board (IRB) of the National Institutes of Environmental Health Sciences.
Results
Examination of the distribution of the study covariates for the study population by exposure to either an oral contraceptive or DES indicated differences in proportion exposed by race and education (Table 1). Specifically, pregnancies to mothers of White race and with a high school education or more were more likely to have been exposed (Table 1). Additionally, pregnancies to women with higher parity were, in general, more likely to have been exposed to an oral contraceptive. The distribution of child overweight or obesity status at age 7 by covariate status indicated a higher proportion of overweight or obese children were born to women of White or Other race or who had a pre-pregnancy BMI ≥ 25.0 (kg/m2) (Table 1). The distribution of covariates among those pregnancies not included in the study, either because of missing covariates or loss to follow-up (n=21,321), compared to the study population (n=34,419) was similar (Table 1S) as was the distribution of exposure status (Table 2S).
Table 1.
Study population covariate distribution and prevalence of exposure and outcome by covariate category
| Study population | Exposed to an OC† months 1–4 |
Exposed to DES‡ months 1–9 |
Overweight or obese (≥85thpercentile) at age 7 |
||
|---|---|---|---|---|---|
| (n* = 34,419) | (n=196) | (n=131) | (n=4,934) | ||
| Characteristic | n | %** | %** | %** | |
| Overall | -- | 0.4 | 0.4 | 14 | |
| Maternal age (years) | |||||
| <20 | 8,192 | 0.3 | 0.1 | 13 | |
| 20–34 | 23,516 | 0.7 | 0.4 | 15 | |
| ≥35 | 2,711 | 0.1 | 0.8 | 19 | |
| Maternal race | |||||
| Black | 15,402 | 0.2 | 0.2 | 12 | |
| White | 17,663 | 0.7 | 0.7 | 17 | |
| Other | 1,354 | 0.1 | 0.1 | 20 | |
| Maternal education (years) | |||||
| <12 | 3,833 | 0.2 | 0.2 | 14 | |
| 12 | 10,913 | 0.6 | 0.6 | 16 | |
| ≥13 | 19,673 | 0.7 | 0.7 | 15 | |
| Maternal pre-pregnancy BMI (kg/m2) | |||||
| <18.5 | 3,103 | 0.6 | 0.6 | 6 | |
| 18.5–24.9 | 23,383 | 0.4 | 0.4 | 13 | |
| 25.0–29.9 | 1,792 | 0.4 | 0.4 | 21 | |
| ≥30.0 | 6,141 | 0.4 | 0.4 | 27 | |
| Maternal smoking | |||||
| No | 18,550 | 0.4 | 0.4 | 14 | |
| Yes | 15,869 | 0.4 | 0.4 | 15 | |
| Parity | |||||
| 0 | 10,103 | 0.3 | 0.3 | 16 | |
| 1 | 7,672 | 0.3 | 0.3 | 15 | |
| 2–3 | 9,607 | 0.5 | 0.5 | 14 | |
| ≥4 | 7,037 | 0.3 | 0.3 | 12 |
complete case analysis sample
denotes percent of study population within a given category who had the given exposure or outcome
oral contraceptive
diethylstilbestrol
Oral contraceptives
Use of an oral contraceptive in the first trimester was positively associated with both overweight or obese (adjusted OR: 1.4, 95% CI: 1.4, 2.0) and obese (adjusted OR: 1.7, 95% CI: 0.9, 3.0). Estimates obtained for exposure in the second trimester were highly imprecise for overweight or obese and not estimable for obese, a reflection of the sparseness of data in this exposure period (Table 2). Evaluation of exposure by month of initiation further illustrated the sparseness of the data for exposure in months 3 and 4, but provided the benefit of increasing precision for estimates in gestational month 1 (Table 3S). Finally, in characterizing exposure in 2 month, overlapping exposure periods, we observed that contraceptive use in early pregnancy was weakly, positively associated with overweight or obese at exposure periods 1–2, 2–3, and 3– 4, and, more strongly, positively associated with offspring obese for exposure during gestational months 1–2 (adjusted OR: 2.0, 95% CI: 1.1, 3.7) (Table 3).
Table 2.
