Abstract
Introduction
Bortezomib, a novel proteasome inhibitor, is approved for the treatment of relapsed multiple myeloma (MM). Efficacy and safety of bortezomib is well known; however, it was necessary to validate the data in patients with different ethnic backgrounds. The efficacy and safety of bortezomib was assessed in patients from China with relapsed/refractory MM in a real-world scenario.
Methods
This prospective, non-interventional, observational study enrolled both male and female Chinese patients, aged ≥18 years and diagnosed with relapsed or refractory MM. Administration of intravenous bortezomib at 1.3 mg/m2 was recommended twice a week for 2 weeks (days 1, 4, 8 and 11), followed by a 10-day rest period (maximum of 8 cycles) and a follow-up every 12 weeks for 3 years. Efficacy assessments included best response, objective response rate (ORR), time to response, duration of response, and overall survival. Safety was also assessed.
Results
A total of 517 patients were enrolled with a median age of 58.7 years. Patients predominantly had immunoglobulin G type (46.2%) and stage III (47.8%) myeloma. Overall, 202 (42.3%) patients had partial response as best response, ORR was 88.9% and the proportion of patients exhibiting complete response was 24.7%. The median time to response observed was 27 (21–40) days. Median time to progression was 415 days and median overall survival was 475 days. Thrombocytopenia (14.4%) was the most common adverse event.
Conclusion
Bortezomib demonstrated clinical response in majority of patients and was well tolerated in this observational study in Chinese patients with relapsed/refractory MM.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-014-0159-z) contains supplementary material, which is available to authorized users.
Keywords: Bortezomib, Chinese, Multiple myeloma, Observational study, Refractory, Relapsed, Response
Introduction
Multiple myeloma (MM) is a relatively rare hematological malignancy that affects ~1–9 in 100,000 individuals each year worldwide with a higher incidence in North America (7.1 in 100,000 per year) [1]. Although considerably prevalent in developed Western countries, prevalence statistics are substantially lower in developing countries, including Asian countries [2]. An epidemiological study in Taiwan reported an average incidence of 0.75 per 100,000 MM patients with a mortality rate of 0.59 per 100,000 deaths [3].
This incurable disease, which has a median survival of 5 years, poses the major challenge of multiple relapse [4, 5]. The standard of care for MM includes alkylating agents, anthracyclines and corticosteroids with or without hematopoietic stem cell rescue, or high-dose therapy with hematopoietic stem cell rescue [6]. Although this conventional approach offers adequate disease control, treatment benefit durability is limited and disease progression is almost inevitable. Over the last decade, the therapeutic approach for MM has evolved and the treatment paradigm has shifted to novel drugs that target different mechanistic pathways, such as immunomodulatory drugs (thalidomide and lenalidomide) and proteasome inhibitors (e.g., bortezomib) [1, 7–9]. These new agents have been extensively studied in the relapsed or refractory setting, demonstrating higher response rate (up to 50%) than the conventional therapies [10–12]. They are being used as successful salvage therapies (monotherapy or combination) in patients with relapsed MM [13, 14].
With the changing therapeutic landscape for MM, wherein efforts are tailored to formulate the best possible treatment sequence, thalidomide, lenalidomide, and bortezomib are now being introduced as an inductive treatment strategy [15]. The use of thalidomide, lenalidomide, and bortezomib in newly diagnosed MM patients has been illustrated in a few studies, suggesting that early introduction of these drugs as front-line therapy may improve the therapeutic outcomes [16, 17]. The results of the VISTA (NCT00111319) studies confirm the therapeutic advantage of bortezomib use in combination with melphalan–prednisone in patients with newly diagnosed MM who are ineligible for high-dose therapy [18, 19].
Bortezomib is the first proteasome inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of patients with newly diagnosed as well as relapsed MM [20–22]. It is also approved in Europe and several other countries (including China) for the treatment of MM [23, 24]. Bortezomib exhibits a favorable safety profile and overall response rate of up to 67%, when used in combination with dexamethasone in patients with relapsed and refractory MM [17, 25, 26]. Moreover, due to its unique mechanism of action, bortezomib is associated with low incidences of thromboembolic complications, and may provide a better safety profile than immunomodulatory agents (thalidomide and lenalidomide) [27].
Although the efficacy and safety of bortezomib is well established, validation of its benefits in patients of different ethnic backgrounds is warranted. This phase 4 observational study was designed to document the utilization, efficacy and safety of bortezomib in Chinese patients with relapse or refractory MM, with at least one prior chemotherapy regimen, in a real-world practice scenario.
