Abstract
Purpose
Hospitalizations among patients with cancer are common and costly and, if unplanned, may interrupt oncologic treatment. The rate of unplanned hospitalizations in the population of elderly patients with cancer is unknown. We sought to describe and quantify patterns and risk factors for early unplanned hospitalization among elderly patients with GI cancer.
Patients and Methods
We conducted a retrospective cohort study using linked Texas Cancer Registry and Medicare claims data from 2001 to 2009. Texas residents age 66 years or older initially diagnosed with GI cancer between 2001 and 2007 were included in the study. The unplanned hospitalization rate was estimated, and reasons for unplanned hospitalization were evaluated. Risk factors were identified using adjusted Cox proportional hazards modeling.
Results
Thirty thousand one hundred ninety-nine patients were included in our study, 59% of whom had one or more unplanned hospitalizations. Of 60,837 inpatient claims, 58% were unplanned. The rate of unplanned hospitalization was 93 events per 100 person-years. The most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia. Multivariable analysis showed that black race; residing in census tracts with poverty levels greater than 13.3%; esophageal, gastric, and pancreatic cancer; advanced disease stage; high Charlson comorbidity index score; and dual eligibility for Medicare and Medicaid increased the risk for unplanned hospitalization (all P values < .05).
Conclusion
Unplanned hospitalizations among elderly patients with GI cancer are common. Some of the top reasons for unplanned hospitalization are potentially preventable, suggesting that comorbidity management and close coordination among involved health care providers should be promoted.
INTRODUCTION
National expenditures for cancer care amounted to $124 billion in 2010 and are projected to increase to $173 billion in 2020.1 Concern that increasing health care expenditures are unsustainable has resulted in a push to understand the drivers of cancer care costs. The high cost of cancer therapeutic agents and the intensity of care have been identified as reasons for these rising costs.2 Changes in oncologic practice to address these issues have been proposed and have been incorporated in certain quality initiatives.2,3
Hospitalizations have also been identified as a substantial cost in cancer care. Using claims data from almost 720,000 patients with cancer, Yabroff et al4 found that hospitalizations were responsible for more than 50% of net costs during the initial 12 months after diagnosis for most cancers they studied as well as for more than 60% of costs during the final year of life. In 2009, the Agency for Healthcare Research and Quality (AHRQ) estimated that 4.7 million hospitalizations among adults were related to cancer.5 In addition, hospitalizations primarily related to cancer lasted longer than hospitalizations for other conditions and cost $5,700 more per stay. Medicare was the identified payer for almost 50% of these hospitalizations.
It is unclear whether these reported cancer-related hospitalizations are planned; unplanned hospitalizations are not considered to be part of the patient's program of care. We believe that this distinction is important, because unlike planned hospitalizations, unplanned hospitalizations are potentially preventable. In fact, unplanned hospitalizations have been proposed as a quality metric in other countries, with the assumption that they are adverse outcomes of care, especially in ambulatory-sensitive conditions.6 In addition, unplanned hospitalizations may delay scheduled treatment and adversely impact disease outcomes and quality of life.
A better understanding of unplanned hospitalizations in the cancer population, particularly among those older than age 65 years who likely have other comorbid illnesses, is needed.7 Comorbidities may play a role in the use of health resources in this population and may help identify areas of focus for improvement or intervention.
In this study, we sought to describe and quantify patterns of early unplanned hospitalization among elderly Medicare recipients with GI cancer in the state of Texas as well as identify risk factors that contribute to unplanned hospitalization in this population. We focused on GI cancer, because these cancers have some of the highest proportions of cancer care cost attributable to hospitalizations, which suggests that unplanned hospitalizations may be common in this population.4
PATIENTS AND METHODS
The institutional review boards at The University of Texas Medical Branch at Galveston, The University of Texas MD Anderson Cancer Center, and the Texas Department of State Health Services approved this study, as did the Privacy Review Board of the Centers for Medicare and Medicaid Services.
Data Sources
We conducted a retrospective cohort study using linked data from the Texas Cancer Registry (TCR) and the Medicare claims database. The TCR is a population-based registry that collects, maintains, and disseminates cancer data within the state of Texas. It is the fourth largest cancer registry in the United States, containing complete statewide data for cancers diagnosed between 1995 and 2008.8 The Medicare claims database contains all claims filed by qualifying individuals age 65 years or older who have enrolled in the Medicare health insurance plan.9 Medicare data linked with the TCR contain information on health care paid for by the Medicare program for elderly patients with cancer, including hospital stays, physician services, and hospital outpatient visits. The TCR-Medicare linkage is performed under the guidance of the National Cancer Institute, the TCR, and the Centers for Medicare and Medicaid Services, which collect the Medicare claims data. Medicare enrollment and claims files are available for approximately 98% of all patients age 65 years or older whose records are entered into TCR.