Odds of overweight or obese among offspring of women using a pharmacologic sex hormone - by trimester of first use
| Overweight or obese (≥85th percentile) |
Obese (≥95th percentile) | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Exposed n |
Exposed cases n |
Crude OR (95% CI) |
Adjusted* OR (95% CI) |
Exposed cases n |
Crude OR (95% CI) |
Adjusted* OR (95% CI) |
|
| Oral contraceptive** | ||||||||
| None | 34,223 | 4,920 | referent | referent | 1,731 | referent | referent | |
| Trimester 1 | 182 | 31 | 1.3 (0.9, 1.8) | 1.4 (0.9, 2.0) | 12 | 1.3 (0.8, 2.4) | 1.7 (0.9, 3.0) | |
| Trimester 2 | 14 | 3 | 1.7 (0.5, 6.0) | 1.8 (0.5, 7.0) | 0 | --† | --† | |
| DES** | ||||||||
| None | 34,288 | 4,929 | referent | referent | 1,730 | referent | referent | |
| Trimester 1 | 86 | 15 | 1.2 (0.7, 2.1) | 0.8 (0.5, 1.4) | 8 | 2.0 (0.9, 4.0) | 1.3 (0.6, 2.8) | |
| Trimester 2 | 39 | 9 | 1.6 (0.7, 3.4) | 1.5 (0.7, 3.2) | 4 | 2.0 (0.7, 6.0) | 2.0 (0.6, 6.5) | |
| Trimester 3 | 6 | 1 | 1.2 (0.1, 10.1) | 1.2 (0.1, 12.3) | 1 | 3.7 (0.4, 31.9) | 4.4 (0.3, 55.9) | |
adjusted for maternal smoking, education, parity, race, pre-pregnancy BMI, maternal age, and study center
trimester of first documented use in pregnancy -- trimester 1 corresponds to months 1–3, trimester 2 corresponds to months 4–6, and trimester 3 corresponds to months 7–9
not estimable
Table 3.
Odds of overweight or obese among offspring of women using a pharmacologic sex hormone - by months of first use
| Overweight or obese (≥85th percentile) |
Obese (≥95th percentile) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Exposed n |
Exposed cases n |
Crude OR (95% CI) |
Adjusted* OR (95% CI) |
Exposed cases n |
Crude OR (95% CI) |
Adjusted* OR (95% CI) |
|
| OC months of first use** | ||||||||
| None | 34,223 | 4,920 | referent | referent | 1,731 | referent | referent | |
| 1–2 | 159 | 27 | 1.3 (0.8, 1.9) | 1.4 (0.9, 2.1) | 12 | 1.6 (0.9, 2.8) | 2.0 (1.1, 3.7) | |
| 2–3 | 52 | 10 | 1.4 (0.7, 2.8) | 1.6 (0.8, 3.1) | 2 | 0.7 (0.2, 3.1) | 0.9 (0.2, 4.1) | |
| 3–4 | 37 | 7 | 1.3 (0.6, 3.1) | 1.3 (0.5, 3.2) | 0 | --† | --† | |
| DES months of first use** | ||||||||
| None | 34,288 | 4,929 | referent | referent | 1,730 | referent | referent | |
| 1–2 | 51 | 6 | 0.8 (0.4, 1.9) | 0.5 (0.2, 1.3) | 3 | 1.2 (0.4, 3.7) | 0.8 (0.3, 2.5) | |
| 2–3 | 69 | 12 | 1.2 (0.6, 2.3) | 0.7 (0.4, 1.4) | 7 | 2.2 (1.0, 4.7) | 1.4 (0.6, 3.0) | |
| 3–4 | 52 | 16 | 2.4 (1.3, 4.3) | 1.7 (0.9, 3.2) | 9 | 3.8 (1.8, 7.8) | 2.8 (1.3, 6.3) | |
| 4–5 | 32 | 9 | 2.1 (1.0, 4.6) | 2.0 (0.9, 3.2) | 4 | 2.5 (0.9, 7.6) | 2.6 (0.8, 8.6) | |
| 5–6 | 22 | 2 | 0.5 (0.1, 2.7) | 0.6 (0.1, 2.6) | 0 | --† | --† | |
| 6–7 | 9 | 1 | 0.6 (0.1, 7.1) | 0.6 (0.1, 5.9) | 1 | 2.4 (0.3, 18.4) | 2.5 (0.3, 20.2) | |
| 7–8 | 6 | 1 | 1.2 (0.1, 10.1) | 1.2 (0.1, 12.3) | 1 | 3.7 (0.4, 31.9) | 4.5 (0.4, 56.6) | |
| 8–9 | 4 | 0 | --† | --† | 0 | --† | --† | |
| by month of start‡ | -- | -- | 1.1 (0.9, 1.2) | 1.0 (0.9, 1.1) | -- | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.4) | |
adjusted for maternal smoking, education, parity, race, pre-pregnancy BMI, maternal age, and study center
months of first documented use in pregnancy
not estimable
represents OR for one unit increase in month for month of start of use
DES
Use of DES was positively associated with both overweight or obese and obese only for all trimesters of initiation of use. However, estimates were highly imprecise (Table 2). Evaluation of DES use by month was suggestive of a higher period of susceptibility in months 3 or 4, although again, estimates were imprecise and cell counts were sparse (Table 3S). For several months of exposure, data were too sparse for estimation. Applying the 2-month, overlapping periods of exposure approach, described above, we observed more stable and more precise estimates (Table 3). These