Methods
Study Population
Male and female Chinese patients aged ≥18 years, diagnosed with relapsed or refractory MM and having undergone at least one prior chemotherapy regimen were enrolled. All patients participating in the study had already initiated bortezomib therapy. Patients having contraindications listed in package insert (VELCADE®, registered trademark of Millennium Pharmaceuticals, Inc., Cambridge, USA) were disqualified. Patients with severe hepatic/renal impairment and platelet count below 25,000/µL were also excluded.
Study Design and Treatment
This phase 4 study was conducted in China (43 centers) between 17 March 2006 and 31 May 2010 (NCT01675245). The study consisted of a screening phase, treatment phase, and follow-up phase (3 years from the date of bortezomib initiation). Bortezomib was administered as an intravenous bolus twice weekly for 2 weeks (days 1, 4, 8 and 11) followed by a 10-day rest period (21-day total treatment cycle). A lapse period of atleast 72 h was to be maintained between 2 doses. Bortezomib (monotherapy/combination), 1.3 mg/m2 (recommended dose) was administered for a maximum of 8 treatment cycles. The dose modification was allowed based on the treating physician’s judgment (Table 1).
Table 1.
Utilization of bortezomib (safety analysis set)
| Parameters | No. of patients |
|---|---|
| N = 515 | |
| n (%) | |
| Lines of initiating bortezomib treatment | |
| 3 | 248 (48.2) |
| 2 | 150 (29.1) |
| ≥4 | 108 (21.0) |
| Others | 5 (1.0) |
| Missing | 4 (0.8) |
| Dose of bortezomib | Total = 524a |
| Mean, mg/m2 (SD) | 1.18 (0.45) |
| Mean maximum dose, mg/m2 (SD) | 1.24 (0.46) |
| Dose distribution, n (%) | |
| 1.0 to <1.3 mg/m2 | 355 (67.7) |
| ≥1.3 mg/m2 | 88 (16.8) |
| <1.0 mg/m2 | 81 (15.5) |
| Missing | 8 |
Safety analysis set included patients who received at least 1 dose of bortezomib (with 1 or more prior treatments)
aNumber of patients who received particular dose: distribution of bortezomib (1.0 to <1.3 mg/m2 + ≥ 1.3 mg/m2 + < 1.0 mg/m2)
Prospective observational data were collected at baseline and at the end of each treatment cycle up to 8 cycles. Subsequently, the patients were followed up every 12 weeks for up to 3 years (from the date of initiation of bortezomib treatment) to collect the survival and future disease progression data. All concomitant medications, except use of bortezomib, were allowed.
Assessments
Primary Analyses
Retrospective data of prior usage of bortezomib were analyzed to determine treatment sequence (line of therapy), treatment cycles employed and average dose used (mg/m2). Efficacy assessments included: best response (complete response [CR], near CR [i.e., CR with positive immunofixation; nCR], partial response [PR], minimal response [MR], stable disease or progressive disease [PD]); objective response rate (ORR [CR + nCR + PR + MR]); time to response (date of first dose of bortezomib until the date of the first response [CR/nCR/PR/MR]); duration of response (date of first response until PD, relapse from CR [RCR], or death); time to progression (date of first dose of bortezomib until PD or RCR); and overall survival (OS [date of first dose of bortezomib until death]).
Safety assessments included: adverse events (AEs), clinical laboratory parameters, electrocardiograms, vital sign measurements, and physical examination.
Exploratory Analyses
The extent of healthcare resource utilization (emergency room visits, inpatient hospital stays [and reasons for hospitalization], and days of each hospital stay) associated with bortezomib therapy was determined.
Statistical Methods
As this was an observational study, no formal sample size calculation was performed. The data were analyzed using SAS, version 9.1.3 (Cary, NC, USA). The efficacy and safety data was summarized descriptively. Kaplan–Meier method (2-sided 95% confidence intervals [CI]) was used for time-to-event data. The product limit estimator method was used to calculate the median OS, 25th, 50th (median) and 75th percentiles of time to progression, as well as the progression rate at different time points and the median duration of response in subgroups. Cox proportional hazards model (multiple factor analysis) was used for duration of response, time to progression, and OS. A multiple regression model was performed for time to response.
Compliance with Ethics
The Independent Ethics Committee or Institutional Review Board at each study site approved the protocol. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Written informed consent was obtained from all patients for being included in the study.
Results
Patient Demographics and Baseline Characteristics
Of the 517 patients enrolled in this study, 515 received the study drug. The enrolled patients had a median age of 58.7 years (range 31.3–82.5 years) and there was a marginally higher proportion of men than women. A total of 239 (46.2%) patients had immunoglobulin G (IgG) and 247 (47.8%) patients had stage IIIa MM (as per Durie–Salmon [DS] criteria) (Table 2). A total of 231 (44.7%) patients were treated with 2 prior lines of chemotherapy and 135 (26.1%) patients received ≥3 lines of chemotherapy. Overall, 475 (32.3%) patients received a combination of vincristine, adriamycin, and dexamethasone (VAD) as prior therapy. A small minority of patients had been previously treated with bortezomib (2.5%). The greatest proportion of patients (24.4%, n = 126) obtained PR as best response to previous chemotherapy.
