Abstract
Background and Aims
The prevalence of heterotopic gastric mucosa of the upper esophagus (inlet patch) has a wide range depending on the method and detail of examination. The inlet patch is believed to be a congenital malformation that rarely leads to symptoms. We aimed to quantify the prevalence of the inlet patch in a non-referred population, and determine if there are any risk factors or associated symptoms.
Methods
Men between ages 50 and 79 presenting for routine colonoscopy at two clinical sites were recruited to undergo an upper endoscopy. Endoscopists were prompted to examine for the presence of the inlet patch.
Results
Of the 822 enrolled patients, 795 had data regarding the presence of an inlet patch. Of these, 55 (6.9%) had an inlet patch identified. Education was inversely associated (odds ratio [OR] advanced degree vs. high school or less = 0.310; 95% confidence interval [CI] = 0.111, 0.869), and tobacco use was positively associated with the presence of an inlet patch (Current vs. never smokers OR= 2.87; 95% CI= 1.23, 6.69; Former vs. never smokers OR = 1.93; 95% CI= 0.922, 4.02). No association between the inlet patch and symptoms of heartburn, globus or dysphagia was found.
Conclusions
In a cross-sectional study of colon cancer screenees, inlet patches were common, and were not associated with symptoms. Tobacco use appears to be associated with the presence of an inlet patch.
Keywords: Inlet patch, heterotopic gastric mucosa, tobacco
Introduction
An inlet patch is defined as gastric heterotopic mucosa in the upper esophagus; the condition was first described more than a century ago (1). In prior studies, the prevalence ranges broadly from 0.3% to 10% of endoscopies, likely due to how carefully the upper esophagus is examined (2–4). While a majority patients are asymptomatic, symptoms of globus and dysphagia have been associated with these endoscopic findings (5) and complications associated with the inlet patch in the literature include stricture, fistulas, adenocarcinoma and perforation(6–8).
The finding itself is believed to be congenital, but associations with alcohol use and Barrett’s esophagus have been found(9). To our knowledge, no association with tobacco has been reported in the literature.
We recently completed a large prospective study designed to assess risk factors for Barrett’s esophagus. In that study, 822 men between the ages of 50 and 79 undergoing routine colonoscopy for colorectal cancer screening also underwent an upper endoscopy regardless of symptoms and a battery of surveys. As part of that study, endoscopists were directed by a research coordinator specifically to examine for the presence of an inlet patch. This study provided a unique opportunity to quantify the prevalence of the inlet patch and determine if there are any risk factors to suggest it is an acquired condition, and to determine whether inlet patches are associated with symptoms.
Methods
Men between the ages of 50 and 79 were enrolled in the Newly Diagnosed Barrett’s Esophagus Study from February 2008 to December 2011. These patients were presenting for screening or surveillance colonoscopy at the Ann Arbor Veteran’s Affairs Medical Center (AAMC) or the University of Michigan’s East Ann Arbor Medical Procedures Center (MPC). The study design is described in detail elsewhere (10,11). Briefly, patients were excluded if their age was not within 50–79 years, if they were female, if they had undergone prior upper endoscopy or had a prior history of Barrett’s esophagus or esophagectomy. If the colonoscopy was being done for bleeding (including occult blood), iron deficiency anemia, diarrhea, inflammatory bowel disease related surveillance, patients were also excluded. Those with a history of ascites, esophageal varices, history of cancer in the last 5 years (except non-melanomatous skin cancers), or those who were inpatients were excluded.
Prior to endoscopy, patient’s height, weight, and hip circumference were measured in duplicate. Patients were then questioned regarding alcohol, tobacco and medication use in addition to gastrointestinal symptoms. A novel questionnaire regarding GERD symptoms was administered as instruments available at the time of initiation of the study, such as the Mayo Gastroesophageal Reflux Questionnaire (GERQ), did not discriminate between past history of reflux symptoms while using and not using histamine receptor blocker and proton pump inhibitors. Portions of this questionnaire can be found in the supplementary material for the original paper (10). For the purposes of this analysis, we utilized responses to queries regarding time since onset of heartburn symptoms, and typical frequency of either heartburn or regurgitation while not taking acid reducing medications. Questions regarding globus sensation, defined as the sensation of something lodged in the patient’s throat when not eating or drinking, and dysphagia were also included in the questionnaire. Detailed questions regarding dysphagia to type (solids, liquids or pills), location, and frequency were asked; for the purpose of our analysis, we characterized dysphagia only as the dichotomous answer to a query regarding problems with swallowing. The questions regarding heartburn, regurgitation, globus and dysphagia were administered by a research assistant prior to the endoscopy procedure. The remaining questions, including regarding tobacco use, were typically completed by the subjects after the procedure at home, and returned by postal mail.
