Abstract
Purpose
Intergroup Rhabdomyosarcoma Study Group (IRSG) studies III and IV showed improved failure-free survival (FFS) rates with vincristine, dactinomycin, and cyclophosphamide (VAC; total cumulative cyclophosphamide dose, 26.4 g/m2) compared with vincristine and dactinomycin (VA) for patients with subset-one low-risk embryonal rhabdomyosarcoma (ERMS; stage 1/2 group I/II ERMS or stage 1 group III orbit ERMS). The objective of Children's Oncology Group ARST0331 was to reduce the length of therapy without compromising FFS for this subset of low-risk patients by using VA in combination with lower-dose cyclophosphamide (total cumulative dose, 4.8 g/m2) plus radiotherapy (RT).
Patients and Methods
This noninferiority prospective clinical trial enrolled newly diagnosed patients with subset-one clinical features. Therapy included four cycles of VAC followed by four cycles of VA over 22 weeks. Patients with microscopic or gross residual disease at study entry received RT.
Results
With a median follow-up of 4.3 years, we observed 35 failures among 271 eligible patients versus 48.4 expected failures, calculated using a fixed outcome based on the FFS expected for similar patients treated on the IRSG D9602 protocol. The estimated 3-year FFS rate was 89% (95% CI, 85% to 92%), and the overall survival rate was 98% (95% CI, 95% to 99%). Patients with paratesticular tumors had the most favorable outcome. Three-year cumulative incidence rates for any local, regional, or distant failures were 7.6%, 1.5%, and 3.4%, respectively.
Conclusion
Shorter-duration therapy that included lower-dose cyclophosphamide and RT did not compromise FFS for patients with subset-one low-risk ERMS.
INTRODUCTION
The Soft Tissue Sarcoma Committee of the Children's Oncology Group (COG) established risk groups for rhabdomyosarcoma (RMS) treatment stratification based on Intergroup RMS Study Group (IRSG) stage, group, and histologic subtype.1 The low-risk group includes patient subsets with 5-year failure-free survival (FFS) rates ≥ 83% in Intergroup Rhabdomyosarcoma Study (IRS) III or IV.2,3 The overall survival (OS) rate for these patients is approximately 95%.2,3 The low-risk group is composed of patients with localized favorable-histology RMS arising in favorable primary sites (stage I embryonal RMS [ERMS]) or localized favorable-histology tumors arising in unfavorable primary sites that have been grossly resected before initiation of chemotherapy (stage II/III group I/II ERMS). The low-risk group is further subclassified into two subsets based on whether a 5-year FFS rate ≥ 83% was achieved with either vincristine and dactinomycin (VA) alone (subset one: stage I/II group I/II or stage I group III orbit ERMS) or a more intense regimen of vincristine, dactinomycin, and cyclophosphamide (VAC; total cumulative cyclophosphamide dose, 26.4 g/m2; subset two: stage I group III nonorbit or stage III group I/II ERMS).1,4
Therapy changes for patients in subset one of ARST0331 compared with IRS-III and -IV were based on the following factors: First, FFS improved significantly in IRS-IV for patients defined as subset one with the addition of cyclophosphamide (total cumulative cyclophosphamide dose, 26.4 g/m2) to VA, which had been administered in IRS-III.2,3 Second, European trials suggested that shorter-duration therapy compared with 46 to 54 weeks of therapy in IRS-III or -IV was effective for some subset-one low-risk patients.5,6 Third, data from single institutions demonstrated satisfactory local control of microscopic residual disease using radiotherapy (RT) doses down to 30 Gy.7,8 Finally, IRSG data showed no significant dose-response relationship with RT doses down to 40 Gy for group III orbit tumors.9 Outcome was not yet available for IRSG low-risk RMS study D9602 (1997 to 2004) at the time that ARST0331 was designed, which treated similar patients (subgroup A) with 45 weeks of VA and RT.4
