Abstract
Background
The extent of initial surgical resection has been identified as the strongest prognostic indicator for survival in child and adolescent meningioma. Given the paucity of data concerning long-term outcome, the authors undertook a meta-analysis to analyze morbidity in survivors of this disease.
Methods
Individual patient data were obtained from 19 case series published over the last 23 years through direct communication with the authors. Ordinal logistic regression models were used to assess the influence of risk factors on morbidity.
Results
Of 261 patients, 48% reported a completely normal life with no morbidity, and 25% had moderate/severe meningioma-associated morbidity at last follow-up. Multivariate analysis identified relapse as the only independent variable associated with an increased risk of morbidity (odds ratio, 4.02; 95% confidence interval, 2.11-7.65; P ≤ .001). Univariate analysis also revealed an increased risk for patients with neurofibromatosis (odds ratio, 1.90; 95% confidence interval, 1.04-3.48; P = .04). Subgroup analysis identified a higher incidence of morbidity among patients who had intracranial tumors with a skull base location compared with a nonskull base location (P ≤ .001). Timing at which morbidity occurred was available for 70 patients, with persistence of preoperative tumor-related symptoms in 67% and as a result of therapy in 20%.
Conclusions
The majority of survivors of child and adolescent meningioma had no or only mild long-term morbidity, whereas 25% had moderate/severe morbidity, with a significantly increased risk in patients with relapsed disease. In the majority, morbidity occurred as a consequence of the tumor itself, justifying aggressive surgery to achieve gross total resection. However, for patients with neurofibromatosis and skull base meningioma, a more cautious surgical approach should be reserved.
Keywords: morbidity, meningioma, child, adolescent, survivorship
Introduction
Central nervous system (CNS) tumors have the second highest frequency of all childhood cancers.1 With advances in neurosurgery, radiotherapy techniques, the use of combination chemotherapy, and improved supportive care, the last 40 years have witnessed a significant improvement in the survival of these children. However, the price of survival has not been inconsequential and has been accompanied by an increased burden of long-term morbidity. Subsequently, the long-term follow-up of survivors has become essential. Several collaborative groups have been established to study large childhood cancer survivorship cohorts with the objective of identifying, characterizing, and ultimately reducing such complications.2–5 Notably, 1 of the largest groups, the Childhood Cancer Survivor Study,3 excludes meningioma within its selection criteria.
Meningiomas constitute 2.2% of all CNS tumors in children and adolescents.6 We previously undertook a multivariate meta-analysis of individual patient data and identified the extent of initial surgical resection as the strongest prognostic indicator for relapse-free and overall survival in child and adolescent meningioma; and we concluded that aggressive surgical management, to achieve gross total resection, was the optimal initial treatment of choice.6 Given our previous findings and the paucity of data concerning the long-term outcome in survivors of child and adolescent meningioma, we sought to identify the morbidity profile associated with the treatment of this group of patients. We undertook a pooled analysis, using the PRISMA reporting guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses),7 of every child and adolescent meningioma case series published over the last 23 years, with the aim of defining the overall morbidity in survivors of this disease.
Materials and Methods
Search Strategy and Selection Criteria
We searched PubMed, Medline, and Embase for articles published from January 1989 to January 2013 that included the following terms: “meningioma,” “childhood,” “pediatric,” “paediatric,” and “adolescent.” All single case reports and case series of radiation-associated meningioma were excluded. No study was excluded on the basis of language. Two reviewers independently assessed full text copies of all case series for entry into the meta-analysis (R.S.K. and N.G.G.). Additional studies were traced by checking the references of the selected publications. The search strategy has been previously described in detail.6
Figure 1 illustrates the selection of studies and patient data. Forty-six studies initially were identified,8–53 5 of which were excluded49–53 because they reported duplicate data. Morbidity was reported in 21 studies,8–28 all of which were exclusive to children and adolescents. Ten patients who had meningiomas that could not be categorized histologically according to the 2007 World Health Organization (WHO) grading system54 (eg, angioblastic, sclerosing) or that were of unknown histology were excluded. Eleven patients with radiation-induced meningioma, 24 patients for whom outcome was unknown, and 3 patients24 for whom there were duplicate data in another included publication25 also were excluded. Of the remaining 326 patients, 52 had died, leaving 274 patients who were eligible for the selection of individual patient data.
Figure 1.