The research assistant queried the endoscopist in each case regarding findings and measurements. The endoscopist was asked specifically to examine for the presence of an inlet patch using both white light and narrow band imaging. If one was found, the location, size (circumferential aspect), and distance to proximal and distal aspects of the patch were recorded. Biopsies were not routinely obtained of an inlet patch. If Barrett’s esophagus was suspected endoscopically, biopsies were obtained and reviewed by a pathologist with expertise in Barrett’s esophagus. Patients with columnar mucosa in the distal esophagus identified endoscopically and confirmed to harbor specialized intestinal metaplasia on histology were classified as Barrett’s esophagus. There were trainees involved in some procedures, but this information was not recorded so the percentage of procedures and how this affected the findings of an inlet patch are unknown. An attending physician was always present for these endoscopies.
Analysis
Data was recorded in Microsoft Access and then imported to SAS 9.3 for analysis. Chi-square or Fisher’s exact tests were used where statistically appropriate to compare categorical variables, and the student’s t test was used to analyze continuous variables. Linear trends where analyzed by the Mantel-Haenszel chi-square test after ensuring that all cells had expected values greater than 5. Logistic regression models were fitted adjusting for potential confounders.
Results
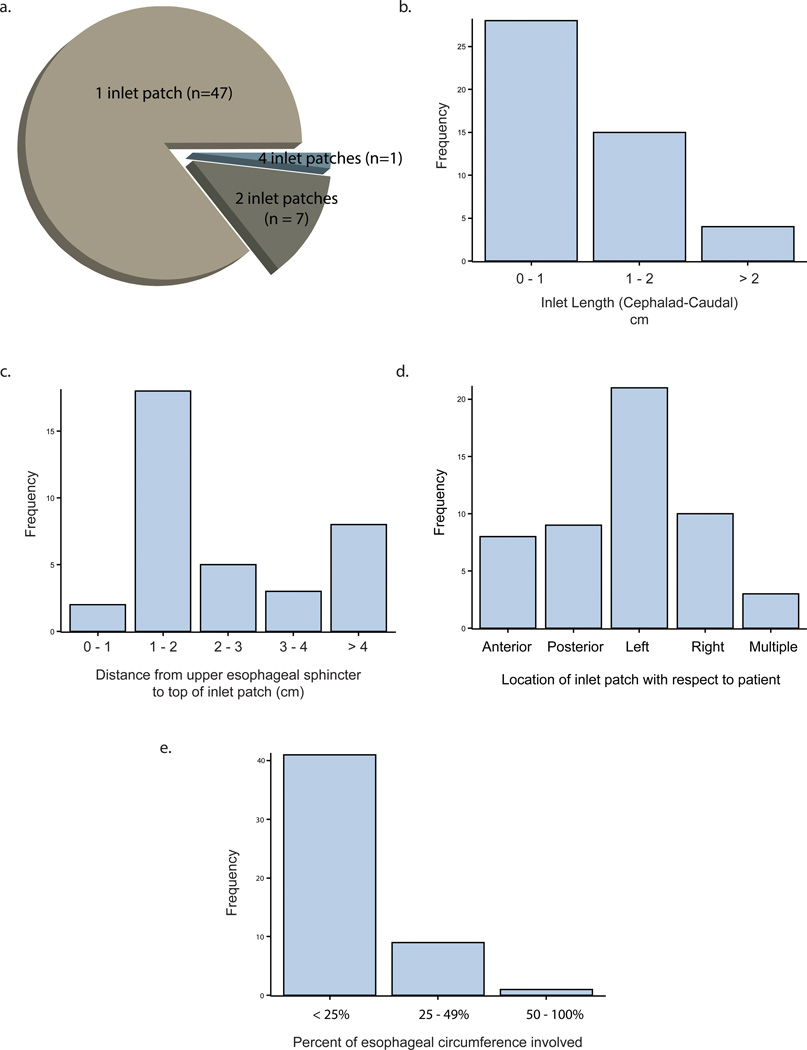
Of the 822 patients enrolled, 463 were from the University site and 359 were from the VA. Of these 822, 27 patients had missing data on the presence of the inlet patch (3.3%). Of these 27 patients, 3 cases specifically noted difficulty in assessing for the presence of a patch due to patient intolerance. Of the remaining 795, 55 had an inlet patch on endoscopy (6.9%). While most patients had only 1 patch (85.5%), 1 patient did have 4 distinct patches (Figure 1). The majority of patients had a patch that encompassed less than 25% of the circumference and most were less than 2cm in length (Figure 1). Inlet patch was most likely to be found on the patient’s left than any other location (p=0.03). Further details about number of patches per patient, location, length, circumference, and distance from the upper esophageal sphincter are found in Figure 1.