Table 2.
Demographic and baseline characteristics (all enrolled analysis set)
| Parameters | Bortezomib |
|---|---|
| N = 517 | |
| Male, n (%) | 300 (58.0) |
| Age, years | 488; 58.7 (31.3–82.5) |
| Weight, kg, n; mean (SD) | 512; 64.0 (10.8) |
| Myeloma type, n (%) | |
| Immunoglobulin G | 239 (46.2) |
| Immunoglobulin A | 124 (24.0) |
| Light chain | 98 (19.0) |
| Non-secretory | 20 (3.9) |
| Immunoglobulin D | 19 (3.7) |
| Immunoglobulin M | 5 (1.0) |
| Plasma cell leukemia | 4 (0.8) |
| Solitary plasmacytoma | 3 (0.6) |
| Smoldering | 1 (0.2) |
| Biclonal | 1 (0.2) |
| Missing | 3 (0.6) |
| Time from initial diagnosis to first dose, years, n; mean (SD) | 475; 2.0 (3.1) |
| Durie–Salmon staging at initial diagnosis, n (%) | |
| IIIa | 247 (47.8) |
| IIIb | 100 (19.3) |
| IIa | 76 (14.7) |
| Unclear | 50 (9.7) |
| I | 24 (4.6) |
| IIb | 18 (3.5) |
| Serum beta-2 microglobulin, mg/L | 368; 3.9 (0.9–39.9) |
| C reactive protein, mg/L | 252; 6.0 (0–189.6) |
| Hemoglobin, g/L | 500; 92.0 (11.0–175.0) |
| Platelet count, 109/L | 453; 163.0 (50.0–461.0) |
| Serum creatinine, mg/dL | 191; 1.4 (1.0–7.6) |
| WBC, 109/L | 493; 4.3 (1.2–28.4) |
Data presented as n; median (range), unless otherwise specified. All enrolled analysis set included patients who were enrolled in the study
SD standard deviation, WBC white blood cells
A total of 503 patients out of 515 discontinued during the treatment phase (including discontinuations due to physician’s decision). Treatment discontinuations were primarily due to financial reasons (14.5%, n = 73) followed by disease remission (13.7%, n = 69) and AEs (10.7%, n = 54). Discontinuations were unclear for 48 (9.5%) patients; whereas, other discontinuations were due to death (4%), loss to follow-up/non-compliance/voluntary withdrawal (4%), transplant (3.2%), no response/progression (3.2%), use of other chemotherapy (1.2%), others (1.0%) and hospital beds (0.2%). Overall, the reasons for discontinuations of 176 (35.0%) patients who maintained bortezomib after 8 cycles were unknown.
Utilization of Bortezomib
A total of 248 (48.2%) enrolled patients used bortezomib as third-line treatment (Table 2). The majority (75.6% [n = 214/283]) of patients had PD at the start of the study who received initial bortezomib treatment.
Extent of Exposure
A mean of 3.3 cycles of bortezomib treatment was administered. More than half of patients (n = 345, 67.0%) used bortezomib at a dose of 1.0 to <1.3 mg/m2; 18.1% received ≥1.3 mg/m2 dose (very few patients received 1.6 mg/m2, bi-weekly). The mean dose administered was 1.2 mg. Most patients received bortezomib as combination therapy (461/515 patients); the majority (n = 282) received bortezomib with dexamethasone. Other combination therapies administered during the study were bortezomib, adriamycin, and dexamethasone (VAD; n = 36); bortezomib, cyclophosphamide, and dexamethasone (VCD; n = 10); bortezomib, melphalan, and prednisone (VMP; n = 17); bortezomib and thalidomide (VT; n = 3); bortezomib, thalidomide, adriamycin and dexamethasone (VTAD; n = 8); bortezomib, thalidomide, and dexamethasone (VTD; n = 44); and bortezomib, thalidomide, melphalan, and prednisone (VTMP; n = 7).
Efficacy
Best Response to Bortezomib Treatment
Overall, majority of patients had PR as best response (42.3%, n = 202). About 118 (24.7%) patients demonstrated CR, 56 (11.7%) demonstrated nCR, 49 (10.3%) demonstrated MR and 35 (7.3%) had stable disease. Few patients demonstrated PD (3.8%, n = 18) (Table 3). The ORR (CR + nCR + PR + MR) in 478 evaluable patients was 88.9%.