Study Population
We identified Texas residents age 66 years or older who were diagnosed with a GI malignancy (esophageal, gastric, liver or intrahepatic ductal, pancreatic, colon, or anorectal cancer) between 2001 and 2007 from the Medicare-linked TCR data. Because the database contained Medicare claims data through 2009, we excluded patients who were diagnosed after 2007, thus giving each patient a 2-year observation period for claims analysis. Figure 1 illustrates the algorithm for cohort selection.
Fig 1.
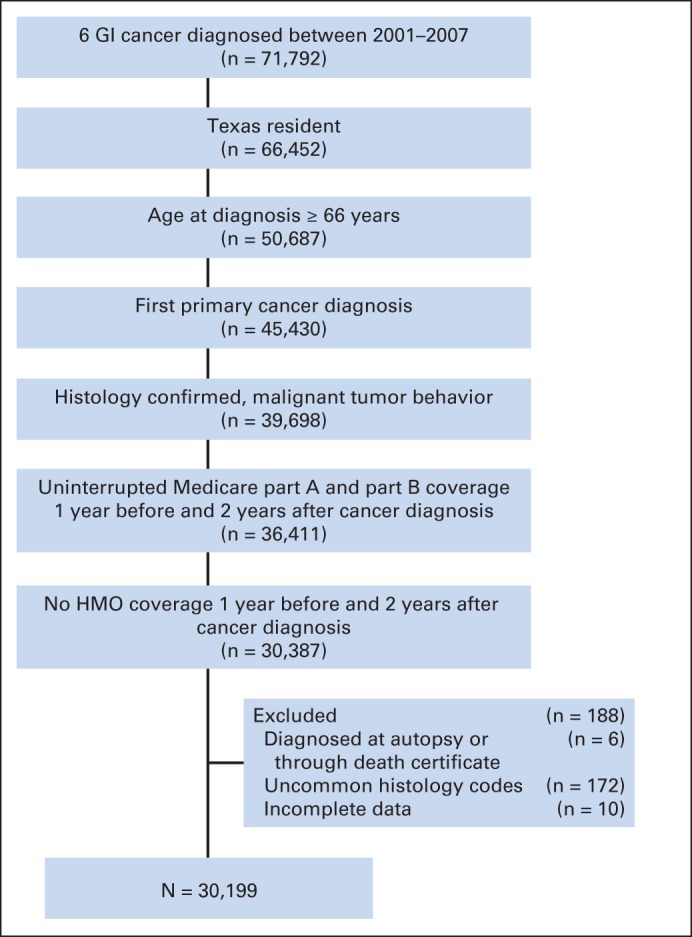
Algorithm for selection of the study cohort. HMO, health maintenance organization.
Study Variables
We reviewed Medicare claims data within the Medicare Provider Analysis and Review hospital stay file for each patient to determine whether the patient had an early unplanned hospitalization within the first 2 years after cancer diagnosis. Skilled nursing facility claim records, which are also located in the Medicare Provider Analysis and Review file, were excluded from the analysis. We defined “unplanned hospitalization” as a hospitalization with an admission type of urgent or emergent, excluding those that were primarily for chemotherapy or radiotherapy (International Classification of Disease, 9th revision [ICD-9] codes V58.0, V58.1, V58.11, and V58.12) or for rehabilitation services (ICD-9 code V57.xx).6,10–12 If the admission type for a hospitalization was unknown, we used an emergency room charge greater than $0 to identify patients who came in through the emergency room.13 Hospitalizations that did not meet our criteria for unplanned hospitalization were classified as “other” hospitalizations.
Information on length of stay and timing of claim (≤ 1 year after diagnosis or > 1 year after diagnosis) were also collected. We considered the first noncancer diagnosis in the claims data to be the reason for admission, recognizing that a cancer diagnosis may be coded before other medical issues, especially if the other medical issues are related to the cancer. We then used the AHRQ Clinical Classifications Software to assign ICD-9 diagnoses into more meaningful clinical categories.
We also collected demographic and clinical information about each patient at the time of diagnosis. Study variables were selected on the basis of risk factors for unplanned admissions in the general population, as identified from related literature.12,14,15 The Charlson comorbidity index (CCI) was calculated for each patient by searching all Medicare claims files for ICD-9 codes pertaining to Charlson comorbidities in the 12 months before cancer diagnosis.16–19 Patients without inpatient or outpatient claims during this 12-month period were initially assigned a separate Charlson category but were later combined with those who had a CCI score of 0 (ie, no comorbidities) after statistical analyses showed no significant difference between these two groups.
Statistical Analysis
The unplanned hospitalization rate was calculated as the number of all unplanned hospitalization events per person-time (years) for the total cohort. We used the Mann-Whitney U test to compare the length of stay between unplanned and other hospitalizations. We also calculated the proportion of unplanned hospitalizations that were preceded within 30 days by a treatment procedure (chemotherapy, radiotherapy, or surgery).