Figure 1.
Characteristics of the inlet patch findings. A pie chart of the number of inlet patches found per patient is located in panel a. Panel b shows a histogram (absolute counts) of the inlet patch length from proximal to distal edge in centimeters. Panel c shows the distance from the upper esophageal sphincter to the proximal edge of the patch in centimeters. Panel d shows the distribution of the patch location, with respect to the patient. Finally, panel e shows the percentage circumference that the patch encompasses in this population.
Demographics were similar for those with and without inlet patch with the exception of education, which was inversely associated with inlet patch (33% had high school education or less vs. 20%, Table 1). There was no difference in age, race, household income, BMI or waist-hip-ratio between the 2 groups. Of the 822 patients, 16 (2%) did not complete any portion of our GERD questionnaire.
Table 1.
Comparison of characteristics between patients with an inlet patch and those without a patch.
| Inlet patch (n=55) | No Inlet Patch (n=740) | p value | |
|---|---|---|---|
| Age (SD) | 58.5 (6.5) | 58.8 (6.8) | 0.72 |
| Race (%) | |||
| Caucasian | 43 (93.5) | 635 (89.8) | 0.61 |
| Not Caucasian | 3 (6.5) | 72 (10.2) | |
| Education | |||
| High School or Less | 16 (33.3) | 145 (20.4) | |
| Some College up to Bachelors | 0.05 | ||
| Degree | 27 (56.3) | 421 (59.1) | |
| Advanced Degree | 5 (10.4) | 146 (20.5) | |
| Annual Income ($) | |||
| <40,000 | 21 (44.7) | 259 (38.8) | 0.64 |
| 40,000–100,000 | 15 (31.9) | 215 (32.2) | |
| >100,000 | 11 (23.4) | 194 (29.0) | |
| Site (%) | |||
| MPC | 28 (50.9) | 421 (56.9) | 0.39 |
| VA | 27 (49.1) | 319 (43.1) | |
| BMI (%) | |||
| BMI<20 | 1 (1.9) | 7 (1.0) | |
| BMI 20–25 | 11 (20.4) | 123 (16.8) | 0.32 |
| BMI 25–30 | 24 (44.4) | 266 (36.2) | |
| BMI >30 | 18 (33.3) | 338 (46.1) | |
| Waist to Hip Ratio by tiles Tertiles | |||
| 1st (< 0.979) | 21 (38.2) | 246 (33.3) | 0.73 |
| 2nd (0.980–1.024) | 16 (29.1) | 245 (33.1) | |
| 3rd (> 1.024) | 18 (32.8) | 248 (33.6) | |
| Biopsy Proven Barrett's (%) | |||
| Yes | 5 (9.1) | 63 (8.5) | 0.8 |
| No | 50 (90.9) | 676 (91.5) | |
| Hiatal hernia >2cm | |||
| Yes | 6 (11.1) | 87 (11.8) | 0.88 |
| No | 48 (88.9) | 651 (88.2) | |
| GERD frequency off meds | |||
| >1 weekly | 13 (24.1) | 135 (18.6) | 0.32 |
| Not weekly | 41 (75.9) | 590 (81.4) | |
| Heartburn for 3 continuous months | |||
| Never | 45 (83.3) | 573 (79.3) | |
| In the last year only | 0 | 24 (3.3) | 0.7 |
| Started 1–3 years ago | 2 (3.7) | 27 (3.7) | |
| Started >3 years ago | 7 (13.0) | 99 (13.7) | |
| Globus | 0.75 | ||
| Yes | 5 (9.3) | 77 (10.6) | |
| No | 49 (90.7) | 648 (89.4) | |
| Dysphagia | |||
| Yes | 4 (7.4) | 75 (10.3) | 0.49 |
| No | 40 (92.6) | 650 (89.7) | |
| ASA use | |||
| None | 34 (61.8) | 412 (55.7) | 0.61 |
| For <5 years | 8 (14.6) | 143 (19.3) | |
| For >=5 years | 13 (23.6) | 185 (25.0) | |
| NSAID use | |||
| None | 43 (78.2) | 552 (74.6) | 0.81 |
| For <5 years | 8 (14.6) | 118 (16.0) | |
| For >=5 years | 4 (7.3) | 70 (9.0) | |
| PPI use (%) | |||
| None | 50 (90.9) | 646 (87.3) | 0.8 |
| PPI<5 years | 3 (5.5) | 64 (8.7) | |
| PPI>5 years | 2 (3.6) | 30 (4.0) | |
| H2RA use (%) | |||
| None | 50 (94.6) | 693 (93.7) | 0.77 |
| H2RA <5 years | 2 (3.6) | 38 (5.1) | |
| H2RA >5 years | 1 (1.8) | 9 (1.2) | |