results indicated that DES was positively associated with overweight or obesity for exposure beginning at 3–4 months and 4–5 months. Similarly, for DES and obesity, there was a positive association between initiation of use at 2–3, 3–4, and 4–5 months, with the strongest association observed at 3–4 months (adjusted OR: 2.8, 95% CI: 1.3, 6.3) (Table 3). With exposure modeled as a continuous measure, a positive trend was observed in the association between timing of use and offspring obesity, with a 10% increase in odds of obesity for each increase in month of initiation of use (Table 3). Inclusion of a quadratic term for month of initiation did not improve model fit.
A higher proportion of women prescribed DES had vaginal bleeding during pregnancy, a history of prior pregnancy loss, a longer period of time to achieve pregnancy, or a history of infertility investigation. Women using DES in early pregnancy were also more likely to report that the pregnancy was planned (Table 4S). We also observed differences in the indications for prescribing DES in early versus later pregnancy (trimester 1 versus trimester 2). Women initiating DES use in the first trimester were more likely to have a history of infertility (54% for first trimester users versus 15% for second trimester users) and were somewhat less likely to have experienced vaginal bleeding in the index pregnancy (58% for first trimester users versus 72% for second trimester users). To explore whether the association between DES use and offspring obesity could be confounded by these differences, we conducted a sensitivity analysis whereby we adjusted for these possible confounding factors. An additional 414 participants had to be excluded from the sensitivity analyses for missing one or more of these study covariates. The estimates obtained from these analyses (Table 5S) were similar to estimates observed in our primary analyses.
Supplementary analyses
In our supplementary analyses, evaluating change in estimate with inclusion of model terms for birthweight, year of birth, and gestational diabetes, an additional 156 participants were excluded from the analysis due to missing data on one or more of these covariates. Estimates obtained, for both DES or OCs, were substantively unchanged from estimates obtained in analyses including model terms for maternal age, race, education, prepregnancy BMI, and smoking (Table 6S). There was also no evidence to support effect modification by maternal race for either DES or OC use.
Discussion
In our study, exposure to either an oral contraceptive or to DES during specific periods of pregnancy was associated with offspring overweight or obese or obese only. This result is compatible with effects observed in animal studies. Although estrogenic agents are believed to sometimes act in non-receptor driven pathways, their primary action is through binding with nuclear receptors, e.g. estrogen receptors (ER) and peroxisome proliferator-activated receptor gamma (PPAR-γ), and activating or deactivating steroid receptor-mediated transcription29. In in vitro models DES has activated expression of both ER and PPAR-γ receptors required for adipogenesis11. Similarly, 17-β estradiol has resulted in increased preadipocyte proliferation, likely through up-regulation of PPAR-γ30. The process of preadipocyte formation can be initiated at any stage of life, but perturbation has been demonstrated to occur as early as in the blastocyst stage29. In humans, adipocyte formation has been identified to be most active in gestational weeks 14 through 2213. Recently, there has been interest in possible epigenetic effects of obesogens, whereby alterations in gene expression are driven by DNA methylation or histone modifications. These epigenetic effects may perturb priming of multipotent stem cells to preadipocyte formation31.