To identify factors significantly associated with an early unplanned hospitalization, we used Cox proportional hazards modeling with the first unplanned hospitalization event as the dependent variable. This allowed comparison of patients with different survival times. Patients were censored at date of death or at the end of the 2-year observation period if they had not experienced an unplanned hospitalization at that point. Independent variables included: age, sex, race, area of residence, census tract poverty level, cancer type, disease stage, comorbidity index, and dual eligibility for Medicare and Medicaid, which was ascertained using a covariate indicating whether the patient was a participant in the state's buy-in program. Using the full Cox model, we estimated hazard ratios for first unplanned hospitalization in two hypothetical patients with different levels of risk compared with a reference patient, by exponentiating the sum of the regression coefficients of each of the covariates in the model.16,20 The adjusted Cox proportional hazards model was also used to estimate time to first unplanned hospitalization for some of the independent variables. A P value less than .05 was considered statistically significant. Statistical analyses were performed using Statistical Analysis Software version 9.3 (SAS Institute, Cary, NC).
RESULTS
A total of 30,199 patients were included in the cohort. Patient characteristics at the time of diagnosis and association of these characteristics with unplanned hospitalization are listed in Table 1. The most common comorbidities in our study population were diabetes without complications (18.0%), chronic pulmonary disease (10.7%), and congestive heart failure (9.5%).
Table 1.
Demographic and Clinical Characteristics of the Entire Study Population (n = 30,199) at Diagnosis, as Well as Those of Patients Who Experienced Unplanned Hospitalizations (n = 17,842)
| Characteristic | All Patients |
Patients Who Experienced Unplanned Hospitalization |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age group, years | ||||
| 66–69 | 5,547 | 18.4 | 3,191 | 57.5 |
| 70–74 | 7,311 | 24.2 | 4,313 | 59.0 |
| 75–79 | 7,184 | 23.8 | 4,257 | 59.3 |
| ≥ 80 | 10,157 | 33.6 | 6,081 | 59.9 |
| Sex | ||||
| F | 15,149 | 50.2 | 9,020 | 59.5 |
| M | 15,050 | 49.8 | 8,822 | 58.6 |
| Race | ||||
| White | 24,750 | 82.0 | 14,393 | 58.2 |
| Hispanic | 1,848 | 6.1 | 1,176 | 63.6 |
| Black | 2,921 | 9.7 | 1,879 | 64.3 |
| American Indian | 47 | 0.2 | 22 | 46.8 |
| Other | 633 | 2.0 | 372 | 58.8 |
| Cancer type | ||||
| Colon | 14,548 | 48.2 | 8,272 | 56.9 |
| Esophageal | 1,695 | 5.6 | 1,123 | 66.3 |
| Liver or intrahepatic ductal | 2,059 | 6.8 | 1,240 | 60.2 |
| Pancreatic | 4,085 | 13.5 | 2,555 | 62.5 |
| Anorectal | 4,979 | 16.5 | 2,853 | 57.3 |
| Gastric | 2,833 | 9.4 | 1,799 | 63.5 |
| Disease stage | ||||
| Localized | 10,003 | 33.1 | 5,406 | 54.0 |
| Regional | 10,080 | 33.4 | 6,204 | 61.5 |
| Distant | 6,652 | 22.0 | 4,211 | 63.3 |
| Unknown | 3,464 | 11.5 | 2,021 | 58.3 |
| Area of residence* | ||||
| Big metropolis | 14,266 | 47.2 | 8,415 | 59.0 |
| Metropolis | 8,817 | 29.2 | 5,169 | 58.6 |
| Urban | 2,148 | 7.1 | 1,313 | 61.1 |
| Urban less | 4,340 | 14.4 | 2,575 | 59.3 |
| Rural | 628 | 2.1 | 370 | 58.9 |
| Census tract poverty level† | ||||
| Q1 | 7,562 | 25.0 | 4,316 | 57.1 |
| Q2 | 7,547 | 25.0 | 4,321 | 57.3 |
| Q3 | 7,551 | 25.0 | 4,503 | 59.6 |
| Q4 | 7,539 | 25.0 | 4,702 | 62.4 |
| Charlson comorbidity index | ||||
| 0 | 19,424 | 64.3 | 10,850 | 55.9 |
| 1 | 5,828 | 19.3 | 3,614 | 62.0 |
| 2 | 2,571 | 8.5 | 1,685 | 65.5 |
| ≥ 3 | 2,376 | 7.9 | 1,693 | 71.2 |
| State buy-in‡ | 6,080 | 20.1 | 3,957 | 65.1 |
Abbreviation: Q, quartile.
Big metropolis, population ≥ 1,000,000; metropolis, population of < 250,000 to 1,000,000; urban, population of ≥ 20,000; urban less, population of 2,500 to 19,999; rural, population < 2,500.
Percentage of people living below the poverty line in the patient's census tract: Q1, 0.00%-7.48%; Q2, 7.49%-13.30%; Q3, 13.31%-21.04%; Q4, ≥ 21.05%.
State buy-in variable is used to determine dual eligibility of patients for both Medicare and Medicaid.