Table 3.
Best response with bortezomib treatment in patients with relapsed/refractory multiple myeloma (efficacy analysis set)
| Overall best response, n (% of total) | CR | nCR | PR | MR | Stable disease | PD | Total |
|---|---|---|---|---|---|---|---|
| 118 (24.7) | 56 (11.7) | 202 (42.3) | 49 (10.3) | 35 (7.3) | 18 (3.8) | 478 (100) | |
| Best response in different subgroups, n (% of total) | |||||||
| Lines of treatment | |||||||
| 2 | 35 (25.9) | 16 (11.9) | 50 (37.0) | 18 (13.3) | 9 (6.7) | 7 (5.2) | 135 (100) |
| 3 | 54 (23.0) | 38 (16.2) | 97 (41.3) | 23 (9.8) | 17 (7.2) | 6 (2.6) | 235 (100) |
| ≥4 | 28 (28.0) | 2 (2.0) | 53 (53.0) | 8 (8.0) | 6 (6.0) | 3 (3.0) | 100 (100) |
| Othersa | 1 (25.0) | 0 | 1 (25.0) | 0 | 2 (50.0) | 0 | 4 (100) |
| Durie–Salmon staging | |||||||
| I | 7 (29.2) | 2 (8.3) | 9 (37.5) | 2 (8.3) | 2 (8.3) | 2 (8.3) | 24 (100) |
| IIA | 26 (36.1) | 8 (11.1) | 22 (30.6) | 11 (15.3) | 5 (6.9) | 0 (0.0) | 72 (100) |
| IIB | 3 (17.6) | 2 (11.8) | 10 (58.8) | 0 (0.0) | 1 (5.9) | 1 (5.9) | 17 (100) |
| IIIA | 56 (24.9) | 26 (11.6) | 103 (45.8) | 21 (9.3) | 10 (4.4) | 9 (4.0) | 225 (100) |
| IIIB | 18 (18.9) | 14 (14.7) | 38 (40.0) | 12 (12.6) | 9 (9.5) | 4 (4.2) | 95 (100) |
| Average dose of bortezomib | |||||||
| <1.0 mg/m2 | 10 (16.4) | 7 (11.5) | 26 (42.6) | 9 (14.8) | 5 (8.2) | 4 (6.6) | 61 (100) |
| 1.0 to <1.3 mg/m2 | 72 (24.7) | 36 (12.4) | 127 (43.6) | 27 (9.3) | 19 (6.5) | 10 (3.4) | 291 (100) |
| ≥1.3 mg/m2 | 35 (29.2) | 12 (10.0) | 47 (39.2) | 12 (10.0) | 10 (8.3) | 4 (3.3) | 120 (100) |
| Best response by cycle | |||||||
| 1 | 43 (9.9) | 31 (7.1) | 217 (50.0) | 66 (15.2) | 53 (12.2) | 24 (5.5) | 434 (100) |
| 2 | 56 (16.2) | 43 (12.5) | 155 (44.9) | 33 (9.6) | 27 (7.8) | 31 (9.0) | 345 (100) |
| 3 | 47 (21.3) | 30 (13.6) | 92 (41.6) | 22 (10.0) | 18 (8.1) | 12 (5.4) | 221 (100) |
| 4 | 54 (31.4) | 18 (10.5) | 71 (41.3) | 12 (7.0) | 8 (4.7) | 9 (5.2) | 172 (100) |
| 5 | 32 (30.2) | 11 (10.4) | 43 (40.6) | 5 (4.7) | 7 (6.6) | 8 (7.6) | 106 (100) |
| 6 | 24 (35.3) | 2 (2.9) | 30 (44.1) | 3 (4.4) | 4 (5.9) | 5 (7.4) | 68 (100) |
| 7 | 14 (33.3) | 3 (7.1) | 15 (35.7) | 3 (7.1) | 3 (7.1) | 4 (9.5) | 42 (100) |
| 8 | 14 (45.2) | 0 (0) | 12 (38.7) | 1 (3.2) | 2 (6.5) | 2 (6.5) | 31 (100) |
Efficacy analysis set: included patients who received at least 1 dose of bortezomib (with 1 or more prior treatments)
CR complete response, MR minimal response, nCR near complete response (complete response with positive immunofixation), PD progressive disease, PR partial response
aConsolidation therapy (treatment after autologous stem cell transplant) or missing
The subgroup analysis of the response data by lines of treatment, disease stage (as per DS staging), average dose of bortezomib, and treatment cycle showed similar trend of best response across subgroups (Table 3); the highest number of patients had PR, with few demonstrating stable disease or PD. A higher proportion of patients receiving bortezomib at dose ≥1.3 mg/m2 achieved CR (29.2%), compared to those receiving 1.0 to <1.3 mg/m2 and <1.0 mg/m2 doses (24.7% and 16.4%, respectively).