The primary objective of ARST0331 for patients in subset one was to estimate the FFS rate using shorter-duration therapy (22 weeks) that included four cycles of VAC (total cumulative cyclophosphamide dose, 4.8 g/m2) followed by four cycles of VA. The cumulative dose of cyclophosphamide was fixed at a dose likely to preserve fertility in most patients. The secondary objective was to estimate local control rates for patients with microscopic residual tumor and no regional lymph node involvement (group IIA), who received 36 compared with 41.4 Gy in IRS-IV, and those with unresected (group III) orbit tumors, who received 45 compared with 50.4 to 59 Gy in IRS-IV.3 Results for patients enrolled onto subset two of ARST0331 with stage I group III vaginal tumors have already been reported, and results for all other patients enrolled onto subset two of ARST0331 will be reported elsewhere.10
PATIENTS AND METHODS
Patients were eligible for subset one of ARST0331 if they were age < 50 years at the time of diagnosis and previously untreated; had ERMS, the botryoid or spindle-cell embryonal variants, or primitive ectomesenchymoma with ERMS histology confirmed by central pathology review; had a stage I/II group I/II tumor or stage I group III orbit tumor; had started protocol therapy within 42 days of diagnosis; had acceptable organ function for age; and provided (or guardian provided) signed written informed consent according to institutional guidelines, with institutional review board approval according to the Declaration of Helsinki. Extent of disease was assessed by magnetic resonance imaging or computed tomography (CT) scan of the primary site; CT scan of the chest, retroperitoneum, and liver; bone scan; bilateral bone marrow aspirates and biopsies; and biopsies of radiographically or clinically enlarged lymph nodes. Magnetic resonance imaging or CT scan of the head and cerebrospinal fluid cytology were required for patients with parameningeal or orbit tumors. Staging ipsilateral retroperitoneal lymph node dissection was required for boys age > 10 years with paratestis tumors, and regional lymph node sampling was required for histologic evaluation in patients with extremity tumors.
The treatment schema is summarized in Table 1. RT began at week 13 for patients with group II/III tumors and could be administered as three-dimensional conformal RT, intensity-modulated RT, proton-beam RT, or brachytherapy. Total doses were based on the extent of residual disease; no RT for group I tumors, 36 Gy for group IIA tumors, 41.4 Gy for group IIB/C tumors, and 45 Gy for group III orbit tumors in 1.8-Gy fractions. Before August 19, 2009, patients with group IIA vaginal tumors did not follow this RT plan. Instead, RT was delayed for these patients if tumor was undetectable by imaging and/or vaginoscopy at week 12 and was not administered if biopsy or resection of the local site did not show tumor at week 24. As of August 19, 2009, patients with group IIA vaginal primaries followed the same RT guidelines as other patients. This decision was based on increased local recurrences in patients with group III vaginal RMS in subset two who did not receive RT based on biopsy-proven complete response at week 24 with chemotherapy with or without resection. Dactinomycin was held during RT. Myeloid growth factor support was used if chemotherapy had been delayed because of hematologic toxicity in earlier cycles. Trimethoprim/sulfamethoxazole prophylaxis was administered to all patients.
Table 1.
ARST0331 Regimen for Patients in Subset One
| Treatment | Week |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Eval | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | Eval | |
| V* | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| A† | X | X | X | X | X | X‡ | X | X | ||||||||||||||||||
| C§ | X | X | X | X | ||||||||||||||||||||||
| RT | X‖ | |||||||||||||||||||||||||
Abbreviations: A, dactinomycin; C, cyclophosphamide; RT, radiotherapy; V, vincristine.
Age < 1 year, 0.025 mg/kg; age 1 to < 3 years, 0.05 mg/kg; age ≥ 3 years, 1.5 mg/kg (maximum dose, 2 mg).
Age < 1 year, 0.025 mg/kg; age ≥ 1 year, 0.045 mg/kg (maximum dose, 2.5 mg).
Dactinomycin omitted at week 16 in patients receiving RT.
Age < 3 years, 40 mg/kg; age ≥ 3 years, 1.2 g/m2. Cyclophosphamide administered with mesna.
If group II or III.