We included 60,837 claims in our analysis; of these, 35,336 (58.1%) were for unplanned hospitalizations. The unplanned hospitalization rate was 93 unplanned hospitalization events per 100 person-years, with mean observation time of 15 months or 1.3 years per patient. In examining the event rates by year of diagnosis, we found that the rates ranged from 89 to 97 unplanned events per 100 person-years; however, no significant trend was detected (P = .362). The mean time to first unplanned hospitalization among patients who had experienced an unplanned hospitalization (n = 17,842; 59.1% of the cohort) was 2.2 months. These patients had a mean of two unplanned hospitalizations throughout the observation period, and this was similar across the different cancer types. The mean length of stay for unplanned hospitalizations was shorter compared with other hospitalizations (7.4 v 8.9 days; P < .001). Most hospitalizations were unplanned, whether they occurred ≤ 1 year after cancer diagnosis (55.9%) or more than 1 year after diagnosis (66.9%).
Among patients who had experienced an unplanned hospitalization, 19.4% had chemotherapy, radiotherapy, or a surgical procedure in the 30 days before their first unplanned hospitalization. Specifically, 6.9% had chemotherapy, 12.0% had a surgical procedure, and 2.5% had radiotherapy. In addition, 9.4% had visited the emergency department within 30 days before their first unplanned hospitalization.
The most common noncancer reason for unplanned hospitalization was fluid and electrolyte disorders (n = 2,944; 8.3%; Table 2). This was consistent across most cancer types. Intestinal obstruction was the second most common reason (n = 1,871; 5.3%) followed by pneumonia (n = 1,572; 4.4%). Congestive heart failure and chronic obstructive pulmonary disease and bronchiectasis, which were among the top three most common comorbidities in our cohort, were also common reasons for unplanned hospitalization (fourth and ninth most common, respectively).
Table 2.
Most Common Noncancer Reasons for Unplanned Hospitalization by Cancer Type by Clinical Classifications Software
| Reason for Unplanned Hospitalization | All GI Cancer |
Esophageal Cancer |
Gastric Cancer |
Pancreatic Cancer |
Liver or Intrahepatic Ductal Cancer |
Anorectal Cancer |
Colon Cancer |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| All reasons | 35,336 | 2,360 | 3,561 | 4,940 | 2,338 | 5,773 | 16,364 | |||||||
| Fluid and electrolyte disorders | 2,944 | 8.3 | 303 | 12.8 | 340 | 9.6 | 563 | 11.4 | 198 | 8.5 | 426 | 7.4 | 1,114 | 6.8 |
| Intestinal obstruction without hernia | 1,871 | 5.3 | 43 | 1.8 | 97 | 2.7 | 169 | 3.4 | 24 | 1.0 | 323 | 5.6 | 1,215 | 7.4 |
| Pneumonia | 1,572 | 4.4 | 164 | 7.0 | 183 | 5.1 | 167 | 3.4 | 94 | 4.0 | 215 | 3.7 | 749 | 4.6 |
| Congestive heart failure, nonhypertensive | 1,547 | 4.4 | 83 | 3.5 | 122 | 3.4 | 1.2 | 2.1 | 85 | 3.6 | 278 | 4.8 | 877 | 5.4 |
| Complications of surgical procedures or medical care | 1,504 | 4.3 | 98 | 4.2 | 140 | 3.9 | 116 | 2.4 | 37 | 1.6 | 316 | 5.5 | 797 | 4.9 |
| Septicemia | 1,450 | 4.1 | 82 | 3.5 | 181 | 5.1 | 283 | 5.7 | 131 | 5.6 | 232 | 4.0 | 541 | 3.3 |
| Gastrointestinal hemorrhage | 1,330 | 3.8 | 87 | 3.7 | 262 | 7.4 | 138 | 2.8 | 76 | 3.3 | 236 | 4.1 | 531 | 3.2 |
| Deficiency and other anemia | 1,074 | 3.0 | 63 | 2.7 | 161 | 4.5 | 91 | 1.8 | 46 | 2.0 | 161 | 2.8 | 552 | 3.4 |
| Chronic obstructive pulmonary disease and bronchiectasis | 1,032 | 2.9 | 104 | 4.4 | 113 | 3.2 | 119 | 2.4 | 50 | 2.1 | 136 | 2.4 | 510 | 3.1 |
| Urinary tract infection | 1,030 | 2.9 | 44 | 1.86 | 103 | 2.9 | 119 | 2.4 | 72 | 3.1 | 194 | 3.4 | 498 | 3.0 |
| Other common reasons* | ||||||||||||||
| Aspiration pneumonitis, food/vomitus | 97 | 4.1 | ||||||||||||
| Biliary tract disease | 321 | 6.6 | ||||||||||||
| Other liver diseases | 336 | 14.4 | ||||||||||||
≥ 4% of hospitalizations for specific cancer type.
