Abstract
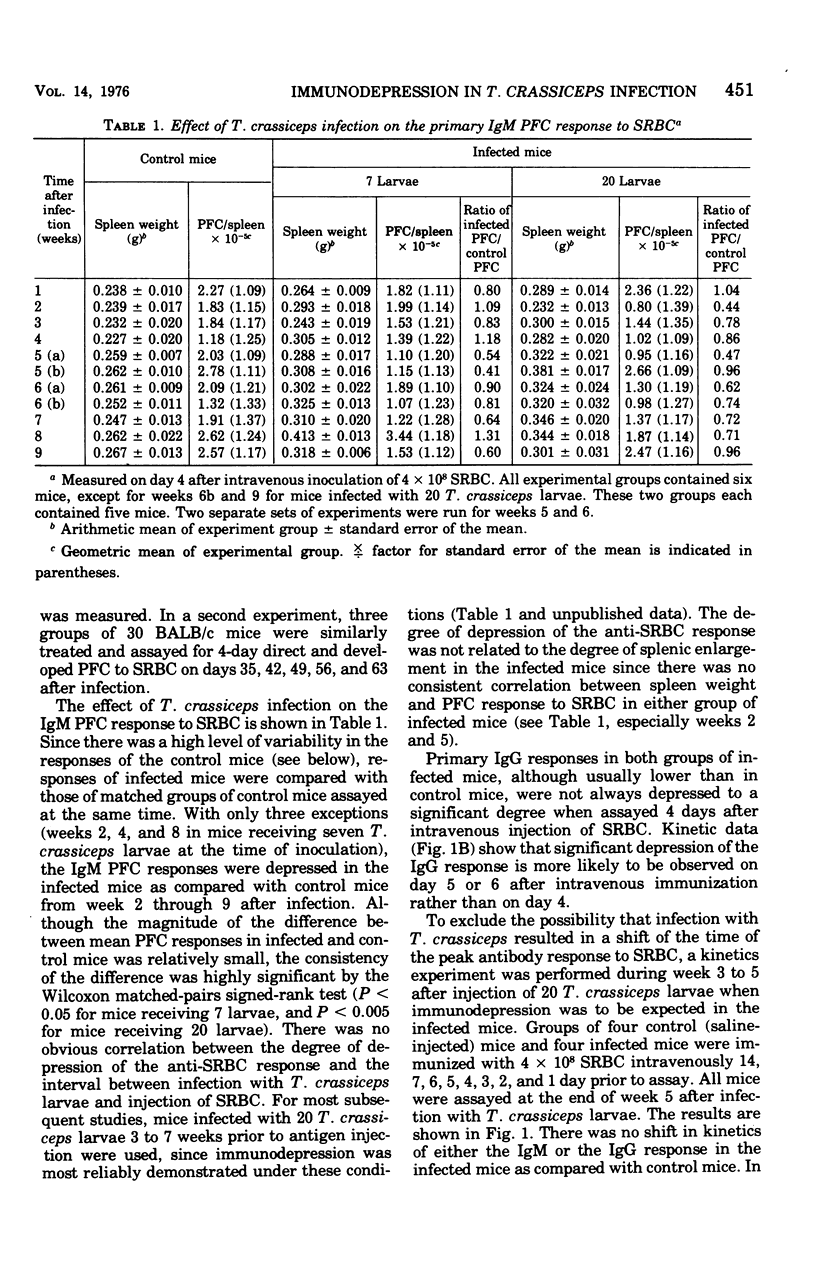
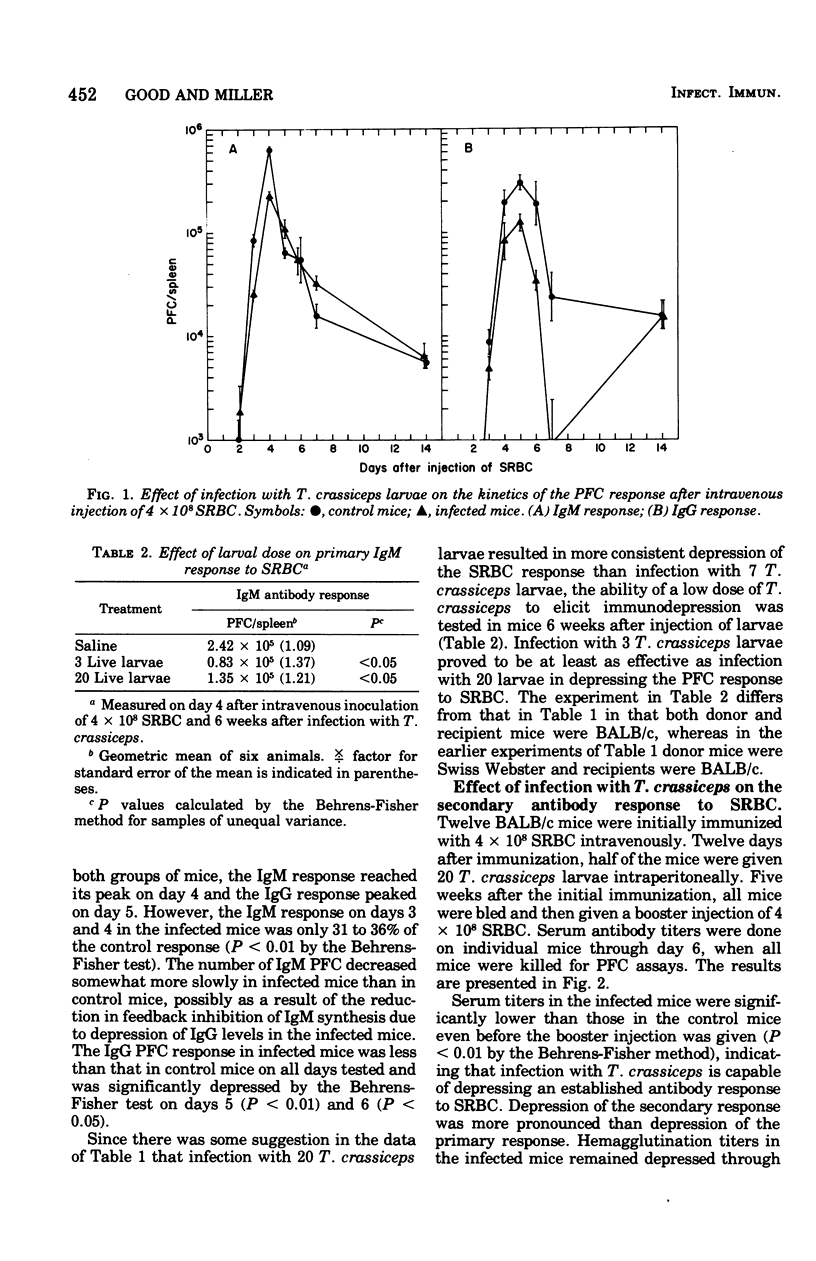
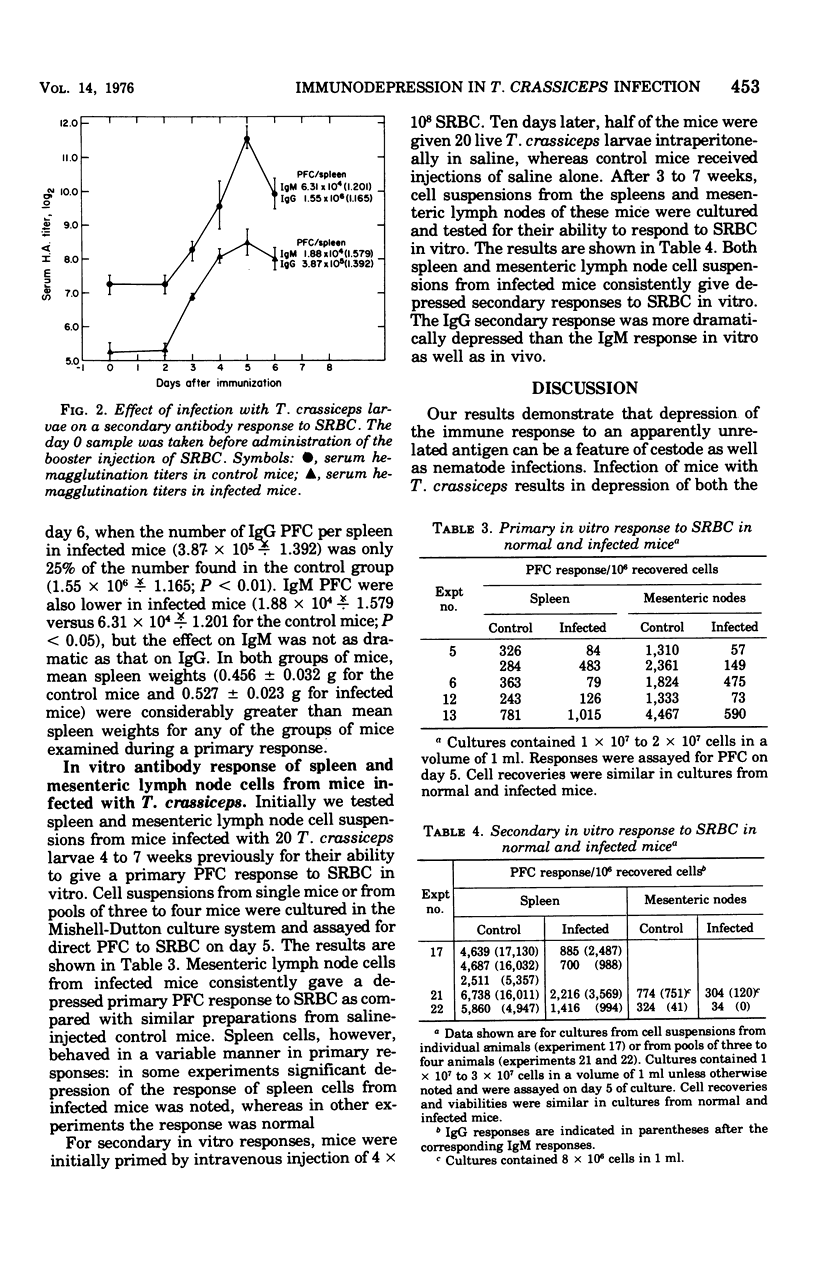
Intraperitoneal infection of mice with larvae of the cestode parasite Taenia crassiceps results in depression of both primary and secondary antibody responses to sheep erythrocytes in vivo. The depression is not associated with a shift in kinetics of the response. Both immunoglobulin M (IgM) and IgG responses are depressed, but IgG is depressed more than IgM in the secondary response. Secondary in vitro sheep erythrocyte responses are consistently depressed in both spleen and mesenteric lymph node cell preparations from infected mice, whereas primary in vitro sheep erythrocyte responses are consistently depressed in mesenteric lymph node cell preparations but not always in spleen cell preparations. These results are consistent with antigenic competition. The cell type or types involved in mediation of the immunological defect in the infected animals remain to be identified.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriga O. O. Selective immunodepression in mice by Trichinella spiralis extracts and infections. Cell Immunol. 1975 May;17(1):306–309. doi: 10.1016/s0008-8749(75)80031-1. [DOI] [PubMed] [Google Scholar]