The study was designed as a nonrandomized, noninferiority prospective clinical trial. The primary end point was FFS, which was defined as the time from start of treatment to disease progression, recurrence, or death as a first event. FFS was compared with the fixed expected outcome—S(t) = 0.83 + 0.17 * exp(−1.00 * t)—based on similar patients from D9602 (subgroup A). This distribution plateaus at an 83% cure rate and assumes that the distribution of failures for those patients who experience an event is exponentially distributed with λ = 1.00. This means that 95% of all failures are expected to be observed in the first 3 years of follow-up. This distribution describes the outcome expected for this population of patients treated in D9602 at the time that ARST0331 was written.4
There was concern that the reduction in the total treatment period as compared with D9602 might increase the risk of treatment failure. Therefore, using S(t) as a historical fixed outcome, the protocol called for the enrollment of a total of 410 subset-one patients, which was expected to provide 90% power (testing at 10% significance level, one sided) to detect a reduction in long-term FFS from 83% to 78%. However, interim analysis based on data available as of April 2010 showed that there was 99% confidence that the true long-term FFS for subset-one patients was no worse than the protocol-specified null hypothesis value of 83%. At that time, the estimated FFS at 2 years was 88% (95% CI, 82% to 92%). In July 2010, these results convinced the COG Data Monitoring Committee that ARST0331 protocol treatment had not had a negative impact on outcome, and it suspended enrollment of subset-one patients in August 2010.
Survival was defined as the time from start of treatment to death resulting from any cause. FFS and survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test.11 CIs for estimates of time-to-event distributions were calculated using Greenwood's formula.12 Failure rates were estimated using cumulative incidence curves. Failure was defined as local if the tumor recurred at the site of primary disease, regional if regional lymph nodes were involved with recurrent tumor, and distant if any metastatic disease was present at the time of recurrence. Only first treatment failures were considered in the analysis. The results were based on data available by June 30, 2013.
RESULTS
Patients
ARST0331 accrued 304 subset-one patients from September 17, 2004, to August 13, 2010. Thirty-three patients were ineligible: 23 because of ineligible pathology, eight because of unavailable pathology, and two for other reasons. The median follow-up for the 271 eligible patients was 4.3 years (range, 0.5 to 8 years).
Patient characteristics for the 271 eligible patients are listed in Table 2. A majority of patients were male and had stage I tumors that were ≤ 5 cm, noninvasive, and without nodal involvement. Approximately half of all patients had group I tumors. The most common primary sites were paratestis (n = 118; 44%) and orbit (n = 82; 30%). Among the 116 patients with group II/III tumors who received RT, the type of radiation used was available for 108; 65 patients (60%) received intensity-modulated RT, 35 (32%) received three-dimensional conformal RT, seven (7%) received proton RT, and one (1%) received brachytherapy. There was only one patient with group IIA vaginal RMS enrolled onto subset one of ARST0331. This patient was enrolled before RT guidelines were revised on August 19, 2009.
Table 2.
Demographic and Clinical Characteristics of Eligible Patients Enrolled Onto Subset One of ARST0331
| Characteristic | Subset One (n = 271) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| < 5 | 101 | 37 |
| 5-9 | 76 | 28 |
| 10-14 | 48 | 18 |
| 15-21 | 41 | 15 |
| > 21 | 5 | 2 |
| Sex | ||
| Male | 202 | 75 |
| Female | 69 | 25 |
| Histology* | ||
| Botryoid | 28 | 10 |
| Embryonal | 186 | 69 |
| Spindle cell | 57 | 21 |
| Stage* | ||
| I | 251 | 93 |
| II | 20 | 7 |
| Group* | ||
| I | 137 | 51 |
| IIA | 52 | 19 |
| IIB | 11 | 4 |
| IIC | 9 | 3 |
| III | 62 | 23 |
| Primary site* | ||
| Extremity | 5 | 2 |
| GU, non-BP | ||
| Other | 18 | 7 |
| Paratestis | 118 | 44 |
| Head and neck | 30 | 11 |
| Orbit | 82 | 30 |
| Other | 14 | 5 |
| PM/PM extension | 4 | 1 |
| Tumor size, cm* | ||
| ≤ 5 | 223 | 82 |
| > 5 | 48 | 18 |
| Tumor invasion* | ||
| T1 | 250 | 92 |
| T2 | 21 | 8 |
| Nodal status * | ||
| N0 | 247 | 91 |
| N1 | 18 | 7 |
| Nx | 6 | 2 |
Abbreviations: BP, bladder/prostate; GU, genitourinary; PM, parameningeal.
Composite variables are values from central review, if available; otherwise, they are values reported by institution.