All variables studied were risk factors for unplanned hospitalization except for population size of the area of residence and sex (Table 3). Patients age 75 to 79 years and older than 80 years (compared with 66-69 years), black race (compared with white), those residing in quartile 3 or quartile 4 census tracts (compared with quartile 1), and those who had dual eligibility for Medicare and Medicaid were at increased risk for unplanned hospitalization (Table 3).
Table 3.
Adjusted Multivariable Analysis Showing Risk Factors for Unplanned Hospitalization
| Variable | Coefficient | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| Age, years (v 66-69) | ||||
| 70-74 | 0.04 | 1.04 | 1.00 to 1.09 | .058 |
| 75-79 | 0.06 | 1.07 | 1.02 to 1.12 | .006 |
| ≥ 80 | 0.11 | 1.12 | 1.07 to 1.17 | < .001 |
| Sex: F (v M) | 0.03 | 1.03 | 1.00 to 1.06 | .073 |
| Race (v white) | ||||
| Hispanic | 0.01 | 1.01 | 0.95 to 1.08 | .689 |
| Black | 0.07 | 1.07 | 1.02 to 1.13 | .007 |
| American Indian | −0.22 | 0.80 | 0.53 to 1.22 | .295 |
| Other | −0.05 | 0.95 | 0.85 to 1.05 | .323 |
| Cancer type (v colon) | ||||
| Esophageal | 0.13 | 1.14 | 1.07 to 1.22 | < .001 |
| Liver or intrahepatic ductal | 0.02 | 1.02 | 0.96 to 1.08 | .517 |
| Pancreatic | 0.07 | 1.08 | 1.03 to 1.13 | .001 |
| Anorectal | −0.03 | 0.97 | 0.93 to 1.01 | .171 |
| Gastric | 0.13 | 1.14 | 1.08 to 1.20 | < .001 |
| Disease stage (v localized) | ||||
| Regional | 0.23 | 1.26 | 1.22 to 1.31 | < .001 |
| Distant | 0.29 | 1.34 | 1.28 to 1.40 | < .001 |
| Unknown | 0.02 | 1.02 | 0.97 to 1.08 | .384 |
| Area of residence* (v big metropolis) | ||||
| Metropolis | −0.04 | 0.97 | 0.93 to 1.00 | .059 |
| Urban | 0.04 | 1.04 | 0.98 to 1.10 | .201 |
| Urban less | −0.01 | 0.99 | 0.95 to 1.04 | .762 |
| Rural | −0.01 | 0.99 | 0.89 to 1.10 | .817 |
| Census tract poverty level† (v Q1) | ||||
| Q2 | 0.001 | 1.00 | 0.96 to 1.05 | .972 |
| Q3 | 0.05 | 1.05 | 1.00 to 1.10 | .043 |
| Q4 | 0.08 | 1.08 | 1.03 to 1.14 | < .001 |
| Charlson comorbidity index (v 0) | ||||
| 1 | 0.16 | 1.17 | 1.13 to 1.22 | < .001 |
| 2 | 0.25 | 1.30 | 1.22 to 1.35 | < .001 |
| ≥ 3 | 0.42 | 1.52 | 1.44 to 1.60 | < .001 |
| State buy in‡ (v none) | 0.12 | 1.13 | 1.09 to 1.18 | < .001 |
Abbreviation: Q, quartile.
Big metropolis, population ≥ 1,000,000; metropolis, population of < 250,000 to 1,000,000; urban, population of ≥ 20,000; urban less, population of 2,500 to 19,999; rural, population < 2,500.
Percentage of people living below the poverty line in the patient's census tract: Q1, 0.00%-7.48%; Q2, 7.49%-13.30%; Q3, 13.31%-21.04%; Q4, ≥ 21.05%.
State buy-in variable is used to determine dual eligibility of patients for both Medicare and Medicaid.
Among patients with different types of GI cancer, those with esophageal cancer had the highest risk for first unplanned hospitalization compared with those with colon cancer. By 1 year after diagnosis, more than half of patients with esophageal cancer had already experienced an unplanned hospitalization (Fig 2A). Advanced-stage disease was associated with an increased risk compared with localized disease. At 6 months after diagnosis, more than 50% of patients with distant or metastatic disease had experienced an unplanned hospitalization (Fig 2B). The risk for unplanned hospitalization also increased with increasing CCI (Fig 2C).
Fig 2.