There were no statistically significant differences between those with and without inlet patches with regard to symptoms, including heartburn, globus, or dysphagia (Table 1). Examining only those with larger inlet patches (>1cm in length or >25% of the esophageal circumference) also did not reveal an association with these symptoms. There was also no difference in PPI use, type 2 histamine receptor blocker use, aspirin use or other NSAID use. Neither group was more likely to have a hiatal hernia (>2cm) or biopsy proven Barrett’s esophagus (Table 1).
Patients with inlet patches had a heavier smoking history (mean 23.7 pack-years versus 16.3 pack-years, p=0.006). Categorizing patients into groups with no smoking history, light smoking history (<35 pack years) and heavy smoking history (>= 35 pack-years), there was also an association with smoking history and the inlet patch (p=0.02) along with evidence of a linear trend between increasing exposure to cigarettes and increased risk of having an inlet patch (p=0.01) (Table 2). Analyzing tobacco use further, we found that there was an positive association between current cigarette use and the finding of an inlet patch (p=0.04), again with a linear trend showing that current cigarette users had a higher likelihood of having an inlet patch than former smokers, who in turn had a higher likelihood than those who never smoked. The OR of inlet patch for those who had quit recently (2.71, 95%CI = 1.01, 7.25) approximated the OR of inlet patch for current smokers (2.84, 95% CI = 1.22, 6.63) while those who quit more than a decade ago had an OR of 1.58 (95% CI= 0.711, 3.50), which was not significantly different than patients who never smoked (Table 2). Average typical alcohol intake per week was not associated with the inlet patch.
Table 2.
The effects of tobacco and alcohol use on the presence of inlet patch.
| Inlet patch (n=55) | No Inlet Patch (n=740) |
OR (95%CI) | p value | |
|---|---|---|---|---|
| Pack years of cigarettes | ||||
| 0 pack years | 12 (25.0) | 241 (34.2) | 1 | 0.02 |
| 1–34 pack years | 12 (25.0) | 246 (34.9) | 1.42 (0.623 – 3.21) | |
| >35 pack years | 24 (50.0) | 218 (30.9) | 2.69 (1.31 – 5.52) | |
| Linear trend | 0.01 | |||
| Smoking status | ||||
| Never | 11 (23.9) | 281 (40.6) | 1 | |
| Former | 23 (50.0) | 305 (44.0) | 1.93 (0.922 – 4.02) | 0.04 |
| Current | 12 (26.1) | 107 (15.4) | 2.87 (1.23 – 6.69) | |
| Linear trend | 0.01 | |||
| Smoker by duration since use | ||||
| Never | 11 (24.4) | 281 (40.3) | 1 | |
| Quit >10 years ago | 15 (33.3) | 243 (34.8) | 1.58 (0.711 – 3.50) | 0.06 |
| Quit <10 years ago | 7 (15.6) | 66 (9.5) | 2.71 (1.01 – 7.25) | |
| Current | 12 (26.7) | 108 (15.5) | 2.84 (1.22 – 6.63) | |
| Linear trend | 0.007 | |||
| Smoking status and duration | ||||
| Never | 11 (23.9) | 281 (40.6) | 1 | 0.04 |
| Former, 1–34 pack years | 11 (23.9) | 188 (27.1) | 1.50 (0.635 – 3.52) | |
| Former, > 35 pack years | 12 (26.1) | 117 (16.9) | 2.62 (1.12 – 6.11) | |
| Current | 12 (26.1) | 107 (15.4) | 2.87 (1.23 – 6.69) | |
| Linear trend | 0.005 | |||
| ETOH (drinks/wk) | 4.7 (7.2) | 5.2 (7.8) | - | 0.65 |
We considered that the education effect might be confounded by smoking on the prevalence of an inlet patch, or vice versa. Smoking (categorized as never, former, and current) was inversely correlated with education (categorized as high school or less, at least some college, or post-graduate degree) (p-value <0.001). Table 3 presents the odds ratios for these effects considered with and without mutually adjusting for each other. The estimate of the smoking effect was attenuated toward the null after adjusting for education; likewise, the estimate of the education effect was also attenuated toward the null after adjusting for smoking.