Outcome
With a median follow-up of 4.3 years, we observed 35 failures versus 48.4 expected failures (P = .05) based on the outcome for patients in subgroup A of D9602. Thus, we can be reasonably confident that the long-term FFS rate for these patients is not worse than 83%. The estimated 3-year FFS rate was 89% (95% CI, 85% to 92%), and the estimated 3-year OS rate was 98% (95% CI, 95% to 99%; Fig 1). Patients with stage I group IIB/C (node positive) or stage II group II ERMS (n = 30) were classified as subset one in ARST0331 but had been classified as subgroup B in D96024 and treated with VAC, including a total cumulative cyclophosphamide dose of 28.6 g/m2. Their estimated 3-year FFS and OS rates were 90% (95% CI, 72% to 97%) and 96% (95% CI, 78% to 99%), respectively, in ARST0331 (Fig 2). The estimated 3-year FFS and OS rates were 93% (95% CI, 87% to 96%) and 99% (95% CI, 94% to 99%), respectively, for patients with paratestis tumors (n = 118); 86% (95% CI, 77% to 92%) and 97% (95% CI, 90% to 99%) for patients with orbit tumors (n = 82); and 86% (95% CI, 75% to 92%) and 97% (95% CI, 88% to 99%) for patients with tumors in all other sites combined (n = 71; Fig 3). For patients with stage I/II group IIA tumors (n = 52) who received 36 Gy, the estimated 3-year FFS and OS rates were 90% (95% CI, 77% to 96%) and 96% (95% CI, 84% to 99%), respectively.
Fig 1.
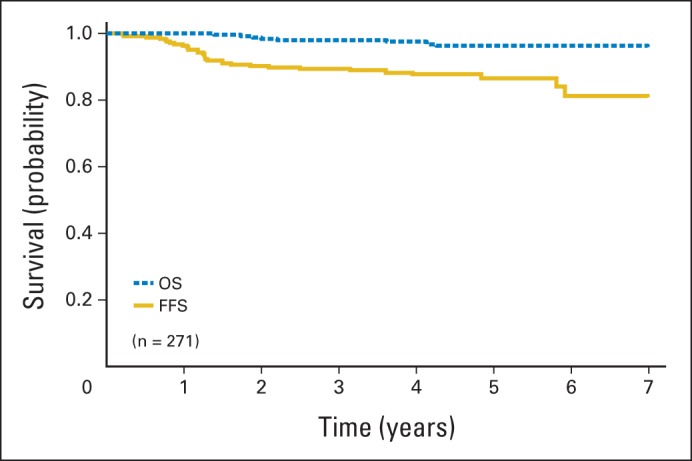
Failure-free (FFS) and overall survival (OS) for subset-one patients in ARST0331.
Fig 2.
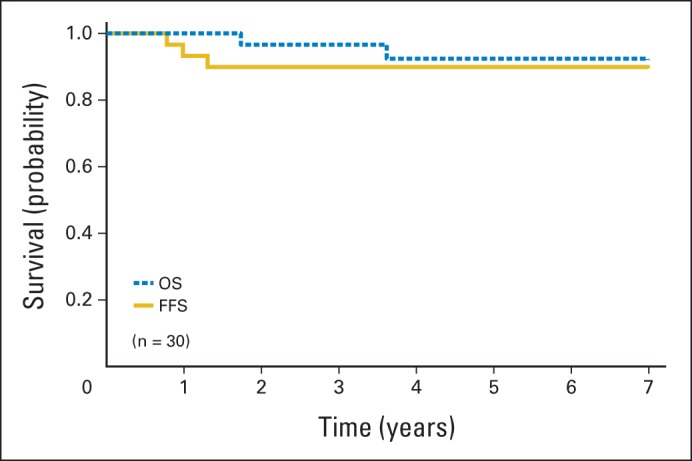
Failure-free (FFS) and overall survival (OS) for patients with stage I group IIB/C or stage II group II rhabdomyosarcoma in ARST0331.
Fig 3.
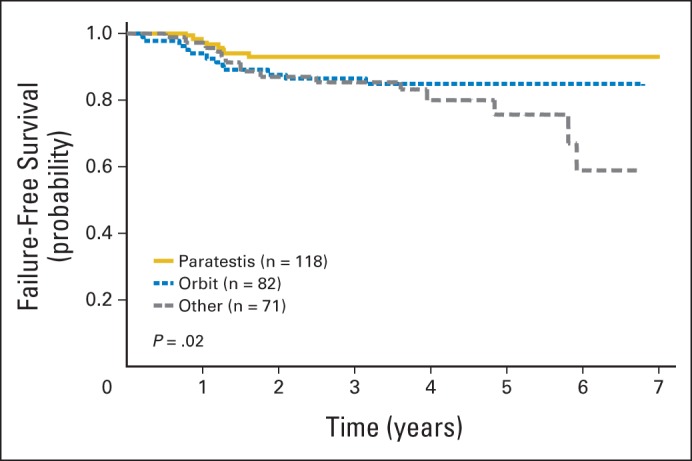
Failure-free survival by primary site for subset-one patients in ARST0331.