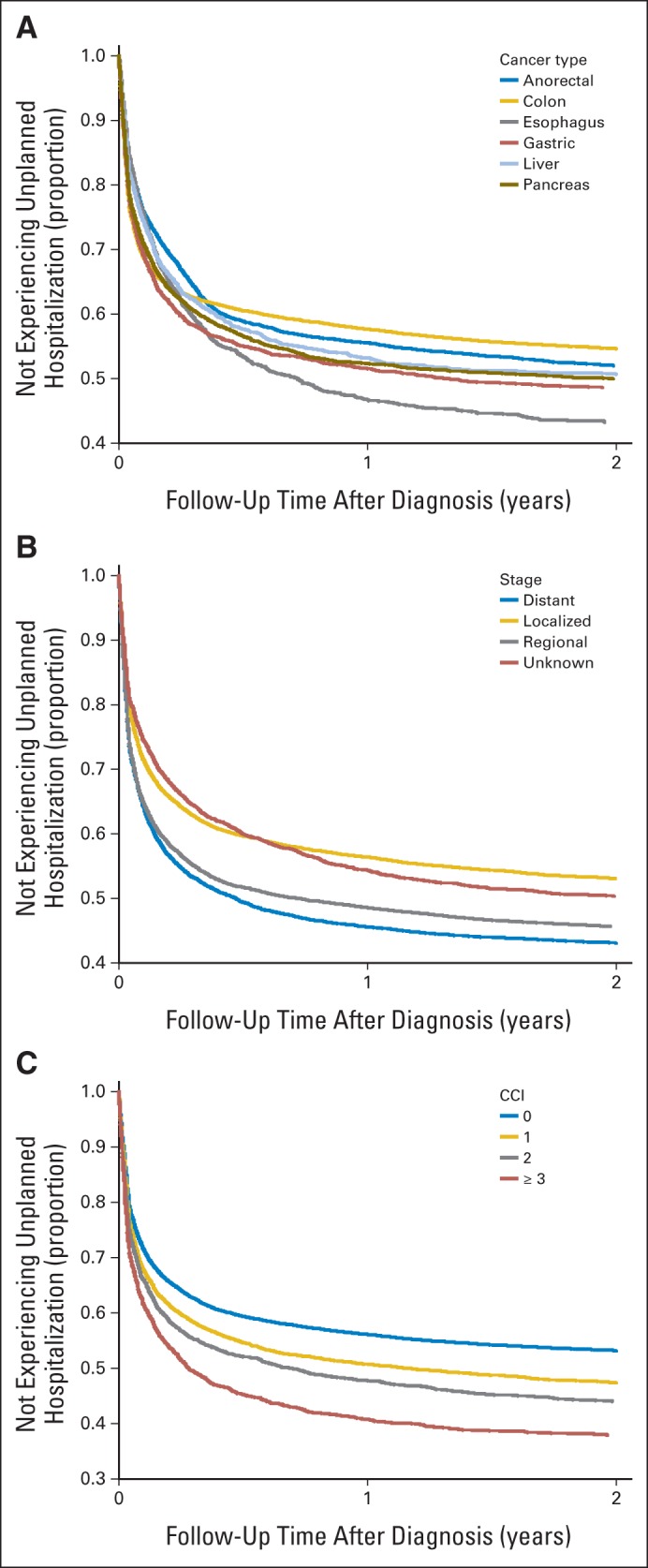
Adjusted survival curves based on Cox proportional hazards regression model for time to first unplanned hospitalization stratified by (A) cancer type, (B) disease stage, and (C) Charlson comorbidity index.
Using the estimated regression coefficients, the fitted model would assign an 80-year-old black woman with metastatic esophageal cancer and a CCI ≥ 3 who resides in an urban area with a poverty level greater than 21.05% and who has dual eligibility (all high-risk variables), a hazard ratio of 3.63. In other words, this hypothetical patient would be 3.63 times more likely to experience an unplanned hospitalization than our reference patient who is male, age 66 to 69 years, and white who has localized colon cancer, a CCI of 0, resides in the most affluent census tract in a big metropolitan area and is not also eligible for Medicaid. Similarly, the model would show that a 75- to 79-year-old Hispanic woman with gastric cancer who has regional disease, has a CCI of 2, lives in a metropolitan area with a poverty level between 13.31% and 21.04% and also has dual eligibility would be 2.32 times more likely to experience an unplanned hospitalization than our reference patient.
DISCUSSION
In our population-based assessment, we observed an unplanned hospitalization rate of 93 events per 100 person-years. To understand the significance of this, we estimated the unplanned hospitalization rate across 2 years among a cohort of elderly individuals derived from a random 5% sample of Medicare beneficiaries without cancer who were enrolled in the Medicare program during the same inclusive years as our patients. In this cohort of 45,459 beneficiaries, we found a significantly lower unplanned hospitalization rate of 11.4 events per 100 person-years, suggesting that unplanned hospitalizations are disproportionately more common in our study cohort compared with the elderly noncancer population (P < .001).
To the best of our knowledge, ours is the first population-based study to report an unplanned hospitalization rate for GI cancer. A recent study by Brooks et al21 done in a single academic medical center found that 19% of 201 hospitalizations among patients with GI cancer were potentially avoidable. Although our defined outcomes were different, potentially avoidable hospitalizations are a subset of unplanned hospitalizations, and the lower proportion observed in Brooks' study implies that not all unplanned hospitalizations are avoidable. In contrast, a 2009 AHRQ statistical brief reported that approximately 55% of patients who were hospitalized with a secondary diagnosis of colorectal cancer were urgently admitted. Although the report did not make a distinction between planned and unplanned hospitalization, our estimates for unplanned hospitalization (58.1% including both urgent and emergent admissions) was similar to the AHRQ report, probably because a large proportion of our cohort had colon and rectal cancer.22 Other studies on patients with cancer have focused on chemotherapy-related hospitalizations, and the observed proportions (8% to 35%) were lower than what we observed in our study.23,24 This is more consistent with our finding that only 6.9% of patients who experienced an unplanned hospitalization had chemotherapy before their first unplanned hospitalization.