Table 3.
Comparison of smoking and education status individually (crude) and in combination (adjusted).
| n (%) | Crude Estimates | Adjusted Estimates | ||
|---|---|---|---|---|
| Inlet patch | No inlet patch | OR (95%CI) | OR (95%CI) | |
| Smoking status (alone) | ||||
| Never | 11 (23.9) | 281 (40.6) | 1 | 1 |
| Former | 23 (50.0) | 305 (44.0) | 1.93 (0.922 – 4.02) | 1.69 (0.788 – 3.62) |
| Current | 12 (26.1) | 107 (15.4) | 2.87 (1.23 – 6.69) | 2.02 (0.809 – 5.06) |
| p value | 0.05 | 0.27 | ||
| Education status (alone) | ||||
| High School or Less | 16 (33.3) | 145 (20.4) | 1 | 1 |
| Some College up to Bachelor Degree | 27 (56.3) | 421 (59.1) | 0.58 (0.304 – 1.11) | 0.573 (0.292 – 1.13) |
| Advanced Degree | 5 (10.4) | 146 (20.5) | 0.31 (0.111 – 0.869) | 0.418 (0.140–1.25) |
| p value | 0.06 | 0.17 | ||
Discussion
Most research has suggested that the inlet patch is a congenital malformation, but there have been associations noted in the past that may suggest it is an acquired condition, including a previously found association with Barrett’s esophagus and with alcohol intake (9). That study failed to show an association between the inlet patch and smoking but only treated smoking as a dichotomous variable in the analysis. The Newly Diagnosed Barrett’s Study provides a unique opportunity to examine the prevalence and risk factors of gastric heterotopic mucosa in the upper esophagus in a population that more closely resembles the general population. The prior endoscopic studies on this topic have typically been a sampling (3,4) of patients presenting for upper endoscopy for clinical indications which likely introduces an element of selection bias into the estimate of the prevalence and associations. Additionally, this study included detailed surveys of patient’s symptoms, medications and habits allowing us to determine if habits such as cigarette smoking are associated with the inlet patch.
Our study did find a relationship between cigarette use and the inlet patch. While the analysis here is performed in a case-control fashion and so is not sufficient to prove causation, there are several features of our analysis that do suggest a causal link. First, there was noted to be an increased risk as the number of pack years of cigarette use increases. Second, former smokers had a lower prevalence of inlet patches than those who currently smoked. Third, those who had quit for more than 10 years had a prevalence of inlet patch that closely approximated that of the population who never smoked. However, the association of inlet patch with tobacco may have been confounded by education, which was also inversely associated with inlet patch.
A number or prior studies have reported the possible association between the presence of an inlet patch and symptoms of globus or dysphagia (4,12,13). There is also limited data to suggest ablation of inlet patch tissue with argon plasma coagulation reduces these symptoms (14). In our observational study, we did not find that patients with an inlet patch were any more likely to have these symptoms than patients without a patch. Results from prior studies may have been biased by selection effects for inclusion in the studies. Alternatively, perhaps only very large inlet patches are associated with symptoms. Our study did not find such an association, but we had limited statistical power to detect associations with large inlet patches as only 10 subjects were found to have a patch occupying more than 25% of the circumference of the esophageal lumen.