Recurrences
There have been 35 failures (12 orbit, eight paratestis, eight head and neck, three other, and one each of kidney, cervix, vagina, and extremity) and eight disease-related deaths (three orbit, two head and neck, and one each of kidney, paratestis, and other). We observed three late recurrences (two local and one locoregional) ≥ 4.8 years from study entry (two patients with stage I group I head and neck RMS and one patient with stage I group IIA vaginal RMS). All three patients are alive after recurrence, with 5.5 to 7.1 years of follow-up from study entry.
The 3-year cumulative incidence rate of local failure alone was 6.7%. To date, there have been 21 failures limited to the local site, occurring in eight patients with group I (6%), five with group II (7%), and eight with group III orbit (13%) tumors. The 3-year cumulative incidence rate was 7.6% for any local failure with or without regional or distant failure, 1.5% for any regional failure with or without distant failure, and 3.4% for any distant failure with or without local or regional failure.
For patients with stage I/II group IIA tumors (n = 52), the 3-year cumulative incidence rate of local failure was 8.1%. Among the five patients with stage I/II group IIA tumors with local failure, there were one minor and two major RT protocol deviations. One of the five patients had a stage I group IIA vaginal primary and according to protocol guidelines did not receive RT, based on an assessment of complete response at second-look surgery. For patients with group III orbit tumors (n = 62), the 3-year cumulative incidence rate of local failure was 11.5%. Among the eight patients with group III orbit tumors with local failure, two developed progressive disease before week 13 RT, and one had a minor RT protocol deviation.
Toxicity
There were no unexpected grade 4 toxicities and no deaths resulting from toxicity. There were five cases of hepatopathy (< 2%). Two cases were classified as mild by protocol guidelines (total bilirubin ≤ 6 mg/dL, or weight gain ≤ 5%, and reversible hepatic dysfunction) and three as moderate (total bilirubin > 6 to < 20 mg/dL, or weight gain > 5%, or clinical or image-documented ascites, and reversible hepatic dysfunction). According to protocol, patients with mild hepatopathy received half doses of dactinomycin and cyclophosphamide in the next chemotherapy cycle but subsequently resumed full doses, and patients with moderate hepatopathy were removed from protocol therapy. All patients with hepatopathy recovered fully.
DISCUSSION
We designed ARST0331 to optimize survival, minimize toxicity, and simplify therapy for patients with low-risk RMS. Treatment changes in ARST0331 for subset-one low-risk patients compared with the predecessor study D9602 for comparable patients included reduced therapy duration (45 to 22 weeks) and addition of modest-dose cyclophosphamide (total cumulative dose, 4.8 g/m2) to VA. RT doses for patients with group IIA tumors (36 Gy) or group III orbit tumors (45 Gy) in ARST0331 were the same as in D9602 but lower than IRS-IV (41.4 or 50.4 to 59 Gy, respectively).3,4 The primary end point for the study was FFS, with the goal of preventing relapse and the subsequent burden of salvage therapy. Outcome was not compromised using this shorter-duration regimen that included lower-dose cyclophosphamide with VA, compared with expected results for subgroup A patients in D9602.