In the present study, the associations were strongest for DES, particularly DES exposure in months 3–4 and 4–5. We were only able to evaluate oral contraceptive use for exposure in early pregnancy, but for exposure in 1–2 months or duration of use 2–3 months, the magnitude of association observed was weaker than that observed for DES. The difference in association for these two agents may be attributable to differences in their capacity to alter receptor signaling, in epigenetic effects, in potency, or in pharmacokinetic changes as pregnancy advances. DES is considered a more potent estrogenic agent than ethinyl estradiol or mestranol, the two forms of synthetic estrogen in oral contraceptives in this study. The relative difference in potency may explain differences observed in the teratogenicity of DES and oral contraceptives. Whereas DES has been consistently reported to have numerous teratogenic effects on offspring32, 33, there is little to no evidence of a teratogenic effect for offspring of oral contraceptive users34.
Another important distinction is that the oral contraceptive formulation includes a progestin component in addition to the estrogen component. Unopposed exposure to an estrogen may elicit a different response than exposure to opposed estrogen. Finally, the results observed may reflect differences in duration in exposure, as opposed to differences in timing of exposure. Once initiated, DES use occurred throughout pregnancy, while oral contraceptive use generally ended early in pregnancy. Duration of DES use during pregnancy and timing of DES initiation was strongly correlated (Spearman r=0.99) and thus the independent effects of each could not be distinguished in these analyses. Given the observation that DES use in months 1–2 and 2–3 were not associated with offspring obesity, timing of use may be more important than duration. However, given the sparse data and the strong correlation between timing and duration, the possibility that both components are necessary cannot be ruled out.
There is very little information in the published literature on the association between oral contraceptive use and offspring overweight or obesity. There has been one study, published as an abstract, suggesting a positive association between DES exposure in utero and offspring obesity in adult women7. A pooled analysis of women exposed in utero to DES indicated no difference (unadjusted) in mean body mass index in adulthood compared to unexposed women35. The difference in the present study findings, and the findings from this pooled analysis, may be attributable to differences in study population, or indicative of a washing out of effect over time. It may be too, that in examining exposure at any point in time, any differences would have been unobservable, as opposed to the present study for which there is indication of differences specific to timing of exposure. In Hatch et al., the association between DES and obesity was strongest for use at ≥15 weeks gestation7. In the present study we assessed for and found no evidence of an interaction between oral contraceptive use or DES use and sex. However, given the relatively small number of women using either of these agents, the study was underpowered to conclude an absence of interaction with sex. Attenuation of effects overtime has been demonstrated in animal models of obesogens, such as that described by Ryan et al. in evaluating the association between BPA and offspring obesity in mice followed to adulthood36. This same study showed that in utero exposure to DES decreased body fat in adult mice relative to controls36.
The results observed, or differences in results observed between DES and oral contraceptives, could be attributable to unmeasured or residual confounding. Underlying maternal metabolic factors could contribute to poorer pregnancy outcomes and DES use. Underlying maternal factors could also contribute to conceiving while taking an oral contraceptive. These factors, if associated with offspring adiposity, could bias estimates. As described previously, adjustment for pregnancy-related factors associated with DES use did not substantively change estimates obtained in the primary analyses (Table 5S). Finally, the estimates observed could reflect chance.
The results might have been influenced by misclassification. Either DES or oral contraceptives could have been under- or over-reported due to poor recall of use. Although the exposure was self-reported, exposure ascertainment occurred relatively close to the time at which exposure occurred. Furthermore, validation studies of self-reported oral contraceptive use indicate women are generally accurate in their recall of oral contraceptive use37, 38. For example, in a validation study of self-reported oral contraceptive use occurring within 12 months of interview, the weighted kappa for self-reported- versus medical record-indicated use was 0.838. Notwithstanding, potential for misclassification of exposure exists, with the greatest potential for misclassification of use in early pregnancy, the most distal point of recall for enrolled women. There is also the potential that initiation of oral contraceptive in months 3 or 4 reflects misclassification of exposure. The 37 women reporting initiation of an oral contraceptive in months 3–4 were generally younger (16% less than 20 years of age versus 9% for those reporting use in months 1 or 2) and somewhat less educated (35% with less than 12 years of education versus 24% for those reporting use in months 1 or 2). Women initiating use in months 3 or 4 were also less likely to be Black (24% versus 36% for women reporting use in months 1 or 2). Any misclassification would likely result in a bias of estimates toward the null. Attenuation of effect estimates in months 3 and 4, for oral contraceptive use, could be an artifact of greater misclassification of exposure in this period.