Time to Response, Duration of Response, Time to Progression and Overall Survival
The mean (standard deviation [SD]) time to response for bortezomib treatment was 36.1 (34.8) days (median 27 days; range 21–40 days). The majority of patients maintained best response to bortezomib treatment up to day 30 (88.3%); however, as time progressed there was gradual decrease in the percentage of patients maintaining response with only 7.2% demonstrating response by 480 days. The median time to progression was 415 days. The disease progression rate was minimal by day 30 (2.1%), and increased gradually to 100% by day 720. Median overall survival time was 475 days. These results corroborated with the OS rate that improved gradually from 97.2% (by day 30) to 25.2% (by day 720) (Table 4). Based on subgroup analysis, patients receiving an average dosage ≥1.3 mg/m2 tended to have longer duration of response and higher OS rate while those receiving average dosage <1.0 mg/m2 had shorter duration of response and low OS rate, compared with the other 2 subgroups (Fig. 1).
Table 4.
Overall duration of response, time to progression and overall survival with bortezomib treatment in patients with relapsed/refractory multiple myeloma (efficacy analysis set)
| Time (days) | Patients maintaining response, % (SE) | 95% CI |
|---|---|---|
| Duration of response | ||
| 30 | 88.3 (1.7) | 84.5, 91.2 |
| 60 | 83.4 (2.2) | 78.6, 87.3 |
| 120 | 81.6 (2.4) | 76.3, 85.8 |
| 240 | 72.5 (3.6) | 64.8, 78.8 |
| 360 | 59.9 (6.1) | 46.8, 70.7 |
| 480 | 55.3 (7.2) | 40.2, 68.0 |
| 600 | 55.3 (7.2) | 40.2, 68.0 |
| 720 | 0.0 (0.0) | – |
| Time to progression | ||
| 30 | 2.1 (0.7) | 1.1, 4.1 |
| 60 | 6.9 (1.3) | 4.7, 10.1 |
| 120 | 16.1 (2.3) | 12.2, 21.2 |
| 240 | 24.1 (3.2) | 18.5, 31.1 |
| 360 | 35.9 (5.4) | 26.4, 47.4 |
| 480 | 59.3 (9.6) | 41.6, 77.7 |
| 600 | 59.3 (9.6) | 41.6, 77.7 |
| 720 | 100.0 (0.0) | – |
| Overall survival | ||
| 30 | 97.2 (0.8) | 95.1, 98.3 |
| 60 | 94.1 (1.2) | 91.3, 96.0 |
| 120 | 89.1 (1.8) | 85.0, 92.1 |
| 240 | 79.0 (2.9) | 72.5, 84.1 |
| 360 | 66.6 (4.5) | 57.1, 74.5 |
| 480 | 49.9 (6.6) | 36.5, 62.0 |
| 600 | 35.3 (7.7) | 20.7, 50.2 |
| 720 | 25.2 (8.2) | 11.2, 42.0 |
| Quantile and 95% CI of time to progression | |
|---|---|
| Quantile (%) | 95% CI |
| 75 | 705.0 (429.00, 705.00) |
| 50 | 415.0 (382.00, 705.00) |
| 25 | 268.0 (186.00, 354.00) |
Efficacy analysis set: included patients who received at least 1 dose of bortezomib (with 1 or more prior treatments)
CI confidence interval, SE standard error
Fig. 1.
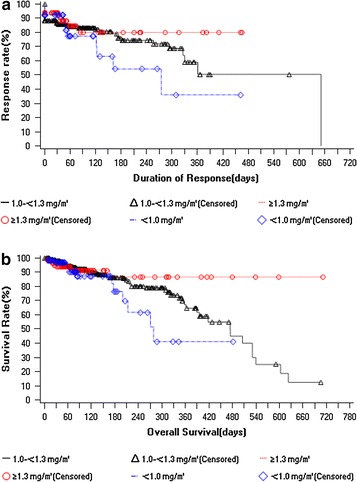
Kaplan–Meier curve of a duration of response and b overall survival in different subgroups of bortezomib dosage in patients with relapsed/refractory multiple myeloma (efficacy analysis set). Efficacy analysis set: included patients who received at least 1 dose of bortezomib (with 1 or more prior treatments)
The duration of response in patients receiving dosage ≥1.0 and <1.0 mg/m2 is as follows (median [95% CI]) ≥1.0 mg/m2 (651 days [361, 651]); <1.0 mg/m2 (275 [123, –]); p = 0.23. The time to progression was shorter in patients who received <1.0 mg/m2 dose compared to patients who received ≥1.0 mg/m2 dose (median [95% CI]) ≥1.0 mg/m2 (415 [386, 705]); <1.0 mg/m2 (329 [186, –]); p = 0.22). The OS (median [95% CI]) ≥1.0 mg/m2 (531 [418, 623]); <1.0 mg/m2 (279 [200, –]); p = 0.059) was shorter for patients who received <1.0 mg/m2 dose compared with patients who received ≥1.0 mg/m2 dose.