We included modest-dose cyclophosphamide in this trial to optimize FFS without significantly increasing toxicity. Three-year FFS was comparable for subset one of ARST0331 (89%; 95% CI, 85% to 92%) and subgroup A of D9602 (90%; 95% CI, 85% to 93%); therefore, the impact of cyclophosphamide on the outcome of this group of patients is not clear. It is possible that an FFS benefit is not apparent because ARST0331 was designed as a noninferiority study and not to detect improved outcome over expected by the addition of cyclophosphamide. It is also possible that cyclophosphamide offset any detrimental effect of shorter-duration VA therapy for subset-one patients. Other cooperative groups have tested shorter-duration VA therapy in a more restricted subset of low-risk patients (T1, N0, group I paratestis ERMS).13–15 It is not possible to reach firm conclusions regarding the impact of cyclophosphamide in ARST0331 based on these earlier reports because of their more restricted subset of low-risk patients.5,14,15
We expect that fertility will be preserved in most patients with a total cumulative cyclophosphamide dose of 4.8 g/m2 and that risks for myelodysplasia or secondary leukemia will be low.16,17 It will be necessary to conduct follow-up studies to establish the incidence of these complications. Cyclophosphamide can also complicate therapy by requiring additional hydration and myeloid growth factor support, both of which may be associated with inconvenience and increased cost. A cost analysis from the perspective of the health care system suggested ARST0331 subset-one therapy incurs fewer costs than D9602 subgroup A therapy.18
The RT doses used in this trial were identical to those used in D9602, but they were modestly reduced compared with IRS-IV.4,19 Local control rates seemed somewhat better in ARST0331 compared with D9602 for patients with group IIA tumors (8.1% in ARST0331 v 11.7% in D9602), supporting a role for cyclophosphamide in local tumor control of microscopic residual disease.4 The 3-year cumulative incidence of local failure was highest in ARST0331 among patients with group III orbit tumors (11.5%) and was similar to the 3-year cumulative incidence of local failure among patients with group III orbit tumors in D9602 (11.9%).4 Overall, 3-year local failure rates in ARST0331 may be somewhat higher than those in IRS-IV (group IIA: 8.1% in ARST0331 v 2% in IRS-IV; group III orbit: 11.5% in ARST0331 v 4% in IRS-IV), which may be the result of VAC (total cumulative cyclophosphamide dose, 26.4 g/m2) or VAC with higher RT doses.4,19 There were not enough patients with group II vaginal tumors (n = 1) enrolled onto subset one of ARST0331 to draw conclusions regarding the local therapy approach for these patients.
Our results support use of ARST0331 for patients with stage I/II group IIB/C (node positive) and stage II group II ERMS (n = 30). Reducing the total cumulative cyclophosphamide dose and shortening duration of therapy compared with VAC (total cumulative cyclophosphamide dose, 28.6 g/m2) as administered to these patients as part of subgroup B in D9602 would be expected to significantly reduce toxicity risks for these patients.4 There have been no second malignant neoplasms reported in D9602 or ARST0331 to date. Fertility was not assessed as part of either study.
We observed three recurrences ≥ 4.8 years from study entry among subset-one patients. Cumulative incidence rates for late recurrence at 5 or 10 years in IRS-III, -IV pilot, and -IV collectively were 2.4% and 2.7%, respectively.20 The rate of late recurrence in ARST0331 was approximately 1% and accounts for 9% of failures on the study. On the basis of the small number of cases, we do not believe it is necessary to modify follow-up guidelines that require surveillance imaging through the fourth year off therapy.
We believe that a balance between FFS, OS, and toxicity that favors quality of outcome should be achieved for low-risk patients with RMS. There were no unexpected or unacceptable acute toxicities reported in this study. Taken together, we believe the balance of FFS, OS, and toxicity may favor ARST0331 or D9602 over the more toxic IRS-IV regimen for subset-one low-risk patients with RMS. Identification of biologic features or biomarkers associated with treatment failure will be important for future novel therapeutic approaches or additional changes in treatment strategy. Simplification of staging evaluations for low-risk patients with RMS and establishment of standard treatment recommendations for low-risk RMS by site based on ARST0331 and earlier IRSG/COG studies represent future goals.21
Acknowledgment
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Glossary Terms
- rhabdomyosarcoma:
a malignant solid tumor arising from mesenchymal tissues, which typically differentiate to form striated muscle. Rhabdomyosarcoma is divided into two primary subtypes: the alveolar rhabdomyosarcoma, primarily arising in adolescents and young adults, and the embryonal rhabdomyosarcoma, predominantly affecting infants and children. It is one of the most frequently occurring soft-tissue sarcomas and the most common in children younger than age 15 years, accounting for 6% to 8% of all childhood cancers.
- overall survival:
the duration between random assignment and death.
- cumulative incidence:
a statistical measure of an event of interest (eg, relapse, death, second malignant neoplasm, a specific disease) occurring in a specified period of time in the population at risk. It is calculated using the formula: (number of new cases of the event of interest)/(total population at risk).