- Cypess R. H., Lubiniecki A. S., Hammon W. M. Immunosuppression and increased susceptibility to Japaneses B encephalitis virus in Trichinella spiralis-infected mice. Proc Soc Exp Biol Med. 1973 Jun;143(2):469–473. doi: 10.3181/00379727-143-37345. [DOI] [PubMed] [Google Scholar]
- Cypess R. H., Lubiniecki A. S., Swidwa D. M. Decreased susceptibility to Listeria monocytogenes in mice after infection with Trichinella spiralis. Infect Immun. 1974 Feb;9(2):477–479. doi: 10.1128/iai.9.2.477-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess R. H., Molinari J. A., Ebersole J. L., Lubiniecki A. S. Immunological sequelae of Trichinella spiralis infection in mice. II. Potentiation of cell-mediated response to BCG after infection with Trichinella spiralis. Infect Immun. 1974 Jul;10(1):107–110. doi: 10.1128/iai.10.1.107-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert G., Tanner C. E. Trichinella spiralis: inhibition of sheep hemagglutinins in mice. Exp Parasitol. 1971 Aug;30(1):120–123. doi: 10.1016/0014-4894(71)90077-4. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D. The thymus dependency of resistance to pinworm infection in mice. J Parasitol. 1974 Dec;60(6):976–979. [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Lubiniecki A. S., Cypess R. H. Immunological sequelae of Trichinella spiralis infection in mice: effect on the antibody responses to sheep erythrocytes and Japanse B encephalitis virus. Infect Immun. 1975 Jun;11(6):1306–1311. doi: 10.1128/iai.11.6.1306-1311.