Safety
Overall, 63.9% patients experienced at least 1 AE. The most frequently reported AE was thrombocytopenia (14.4%, n = 74) (Table 5). Most of the AEs (~35%) were grade 1 or 2 in severity, with few grade 3 (16.7%) or grade 4 (6.4%). Drug-related AEs occurred in 53.8% of patients. The incidence of serious adverse events (SAE) was low (6.4%); deaths and lung infections were reported in nine patients each. Thrombocytopenia as a SAE was reported by two patients.
Table 5.
Adverse events in ≥5% of bortezomib-treated patients with relapsed/refractory multiple myeloma (safety analysis set)
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total patients with AEs | 200 (38.8) | 171 (33.2) | 86 (16.7) | 33 (6.4) | 329 (63.9) |
| Thrombocytopeniaa | 18 (3.5) | 31 (6.0) | 31 (6.0) | 6 (1.2) | 74 (14.4) |
| Diarrhea | 37 (7.2) | 27 (5.2) | 7 (1.4) | 0 | 71 (13.8) |
| Peripheral neuropathy | 34 (6.6) | 20 (3.9) | 3 (0.6) | 0 | 55 (10.7) |
| Hypoesthesia | 33 (6.4) | 18 (3.5) | 5 (1.0) | 0 | 52 (10.1) |
| Asthenia | 41 (8.0) | 13 (2.5) | 2 (0.4) | 0 | 51 (9.9) |
| Lung infection | 7 (1.4) | 22 (4.3) | 10 (1.9) | 8 (1.6) | 40 (7.8) |
| Herpes zoster | 14 (2.7) | 12 (2.3) | 3 (0.6) | 0 | 30 (5.8) |
Safety analysis set: included patients who received at least 1 dose of bortezomib (with 1 or more prior treatments)
AE adverse event
aPlatelet count <50 × 109/L
The most commonly (≥10% of patients) reported AEs of special interest (assessed as per WHO Common Toxicity Criteria) were infection (16.3%, n = 84), thrombocytopenia (14.8%, n = 76), diarrhea (14.0%, n = 72), peripheral sensory neuropathy (10.7%, n = 55), weakness (11.8%, n = 61), and paresthesia (10.7%, n = 55). The majority (55.2%, n = 284) of these AEs were ≤grade 2 in severity, with few grade 3 (15.0%, n = 77) or 4 (4.1%, n = 21) AEs.
Healthcare Utilization
In total, 148 (28.0%) of the enrolled patients did not require any hospital stay. Of the patients who were hospitalized, most (22.8%, n = 118) required only one stay (mean days [SD], 31.9 [55.6]). Of the 1,039 types of hospitalization, the most frequent was voluntary hospitalization (92.8%, n = 964), followed by emergency (6.1%, n = 63), acute (0.7%, n = 7), and unknown (including case report form unfilled) (0.5%, n = 5).
Discussion
The therapeutic paradigm for MM has now shifted in light of the demonstrated therapeutic advantages of proteasome inhibitors and immunomodulators over conventional strategies [28, 29]. As a result, these drugs have emerged as a more feasible treatment option for patients with relapsed/refractory MM, particularly those ineligible for high-dose chemotherapy. Bortezomib is the first proteasome inhibitor approved for treatment of patients with relapsed as well as newly diagnosed MM [30]. The pivotal studies conducted so far establish the efficacy and safety of bortezomib in the Caucasian population [10, 11]. This observational study simulating the real-world practice scenario provides insight into the therapeutic feasibility of bortezomib in Chinese patients with relapsed or refractory MM following at least one prior chemotherapy regimen.