Footnotes
Supported in part by Grants No. CA-98543 and CA-98413 from the National Cancer Institute, Bethesda, MD.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: David O. Walterhouse, Alberto S. Pappo, Jane L. Meza, John C. Breneman, Andrea A. Hayes-Jordan, James R. Anderson, William H. Meyer, Douglas S. Hawkins
Administrative support: William H. Meyer
Collection and assembly of data: David O. Walterhouse, Alberto S. Pappo, Jane L. Meza, Andrea A. Hayes-Jordan, David M. Parham, James R. Anderson
Data analysis and interpretation: David O. Walterhouse, Alberto S. Pappo, Jane L. Meza, John C. Breneman, Andrea A. Hayes-Jordan, David M. Parham, Timothy P. Cripe, James R. Anderson, William H. Meyer
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on Intergroup Rhabdomyosarcoma Studies III and IV: The Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 2.Crist W, Gehan EA, Ragab AH, et al. The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 3.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 4.Raney RB, Walterhouse DO, Meza JL, et al. Results of Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;19:1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flamant F, Rodary C, Rey A, et al. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescences: Results of the second study of the International Society of Paediatric Oncology-MMT84. Eur J Cancer. 1998;34:1050–1062. doi: 10.1016/s0959-8049(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 6.Koscielniak E, Harms D, Henze G, et al. Results of treatment for soft tissue sarcoma in childhood and adolescences: A final report of the German Cooperative Soft Tissue Sarcoma Study, CWS-86. J Clin Oncol. 1999;17:3706–3719. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 7.Mandell L, Ghavimi F, Peretz T, et al. Radiocurability of microscopic disease in childhood rhabdomyosarcoma with radiation doses less than 4,000 cGy. J Clin Oncol. 1990;8:1536–1542. doi: 10.1200/JCO.1990.8.9.1536. [DOI] [PubMed] [Google Scholar]
- 8.Etcubanas E, Rao BN, Kun LE, et al. The impact of delayed surgery on radiotherapy dose and local control of rhabdomyosarcoma. Arch Surg. 1987;122:1451–1454. doi: 10.1001/archsurg.1987.01400240099018. [DOI] [PubMed] [Google Scholar]
- 9.Tefft M, Lindberg RD, Gehan EA. Radiation therapy combined with systemic chemotherapy of rhabdomyosarcoma in children. Natl Cancer Inst Monogr. 1981;56:75–81. [PubMed] [Google Scholar]
- 10.Walterhouse DO, Meza JL, Breneman JC, et al. Local control and outcome in children with localized vaginal rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:76–83. doi: 10.1002/pbc.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Simon R, Wittes RE. Methodologic guidelines for reports of clinical trials. Cancer Treat Rep. 1985;69:1–3. [PubMed] [Google Scholar]
- 13.Ferrari A, Bisogno G, Casanova M, et al. Paratesticular rhabdomyosarcoma: Report from the Italian and German Cooperative Group. J Clin Oncol. 2002;20:449–455. doi: 10.1200/JCO.2002.20.2.449. [DOI] [PubMed] [Google Scholar]
- 14.Stewart RJ, Martelli H, Oberlin O, et al. Treatment of children with nonmetastatic paratesticular rhabdomyosarcoma: Results of the Malignant Mesenchymal Tumors Studies (MMT 84 and MMT 89) of the International Society of Pediatric Oncology. J Clin Oncol. 2003;21:793–798. doi: 10.1200/JCO.2003.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Stevens MCG, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 16.Kenney LB, Laufer MR, Grant FD, et al. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell H, Swint JM, Lal L, et al. Cost minimization analysis of two treatment regimens for low-risk rhabdomyosarcoma in children: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2014;61:970–976. doi: 10.1002/pbc.24950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced dose radiotherapy for low-risk rhabdomyosarcoma: A report from the Children's Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83:720–726. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung L, Anderson JR, Donaldson SS, et al. Late events occurring five years or more after successful therapy for rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Eur J Cancer. 2004;40:1878–1885. doi: 10.1016/j.ejca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Weiss AR, Lyden ER, Anderson JR, et al. Histologic and clinical characteristics can guide staging evaluations for children and adolescents with rhabdomyosarcoma: A report from the Children's Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol. 2013;31:3226–3232. doi: 10.1200/JCO.2012.44.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]