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Eidinger D. Antigenic competition: a review of nonspecific antigen-induced suppression. Adv Immunol. 1974;18:133–168. doi: 10.1016/s0065-2776(08)60309-0. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Salaman M. H. Immunodepression by mammalian viruses and plasmodia. Proc R Soc Med. 1970 Jan;63(1):11–15. [PMC free article] [PubMed] [Google Scholar]
- Shimp R. G., Crandall R. B., Crandall C. A. Heligmosomoides polygyrus (=Nematospiroides dubius): suppression of antibody response to orally administered sheep erythrocytes in infected mice. Exp Parasitol. 1975 Oct;38(2):257–269. doi: 10.1016/0014-4894(75)90028-4. [DOI] [PubMed] [Google Scholar]
- Taussig M. J. Antigenic competition. Curr Top Microbiol Immunol. 1973;60:125–174. doi: 10.1007/978-3-642-65502-9_4. [DOI] [PubMed] [Google Scholar]
- Wakelin D. Genetic control of immune responses to parasites: immunity to Trichuris muris in inbred and random-bred strains of mice. Parasitology. 1975 Aug;71(1):51–60. doi: 10.1017/s0031182000053142. [DOI] [PubMed] [Google Scholar]
- Waterston R. H. Antigen competition: a paradox. Science. 1970 Dec 4;170(3962):1108–1110. doi: 10.1126/science.170.3962.1108. [DOI] [PubMed] [Google Scholar]
- Woodruff A. W. Helminths as vehicles and synergists of microbial infections. Trans R Soc Trop Med Hyg. 1968;62(3):446–452. doi: 10.1016/0035-9203(68)90097-7. [DOI] [PubMed] [Google Scholar]