All height and weight measures were obtained from trained study personnel. Furthermore, the association between DES use and obesity was stronger than the association with overweight or obesity. Obesity is a more sensitive measure of clinically relevant adiposity status as some children who are identified as overweight are children with increased muscle mass, as opposed to fat mass and thus obesity is likely to represent a more valid measure of childhood adiposity39.
Although the present study was large enough to provide an opportunity to study exposure of relatively low occurrence (failed contraception), sample size limitations precluded exploring, in greater detail, the influence of timing, duration, or drug formulation of exposure on observed associations. These details could yield additional etiologic clues in the role of estrogenic agents in development of overweight or obesity. Power calculations after a study has already been conducted may have limited utility40, however we conducted a power calculation to assess adequacy of study sample size and whether there was a potential that lack of association observed at some time periods could be attributable to a Type II error. With a prevalence of obesity of 0.06 at age 7, a proportion exposed to an oral contraceptive of 0.005 for exposure in months 1–2, power of 0.80, and a Type I error of 0.05, we would have needed approximately 27,000 subjects in our study population to detect an odds ratio of 2.0. For DES use, with a proportion exposed of 0.002 in months 3–4, we would have needed approximately 29,000 subjects to detect an odds ratio of 2.8. For exposure periods with fewer exposed or for smaller effect sizes, our study would have been underpowered to reject the null hypothesis of no effect. Therefore, although the study results are suggestive of timing-specific effects, the small sample sizes at some exposure periods may limit the interpretability of results.
Future studies, with a larger number of births followed (>55,000), would benefit from additional exposure details to estimate the association with greater precision and explore possible dose-response relationships. Furthermore, future studies would benefit from assessing the association with contemporary, lower-dose oral contraceptive formulations. The findings from this study generally support those obtained in animal models. However, lower dose formulations may more closely approximate the exposures incurred through environmental sources of estrogenic compounds than the doses documented in this cohort.
Supplementary Material
What is already known about this subject.
In animal models, in utero exposure to exogenous estrogenic agents is associated with offspring adiposity.
In in vitro and animal models, diethylstilbestrol exposure has led to an increase in stem cell differentiation into preadipocytes and adipocytes.
What this study adds
An evaluation of the association between in utero exposure to pharmacologic estrogens and subsequent obesity in humans
A novel approach to studying the potential for developmental origins of obesity as conferred through in utero exposure to estrogenic agents
Acknowledgements
ET Jensen contributed to the development of the research question and was responsible for the analyses and writing of the manuscript. MP Longnecker conceived and provided oversight to the conduct of the research.
This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS).
We acknowledge Dr. Til Sturmer for his input and contribution to the development of the manuscript.
Footnotes
Conflicts of interest statement:
None of the authors have any disclosures.
Contributor Information
Elizabeth T. Jensen, Epidemiology Branch, National Institute of Environmental Health Sciences, 111 T.W. Alexander Drive, Research Triangle Park, North Carolina 27709
Matthew P. Longnecker, Epidemiology Branch, National Institute of Environmental Health Sciences
References
- 1.Bhandari R, Xiao J, Shankar A. Urinary Bisphenol A and Obesity in US Children. American Journal of Epidemiology. 2013;177(11):1263–1270. doi: 10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama. 2012;308(11):1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 3.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304(1–2):84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Research Part A: Clinical and Molecular Teratology. 2005;73(7):478–480. doi: 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- 5.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Molecular Nutrition & Food Research. 2007;51(7):912–917. doi: 10.1002/mnfr.200600259. [DOI] [PubMed] [Google Scholar]
- 6.Werner Fürst R, Pistek VL, Kliem H, Skurk T, Hauner H, Meyer HHD, et al. Maternal low-dose estradiol-17β exposure during pregnancy impairs postnatal progeny weight development and body composition. Toxicology and Applied Pharmacology. 2012;263(3):338–344. doi: 10.1016/j.taap.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hatch E, Troisi R, Palmer J, Wise L, Titus L, Ricker W, et al. Society of Epidemiologic Research. Boston, MA: 2013. Prenatal exposure to diethylstilbestrol and obesity in middle-aged women. [Google Scholar]
- 8.Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3–12. doi: 10.1016/j.beem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Smith OW. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. Am J Obstet Gynecol. 1948;56(5):821–834. [PubMed] [Google Scholar]
