Abstract
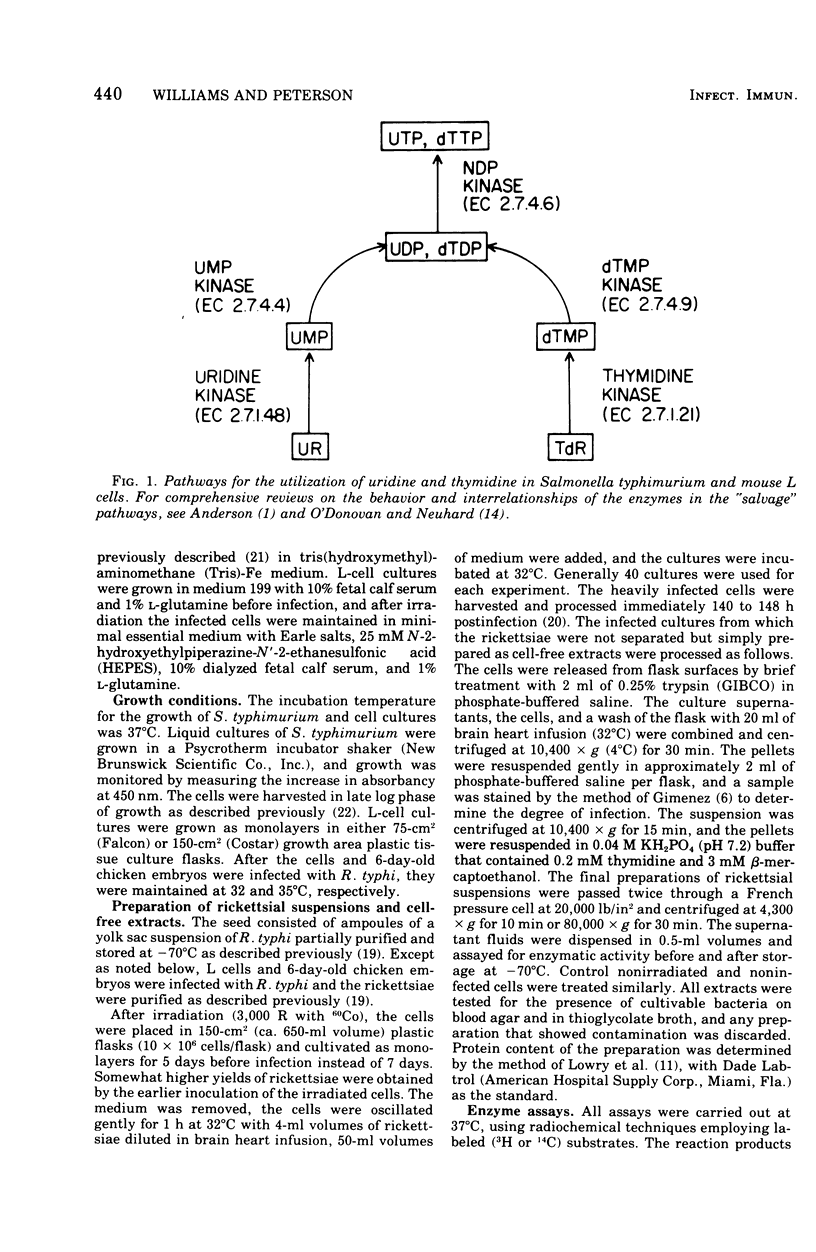
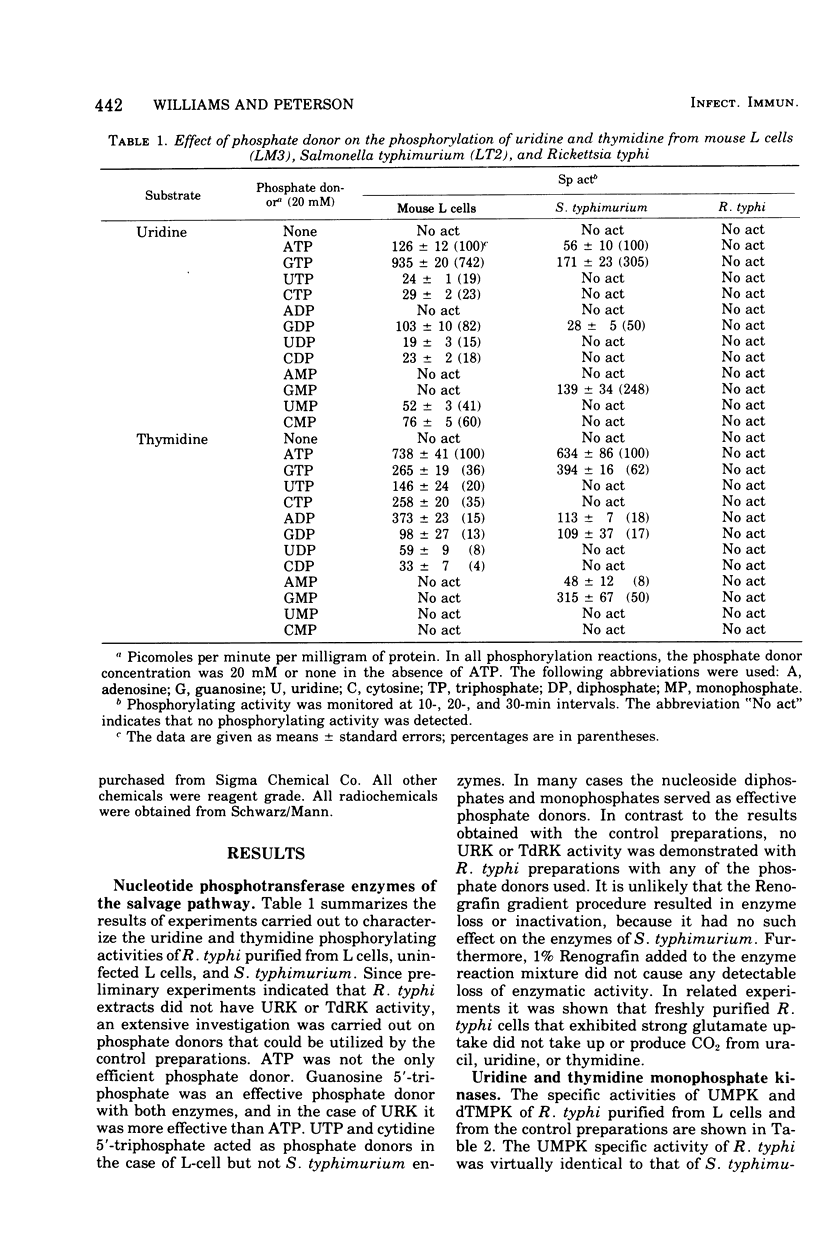
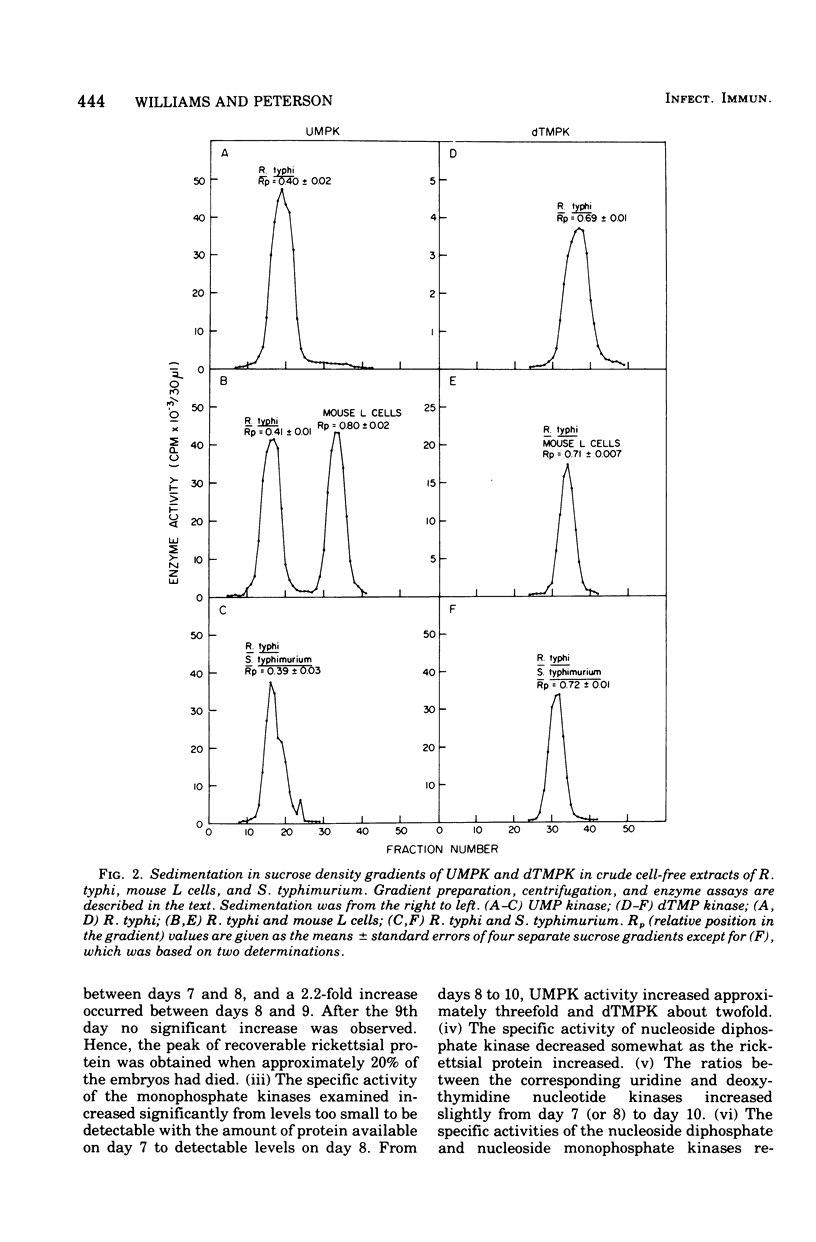
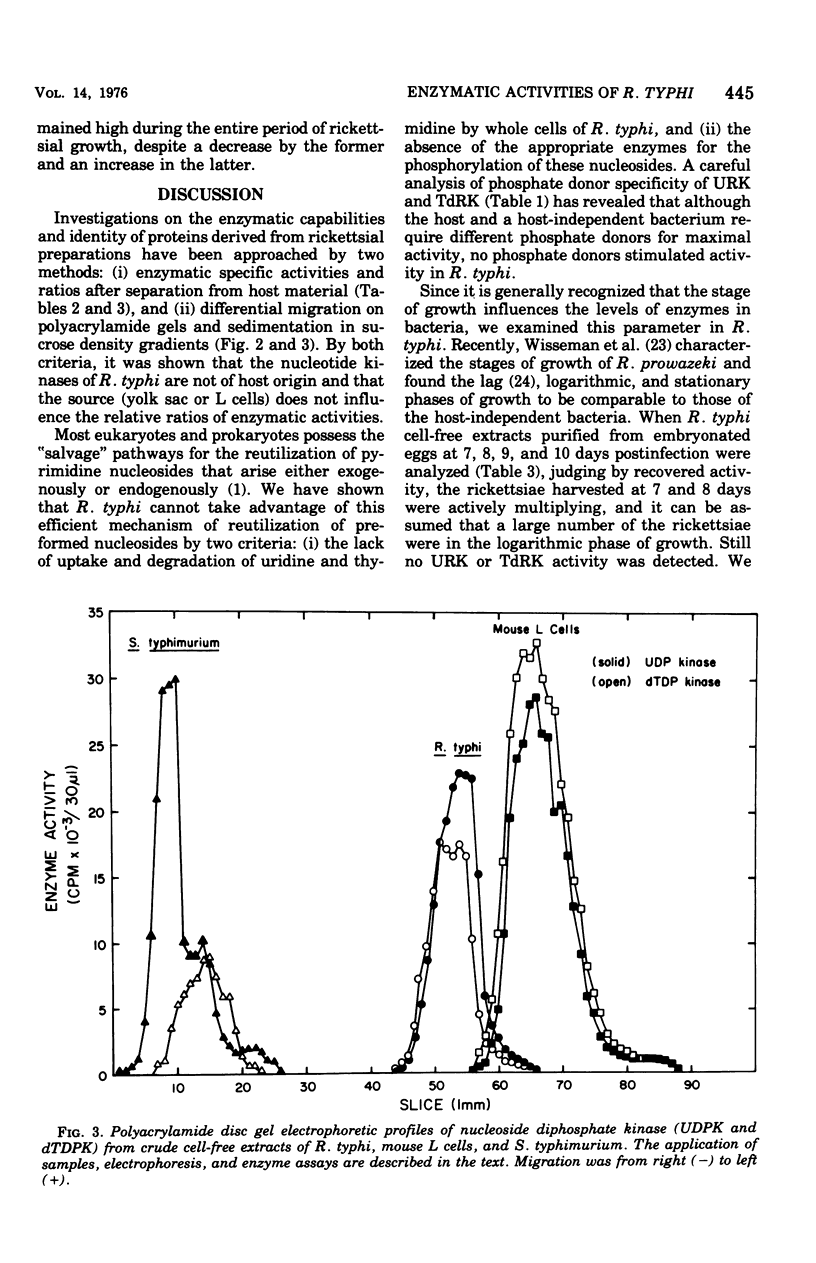
Cell-free extracts from Rickettsia typhi were examined for the presence or absence of pyrimidine phosphotransferase enzymes and compared with the enzymes of mouse L cells and Salmonella typhimurium. The organisms were grown in mouse L cells and in the yolk sacs of chicken embryos, purified by Renografin density gradient centrifugation, and ruptured in a French pressure cell. The enzymes for the reutilization of uridine and thymidine, uridine kinase (EC 2.7.1.48) and thymidine kinase (EC 2.7.1.21), were not detected in R. typhi extracts with the phosphate donors effective for control enzymes. The following enzyme activities were demonstrated in R. typhi: uridine-5'-monophosphate kinase (UMPK, EC 2.7.4.4), deoxythymidine-5'-monophosphate kinase (dTMPK, EC 2.7.4.9), and nucleosidediphosphate kinase (NDPK, EC 2.7.4.6). Physicochemical and enzymatic analyses demonstrated that the pyrimidine nucleotide kinases of R. typhi were not of host origin and that the source (yolk sac and mouse L cells) did not influence the relative enzymatic activities. The specific activities of UMPK and dTMPK were higher when the rickettsiae were harvested before embryo death, whereas NDPK levels were slightly decreased. The specific activities of UMPK, dTMPK, and NDPK were comparable to those of S. typhimurium, and consequently the rickettsiae have potential for the anabolism of monophosphates, as do the host-independent bacteria. These results suggest that R. typhi cannot utilize host uridine or thymidine pools directly but must rely on themonophosphorylated molecules of the host cell or must synthesize the monophosphates de novo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan T. S., Meuth M., Green H. Pyrimidine excretion by cultured fibroblasts: effect of mutational deficiency in pyrimidine salvage enzymes. J Cell Physiol. 1974 Apr;83(2):263–266. doi: 10.1002/jcp.1040830213. [DOI] [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Ginther C. L., Ingraham J. L. Cold-sensitive mutant of Salmonella typhimurium defective in nucleosidediphosphokinase. J Bacteriol. 1974 Jun;118(3):1020–1026. doi: 10.1128/jb.118.3.1020-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther C. L., Ingraham J. L. Nucleoside diphosphokinase of Salmonella typhimurium. J Biol Chem. 1974 Jun 10;249(11):3406–3411. [PubMed] [Google Scholar]
- Hatch T. P. Utilization of exogenous thymidine by Chlamydia psittaci growing in the thymidine kinase-containing and thymidine kinase-deficient L cells. J Bacteriol. 1976 Feb;125(2):706–712. doi: 10.1128/jb.125.2.706-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Long C., Chan T., Levytska V., Kusano T., Green H. Absence of demonstrable linkage of human genes for enzymes of the purine and pyrimidine salvage pathways in human-mouse somatic cell hybrids. Biochem Genet. 1973 Jul;9(3):283–297. doi: 10.1007/BF00485741. [DOI] [PubMed] [Google Scholar]
- MALLAVIA L., PARETSKY D. STUDIES ON THE PHYSIOLOGY OF RICKETTSIAE. V. METABOLISM OF CARBAMYL PHOSPHATE BY COXIELLA BURNETII. J Bacteriol. 1963 Aug;86:232–238. doi: 10.1128/jb.86.2.232-238.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEIS M. S., SILVERMAN M., PARETSKY D. Studies on the physiology of Rickettsiae. IV. Folic acids of Coxiella burnetii. J Bacteriol. 1963 Jan;85:37–41. doi: 10.1128/jb.85.1.37-41.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Newman L. W., Grays R., Green A. E. Metabolism of Rickettsia typhi and Rickettsia akari in irradiated L cells. Infect Immun. 1972 Jul;6(1):50–57. doi: 10.1128/iai.6.1.50-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., O'Donovan G. A. Repression of enzyme synthesis of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1973 Sep;115(3):1071–1076. doi: 10.1128/jb.115.3.1071-1076.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weber G., Morris H. P. Increased UDP kinase activity in rat and human hepatomas. Nature. 1975 Feb 13;253(5492):567–569. doi: 10.1038/253567a0. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Silverman D. J. In vitro studies on Rickettsia-host cell interactions: lag phase in intracellular growth cycle as a function of stage of growth of infecting Rickettsia prowazeki, with preliminary observations on inhibition of rickettsial uptake by host cell fragments. Infect Immun. 1976 Jun;13(6):1749–1760. doi: 10.1128/iai.13.6.1749-1760.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