Notably in this study, bortezomib treatment resulted in ORR of 88.9%, a rate comparatively superior to the ORR achieved in the Global studies assessing the therapeutic benefits of bortezomib plus dexamethasone in a predominantly Caucasian population. (CREST, 62%, SUMMIT, 35% and APEX, 43% [NCT00048230]) [10–12]. The population enrolled in these global studies had baseline characteristics similar to the population of this study (age of ~60 years and majority had Durie–Salmon staging of stage IIIa). When compared with the VOBS trial conducted in the Chinese population, the ORR (>70%) was similar to that noted in this study. Taken together, these studies highlight the difference in treatment sensitivity within populations belonging to diverse ethnic backgrounds (Caucasian and Asian) [31]. Of note, the treatment strategies employed for these two populations were not similar, which might have also contributed to the higher ORR observed in Chinese patients (VOBS trial) compared with Caucasian patients (Global trials). In the Caucasian population, bortezomib was initiated as monotherapy, and dexamethasone was introduced during the course of treatment only if required; while in the Chinese population, bortezomib was initiated as combination therapy in most patients. Of the evaluable patients in this study, the majority demonstrated PR. Although stable disease status was not achieved in most of the patients, those demonstrating disease progression were notably few. These findings were consistent with the APEX study [12] which supports the therapeutic advantage of bortezomib when introduced early as salvage treatment in the course of disease.
The median time to first response was notably shorter in this study (27 days) compared with results from studies in Caucasians (1.3–1.5 months) [10, 11], but was consistent with an earlier study in Chinese population reporting median time to response of 33–38 days [31]. Further, the duration of response was longer in this population (~20 months) compared with Caucasians (12.7 months). A longer duration of response generally translates into improved treatment outcomes [12]. The disease progression rate in this study was minimal by day 30 and increased gradually as time progressed. Overall, this observational study in real-world setting demonstrates the utilization and feasibility of bortezomib, confirming its use in Chinese patients with relapse or refractory MM.
Further, no unexpected safety findings were observed in this Chinese population. The most common AEs (≥10%) were decrease in platelet count, diarrhea, peripheral neuropathy, and hypoesthesia. Overall, bortezomib treatment was associated with manageable AEs and did not limit the continuity of therapy. The incidence of deaths, SAEs and AEs leading to discontinuation were overall low. The safety outcomes indicated that treatment with bortezomib produces a manageable toxicity profile in the Chinese population.
Limitations
One limitation of this study was the short follow-up period. A longer than 3-year follow-up period would help to better interpret the survival data. This corroborated with the VISTA study wherein the follow-up period was similar to our study, and no conclusive results were obtained with respect to OS. This study lacked any novel findings or any additional treatment benefits related to bortezomib. The treatment effects noted in the Chinese population were similar to the known therapeutic outcomes of bortezomib.
Conclusion
This study demonstrates that a bortezomib-based regimen was feasible in Chinese patients with relapse or refractory MM. Bortezomib was associated with good response rates, and a manageable safety profile consistent with previous studies and clinical experience. Notably, patients receiving the standard dosage (1.3 mg/m2) and longer treatment duration demonstrated better survival benefits with bortezomib therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Xian-Janssen Pharmaceuticals, Beijing, China. The sponsor also funded the manuscript development. The authors would like to thank Dr. Ananya Chikramane (SIRO Clinpharm Pvt. Ltd.) for writing assistance, and Dr. Namit Ghildyal (Janssen Research and Development, LLC) for additional editorial assistance. All authors meet the ICMJE criteria for authorship for this manuscript, had full access to all of the data in this study and take complete responsibility for the integrity of the data, of the work as a whole and of the accuracy of the data analysis. All authors have given final approval for the version to be published. The authors thank the study participants, without whom this study would not have been accomplished, as well as the following investigators for their participation in this study: Liu Kaiyan, Yu Li, Qiu Lugui, Shao Zonghong, Wang Chun, Li Juan, Du Xin, Hou Ming, Zhao Hongguo, Ouyang Jian, Wu Peide, Yu Kang, Liu Ting, Zou Ping, Chen Xiequn, Ma Jun, Hu Jianda, Zhou Jianfeng, Zhang Quanying, Chen Baoan, Shao Zonghong, Li Wei, Lu Zhenxia, Liu Nan, Yang Linhua, Ke Xiaoyan, Li Lanping, Ge Linfu, He Xupeng, Li Xiaolin, Ma Jianxia, Ouyang Mufang, Zhang Quanying, Li Xiao, Wang Zhao, Ma Yigai.
Compliance with ethics guidelines
The Independent Ethics Committee or Institutional Review Board at each study site approved the protocol. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Written informed consent was obtained from all patients for being included in the study.
Conflict of interest
Z. Shen, Y. Li, Z. Liu, M. Lin and W. Chen have no conflict of interest. X. Huang received funding from Clinical Subjects Key Project of the Ministry of Health, National Natural Science Foundation of China, and The Key Program of National Natural Science Foundation of China. Y. Zhou received research fund from State Administration of Traditional Chinese Medicine, and Molecular Biology Application Research Funds of Chinese Medicine Association. J. Hou received consultancy from Novartis, received honoraria from Novartis and Xian-Janssen Pharmaceutical, and received research funding from Xian-Janssen Pharmaceutical. T Zhao is an employee of Xian-Janssen Pharmaceutical. L. Wang is an employee of Xian-Janssen Pharmaceutical. K. Wu is an employee of Xian-Janssen Pharmaceutical.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Trial registration: Clinicaltrials.gov #NCT01675245.