Some of the most common reasons for unplanned hospitalization in our study (ie, fluid and electrolyte disorders, pneumonia, congestive heart failure, chronic obstructive pulmonary disease and bronchiectasis, and urinary tract infections) are considered potentially preventable by the AHRQ.25 These conditions are considered ambulatory care–sensitive because hospitalization can be prevented by appropriate outpatient care.11 Although it is difficult to define and confirm preventable hospitalizations among patients with cancer using claims data alone, the diagnoses that most commonly led to unplanned hospitalizations in our study are amenable to outpatient management if addressed early.
We detected socioeconomic disparities in the risk for unplanned hospitalization in our cohort, similar to other health care utilization studies. One United Kingdom study found that Asian ethnicity and residing in areas of deprivation were predictive of an unplanned first-time hospitalization related to cancer.6 In a US study examining hospitalization rates for survivors of childhood cancer, low income was associated with increased hospitalization rates.26 Whether or not these findings are related to access to outpatient care needs further investigation.
Advanced disease stages and increased comorbidity scores were also identified as risks for unplanned hospitalization in our study. A few other studies have identified the same risk factors for unplanned hospitalization in patients with cancer, although these studies were not focused on elderly patients with GI cancer.23,27 Our findings are not surprising because advanced-stage disease and comorbidity may be related to the illness or frailty of a patient. These findings support a renewed emphasis on comorbidity management and follow-up care in patients with cancer. Close coordination among primary care providers, oncologists, and inpatient teams is needed throughout a patient's entire cancer trajectory to limit treatment interruptions caused by unplanned hospitalizations. In addition, careful appraisal of risk factors could help identify patients requiring closer follow-up by their primary care physicians and oncologists.
One of our study's strengths is the use of time-to-event analysis, which allowed us to examine unplanned hospitalization among patients with various follow-up times. Our sample size was also substantial, and although it can be argued that this could have driven the statistical significance in our risk factor analysis, our study findings are in fact consistent with other health care utilization studies.6,23,27 We also provided a hazard estimate for a hypothetical patient given a risk profile using our fitted model. We believe this helps place our results in a clinical context, which will be useful for health care providers who need to stratify their patients by risk.
Our study has some limitations. We included patients age 66 years or older with GI cancer who reside in Texas, and our findings may not be generalizable to all elderly patients with cancer. Additional studies and rate comparisons should be undertaken to determine whether these observations are unique to the state of Texas or to elderly patients with GI cancer. In addition, we presented aggregated data for six GI cancers, recognizing that there may be differences among patients with each of these cancers. To account for this limitation, we presented data adjusted according to cancer type as much as possible, and we stratified results where we felt it was necessary. Although using a large administrative database allowed us to study large populations, our findings are limited by the absence of specific clinical data and hence must rely on our assumptions and definitions of unplanned hospitalization and reasons for hospitalization. Our definition for an unplanned hospitalization, although not validated, has been used in several studies.6,11,12,28 Another limitation of our study is the inability to capture services obtained through the Veterans Affairs Health Care System. Although we do not know what proportion of our cohort are veterans, it should be noted that in a study conducted on Medicare-eligible veterans, the majority of those who had an inpatient stay relied solely on Medicare for their inpatient care.29 Variation in physicians' decisions to admit patients can also introduce unmeasured bias. For example, availability of beds in the admitting hospital may affect a patient's likelihood of being admitted. Lastly, our results are primarily descriptive, and many patterns that we observed cannot be explained by our findings alone.
Nevertheless, we believe that our findings provide useful data for policymakers and health care providers that can be used to inform decisions about resource allocation and coordination efforts among all providers caring for a patient with cancer. Knowing who is at risk for an unplanned hospitalization also opens up opportunities for anticipatory guidance and patient education during the active phase of treatment.
Acknowledgment
We thank Erica Goodoff, ELS, for her help in editing this article. She is a scientific editor at the University of Texas MD Anderson Cancer Center. She did not receive additional compensation for her contribution.
Glossary Terms
- comorbidity:
having two or more diseases at the same time.
Footnotes
Listen to the podcast by Dr Extermann at www.jco.org/podcasts
Supported by the Texas Department of State Health Services and the Cancer Prevention Research Institute of Texas (CPRIT) as part of the statewide cancer reporting program and the Centers for Disease Control and Prevention‘s (CDC) National Program of Cancer Registries Cooperative Agreement (No. 5U58/DP000824-05); by Grant No. RP140020—Comparative Effectiveness Research on Cancer in Texas from CPRIT; a Midcareer Investigator Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant No. K24 AR053593; M.E.S.-A.); and Grant No. CA016672 to the University of Texas MD Anderson Cancer Center from National Institutes of Health Cancer Center Support. The data presented herein are solely the responsibility of the authors and do not necessarily represent the official views of the Department of State Health Services, CPRIT, or the CDC.