Our study has a number of limitations. We did not obtain histologic confirmation of inlet patches, and the symptom questionnaires used were not validated. We limited our analysis of tobacco use to only cigarettes due to difficulty of quantifying equivalent exposure to cigar smoke and pipe smoke. Our survey did not query patients on smokeless tobacco products. Furthermore, we tested for multiple associations, so our finding might be solely due to chance. Finally, our study was limited to a male population and 44% of the population was veterans so these results may not be generalizable to other populations.
In summary, in a cross-sectional study of men undergoing upper endoscopy for research purposes, we found that inlet patches are common, not associated with symptoms, but positively associated with tobacco use and inversely associated with education. Further studies are warranted to understand whether inlet patches are acquired conditions, and whether they cause esophageal symptoms in some individuals.
Acknowledgments
Grant support:
Research and salary funding was provided by the National Institutes of Health (JHR: K23DK079291
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Author contribution:
SMG performed the data analysis, drafted and revised the manuscript
VM collected and cleaned the data
JHR initiated the project, reviewed the data analysis, revised the manuscript and supervised the project
References
- 1.Schmidt FA. De mammalium oesophage atque ventriculo. Inaugural dissertation; Halle, Bethenea, 1805. Cited by von Rahden BHA, Stein HJ, Becker K, et al. Heterotopic Gastric Mucosa of the Esophagus: Literature-Review and Proposal of a Clinicopathlogic Classification. Am J Gastroenterol. 2004;99:543–551. doi: 10.1111/j.1572-0241.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 2.Jabbari M, Goresky CA, Lough J, et al. The inlet patch: heterotopic gastric mucosa in the upper esophagus. Gastroenterology. 1985;89:352–356. doi: 10.1016/0016-5085(85)90336-1. [DOI] [PubMed] [Google Scholar]
- 3.Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut. 1991;32:968–972. doi: 10.1136/gut.32.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maconi G, Pace F, Vago L, et al. Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch) European Journal of Gastroenterology & Hepatology. 2000;12:745–749. doi: 10.1097/00042737-200012070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs E, Dehou MF. Heterotopic gastric mucosa in the upper esophagus: a prospective study of 33 cases and review of literature. Endoscopy. 1997;29:710–715. doi: 10.1055/s-2007-1004294. [DOI] [PubMed] [Google Scholar]
- 6.Yarborough CS, McLane RC. Stricture related to an inlet patch of the esophagus. The American journal of Gastroenterology. 1993;88:275–276. [PubMed] [Google Scholar]
- 7.Sperling RM, Grendell JH. Adenocarcinoma arising in an inlet patch of the esophagus. The American journal of Gastroenterology. 1995;90:150–152. [PubMed] [Google Scholar]
- 8.Sanchez-Pernaute A, Hernando F, Diez-Valladares L, et al. Heterotopic gastric mucosa in the upper esophagus (“Inlet Patch”): a rare cause of esophageal perforation. The American journal of Gastroenterology. 1999;94:3047–3050. doi: 10.1111/j.1572-0241.1999.01458.x. [DOI] [PubMed] [Google Scholar]
- 9.Avidan B, Sonnenberg A, Chejfec G, et al. Is there a link between cervical inlet patch and Barrett's esophagus? Gastrointest Endosc. 2001;53:717–721. doi: 10.1067/mge.2001.114782. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett Esophagus Among Men. The American Journal of Gastroenterology. 2013;108:353–362. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenstein JH, Morgenstern H, Chey WD, et al. Protective role of gluteofemoral obesity in erosive oesophagitis and Barrett's oesophagus. Gut Published Online First March 5th. 2013 doi: 10.1136/gutjnl-2012-304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alagozlu H, Simsek Z, Unal S, et al. Is there an association between Helicobacter pylori in the inlet patch and globus sensation? World Journal of Gastroenterology. 2010;16:42–47. doi: 10.3748/wjg.v16.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alaani A, Jassar P, Warfield AT, et al. Heterotopic gastric mucosa in the cervical oesophagus (inlet patch) and globus pharyngeus–an under-recognised association. The Journal of Laryngology & Otology. 2007;121:885–888. doi: 10.1017/S0022215106005524. [DOI] [PubMed] [Google Scholar]
- 14.Bajbouj M, Becker V, Eckel F, et al. Argon Plasma Coagulation of Cervical Heterotopic Gastric Mucosa as an Alternative Treatment for Globus Sensations. Gastroenterology. 2009;137:440–444. doi: 10.1053/j.gastro.2009.04.053. [DOI] [PubMed] [Google Scholar]