References
- 1.Gupta M, Pal R, Tikoo D. Multiple myeloma: the disease and its treatment. Int J Basic Clin Pharmacol. 2013;2(2):103–121. doi: 10.5455/2319-2003.ijbcp20130302. [DOI] [Google Scholar]
- 2.Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25–35. doi: 10.1007/978-3-540-85772-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Huang SY, Yao M, Tang JL, Lee WC, Tsay W, Cheng AL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. 2007;110(4):896–905. doi: 10.1002/cncr.22850. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88(3):226–235. doi: 10.1002/ajh.23390. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Z, Lin J, Chen J. Bortezomib for patients with previously untreated multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Ann Hematol. 2013;92(7):935–943. doi: 10.1007/s00277-013-1711-7. [DOI] [PubMed] [Google Scholar]
- 6.Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol. 2012;49(Suppl 1):S16–S32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca R, Stewart AK. Targeted therapeutics for multiple myeloma: the arrival of a risk-stratified approach. Mol Cancer Ther. 2007;6(3):802–810. doi: 10.1158/1535-7163.MCT-06-0620. [DOI] [PubMed] [Google Scholar]
- 8.Blade J, Rosinol L. Changing paradigms in the treatment of multiple myeloma. Haematologica. 2009;94(2):163–166. doi: 10.3324/haematol.2008.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larocca A, Palumbo A. Evolving paradigms in the treatment of newly diagnosed multiple myeloma. J Natl Compr Canc Netw. 2011;9(10):1186–1196. doi: 10.6004/jnccn.2011.0096. [DOI] [PubMed] [Google Scholar]
- 10.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 13.van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37(4):266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Richardson P, Mitsiades C, Schlossman R, Ghobrial I, Hideshima T, Chauhan D, et al. The treatment of relapsed and refractory multiple myeloma. Hematology Am Soc Hematol Educ Program. 2007;2007(1):317–23. [DOI] [PubMed]
- 15.Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26(1):73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 16.Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–1505. [PubMed] [Google Scholar]
- 17.Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129(6):776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 18.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 19.Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 20.Velcade®, (bortezomib), Prescribing Information. 2003. www.accessdata.fda.gov/drugsatfda_docs/label/…/021602s015lbl.pdf. Accessed July 14, 2014.
- 21.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: US FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8(6):508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 22.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18):5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 23.www.myeloma-euronet.org. Myeloma Euronet: Treatment with Bortezomib. 2013. http://www.myeloma-euronet.org/en/multiple-myeloma/treatment-with-bortezomibphp. Accessed July 14, 2014.
- 24.LoRusso PM, Venkatakrishnan K, Ramanathan RK, Sarantopoulos J, Mulkerin D, Shibata SI, et al. Pharmacokinetics and safety of bortezomib in patients with advanced malignancies and varying degrees of liver dysfunction: phase I NCI Organ Dysfunction Working Group Study NCI-6432. Clin Cancer Res. 2012;18(10):2954–2963. doi: 10.1158/1078-0432.CCR-11-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol. 2009;144(2):169–175. doi: 10.1111/j.1365-2141.2008.07409.x. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin DH, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: final time-to-event results from the SUMMIT trial. Cancer. 2006;106(6):1316–1319. doi: 10.1002/cncr.21740. [DOI] [PubMed] [Google Scholar]
- 27.Lonial S, Richardson PG, San Miguel J, Sonneveld P, Schuster MW, Blade J, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol. 2008;143(2):222–229. doi: 10.1111/j.1365-2141.2008.07321.x. [DOI] [PubMed] [Google Scholar]
- 28.Katzel JA, Hari P, Vesole DH. Multiple myeloma: charging toward a bright future. CA Cancer J Clin. 2007;57:301–318. doi: 10.3322/CA.57.5.301. [DOI] [PubMed] [Google Scholar]
- 29.Bae J, Munshi NC, Anderson KC. Immunotherapy strategies in multiple myeloma. Hematol Oncol Clin North Am. 2014;28:927–943. doi: 10.1016/j.hoc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med. 2011;12:471–480. [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan ZG, Jin J, Huang XJ, Li Y, Chen WM, Liu ZG, et al. Different dose combinations of bortezomib and dexamethasone in the treatment of relapsed or refractory myeloma: an open-label, observational, multi-center study in China. Chin Med J (Engl). 2011;124(19):2969–2974. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