Presented in part at the 2013 Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, May 31-June 4, 2013.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joanna-Grace M. Manzano, Linda S. Elting, Marina George, Maria E. Suarez-Almazor
Financial support: Linda S. Elting, Maria E. Suarez-Almazor
Provision of study materials or patients: Linda S. Elting
Collection and assembly of data: Joanna-Grace M. Manzano, Ruili Luo
Data analysis and interpretation: Joanna-Grace M. Manzano, Ruili Luo, Linda S. Elting, Maria E. Suarez-Almazor
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Institute of Medicine. Washington, DC: National Academies Press; 2013. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. [PubMed] [Google Scholar]
- 2.Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364:2060–2065. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 5.Anhang Price R, Stranges E, Elixhauser A. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Healthcare Cost and Utilization Project Statistical Brief No. 125: Cancer hospitalizations for adults, 2009. [PubMed] [Google Scholar]
- 6.Bottle A, Tsang C, Parsons C, et al. Association between patient and general practice characteristics and unplanned first-time admissions for cancer: Observational study. Br J Cancer. 2012;107:1213–1219. doi: 10.1038/bjc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 8.Texas Department of State Health Services. Texas Cancer Registry background. http://www.dshs.state.tx.us/tcr/background.shtm.
- 9.Research Data Assistance Center. Medicare claims. http://www.resdac.org/cms-data/file-family/Medicare-Claims.
- 10.Schneider EB, Hyder O, Wolfgang CL, et al. Patient readmission and mortality after surgery for hepato-pancreato-biliary malignancies. J Am Coll Surg. 2012;215:607–615. doi: 10.1016/j.jamcollsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntley AL, Thomas R, Mann M, et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta-analysis. Fam Pract. 2013;30:266–275. doi: 10.1093/fampra/cms081. [DOI] [PubMed] [Google Scholar]
- 12.Payne RA, Abel GA, Guthrie B, et al. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: A retrospective cohort study. CMAJ. 2013;185:E221–E228. doi: 10.1503/cmaj.121349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merriman K, Caldwell D. How to Identify Emergency Room Services in the Medicare Claims Data. Knowledge Base Articles. 2012. http://www.resdac.org/resconnect/articles/144.
- 14.Purdy S. Avoiding hospital admissions: What does the research evidence say. King's Fund. 2010. http://www.kingsfund.ork.uk/publications/avoiding-hospital-admissions.
- 15.Aprile G, Pisa FE, Follador A, et al. Unplanned presentations of cancer outpatients: A retrospective cohort study. Support Care Cancer. 2013;21:397–404. doi: 10.1007/s00520-012-1524-6. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081-1090. [DOI] [PubMed] [Google Scholar]
- 20.Kleinbaum D. The Cox proportional hazards model and its characteristics. In: Dietz K, Gail M, Krickeberg K, et al., editors. Survival Analysis: A Self-Learning Text. 1st ed. Springer-Verlag New York; 1996. pp. 83–115. [Google Scholar]
- 21.Brooks GA, Abrams TA, Meyerhardt JA, et al. Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol. 2014;32:496–503. doi: 10.1200/JCO.2013.52.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo CA, Stocks C. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Healthcare Cost and Utilization Project Statistical Brief No. 69: Hospitalizations for Colorectal Cancer, 2006. [PubMed] [Google Scholar]
- 23.Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol. 2002;20:4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notten P, Boudewijns S, van Wijck H, et al. Unplanned hospital admissions in senior cancer patients receiving chemotherapy: Preliminary evaluation of the use of pre-chemotherapy assessment tools in daily care. Presented at the 12th Meeting of the International Society of Geriatric Oncology; October 25, 2012; Manchester, United Kingdom. abstr P41. [Google Scholar]
- 25.Kruzikas DT, Jiang HJ, Remus D, et al. Rockville, MD: Agency for Healthcare Research and Quality; 2004. Preventable Hospitalizations: A Window Into Primary and Preventive Care, 2000. [Google Scholar]
- 26.Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59:126–132. doi: 10.1002/pbc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunetto AT, Ang JE, Olmos D, et al. A study of the pattern of hospital admissions in a specialist phase I oncology trials unit: Unplanned admissions as an early indicator of patient attrition. Eur J Cancer. 2010;46:2739–2745. doi: 10.1016/j.ejca.2010.06.123. [DOI] [PubMed] [Google Scholar]
- 28.Marcantonio ER, McKean S, Goldfinger M, et al. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. Am J Med. 1999;107:13–17. doi: 10.1016/s0002-9343(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 29.Petersen LA, Byrne MM, Daw CN, et al. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45:762–791. doi: 10.1111/j.1475-6773.2010.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