- 10.Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro. 2013;27(6):1634–1643. doi: 10.1016/j.tiv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor diethylstilbestrol induces adipocyte differentiation and promotes obesity in mice. Toxicology and Applied Pharmacology. 2012;263(1):102–110. doi: 10.1016/j.taap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil Steril. 2008;89(2 Suppl):e55–e56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 13.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10(1–2):1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 14.Niswander KR, Gordon M Collaborative Perinatal Project (U.S.) The women and their pregnancies [by] Kenneth R. Niswander [and] Myron Gordon, with Heinz W. Berendes [et al.] Philadelphia: Saunders; 1972. National Institute of Neurological Diseases and Stroke. [Google Scholar]
- 15.Rich DQ, Zareba W, Beckett W, Hopke PK, Oakes D, Frampton MW, et al. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Perspect. 2012;120(8):1162–1169. doi: 10.1289/ehp.1104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 17.Ross Z, Ito K, Johnson S, Yee M, Pezeshki G, Clougherty JE, et al. Spatial and temporal estimation of air pollutants in New York City: exposure assignment for use in a birth outcomes study. Environ Health. 2013;12:51. doi: 10.1186/1476-069X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaborative Perinatal Project (U.S.), National Institute of Neurological Diseases and Blindness. Perinatal Research Branch. Medical Literature Services., National Institute of Neurological Diseases and Stroke. Perinatal Research Branch. Medical Literature Unit. DHEW publication no (NIH) 72–117. Bethesda, Md: U.S. Dept. of Health, Education, and Welfare, Public Health Service, National Institutes of Health; Bibliography: the collaborative study on cerebral palsy, mental retardation, and other neurological and sensory disorders of infancy and childhood; p. 5. no. [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 20.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21(5):1279–1284. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- 21.Mutsaerts MA, Groen H, Huiting HG, Kuchenbecker WK, Sauer PJ, Land JA, et al. The influence of maternal and paternal factors on time to pregnancy--a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod. 2012;27(2):583–593. doi: 10.1093/humrep/der429. [DOI] [PubMed] [Google Scholar]
- 22.Hall KS, Trussell J. Types of combined oral contraceptives used by US women. Contraception. 2012;86(6):659–665. doi: 10.1016/j.contraception.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weden MM, Brownell P, Rendall MS. Prenatal, perinatal, early life, and sociodemographic factors underlying racial differences in the likelihood of high body mass index in early childhood. Am J Public Health. 2012;102(11):2057–2067. doi: 10.2105/AJPH.2012.300686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog. 2006;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 25.Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32(2):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95(12):5365–5369. doi: 10.1210/jc.2010-0697. [DOI] [PubMed] [Google Scholar]
- 28.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2nd edn. Cary, NC: SAS Institute; 2000. [Google Scholar]
- 29.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 30.Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 31.Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res C Embryo Today. 2011;93(1):34–50. doi: 10.1002/bdrc.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 33.Palmer JR, Herbst AL, Noller KL, Boggs DA, Troisi R, Titus-Ernstoff L, et al. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environ Health. 2009;8:37. doi: 10.1186/1476-069X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waller DK, Gallaway MS, Taylor LG, Ramadhani TA, Canfield MA, Scheuerle A, et al. Use of oral contraceptives in pregnancy and major structural birth defects in offspring. Epidemiology. 2010;21(2):232–239. doi: 10.1097/EDE.0b013e3181c9fbb3. [DOI] [PubMed] [Google Scholar]
- 35.Troisi R, Hyer M, Hatch EE, Titus-Ernstoff L, Palmer JR, Strohsnitter WC, et al. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiology. 2013;24(3):430–438. doi: 10.1097/EDE.0b013e318289bdf7. [DOI] [PubMed] [Google Scholar]
- 36.Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–2612. doi: 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter DJ, Manson JE, Colditz GA, Chasan-Taber L, Troy L, Stampfer MJ, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception. 1997;56(6):373–378. doi: 10.1016/s0010-7824(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 38.Nischan P, Ebeling K, Thomas DB, Hirsch U. Comparison of recalled and validated oral contraceptive histories. American Journal of Epidemiology. 1993;138(9):697–703. doi: 10.1093/oxfordjournals.aje.a116907. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–S34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 40.Smith AH, Bates MN. Confidence limit analyses should replace power calculations in the interpretation of epidemiologic studies. Epidemiology. 1992;3(5):449–452. doi: 10.1097/00001648-199209000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.