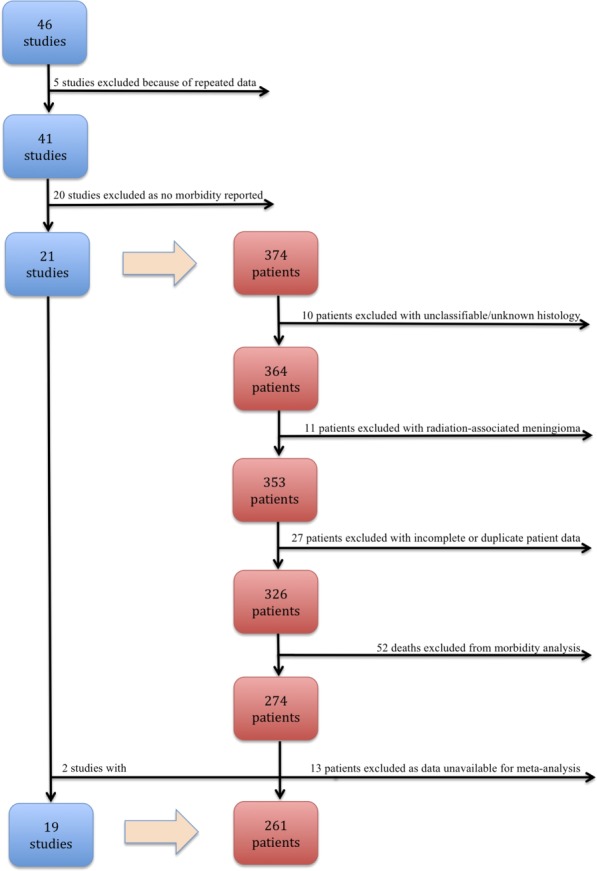
The selection of studies and patient data are illustrated.
Selection of Individual Patient Data
Corresponding authors of the 21 eligible studies were invited to submit their individual patient data. Variables for which data were requested included the extent of initial surgery (subtotal resection, gross total resection), use of upfront radiotherapy (no, yes), tumor grade according to the 2007 WHO classification system (WHO grade I, II, or III),54 tumor location (supratentorial, infratentorial, spinal), neurofibromatosis (no, yes), patient sex (male, female), patient age at diagnosis, and whether the patient suffered a relapse (defined as either progression or recurrence; no, yes). To allow a comparison with previously published data,6 identical categories were selected and included within the multivariate analysis for each variable. Data obtained for clinical endpoints included descriptions of reversible deficits suffered that were no longer apparent at last follow-up, including immediate postoperative complications (defined by the Ibanez classification system55 as occurring within 30 days of the procedure) that were subsequently treated or resolved; any long-term morbidity that existed at last follow-up; the timing at which the morbidity occurred in relation to surgery; and the overall length of follow-up.
Nineteen studies8–26 provided individual patient data for 261 patients. Data returned were verified by independent assessment with the published study by the 2 reviewers. Clarification and confirmation were sought from the originating center for any discrepancies identified. Morbidity was graded independently based on severity into 3 categories—none, mild, or moderate/severe (Table 1)—by the 2 reviewers. If the assessments differed, then a consensus opinion was determined in consultation with a third reviewer (C.H.C.).
Table 1.
Classification of Morbidity According to Severity
| Morbidity Category | Inclusion Criteria |
|---|---|
| No morbidity | KPS 100 |
| GOS 5 with no deficit | |
| No morbidity at last follow-up | |
| Mild morbidity | KPS 80–90 |
| GOS 5 with deficit | |
| Morbidity described as “slight” or “mild” at last follow-up | |
| Moderate/severe morbidity | KPS ≤70GOS ≤4 |
| Persistent neurologic deficit at last follow-up, eg, visual deficit, hemiparesis, seizure disorder |
Abbreviations: GOS, Glasgow Outcome Score; KPS, Karnofsky performance status.
Statistical Analyses
Patients were included in the meta-analysis if complete information for every variable requested was returned. Data were collated and are presented as number (percentage), mean and range, as appropriate for the type of data. To assess whether these patients provided an appropriate representation of the previously published cohort,6 the survivors from the previously published cohort who were included in the current study were compared with those who were excluded using the chi-square test for sex, location, histology, extent of surgery, and relapse and using the t-test for age.
Ordinal logistic regression models using the proportional odds assumption were used to assess the influence of prognostic factors on morbidity. Univariate and multivariate odds ratios (ORs) were calculated for each prognostic factor. Subgroup analysis to assess the effect of intracranial tumor location (recategorized as skull base vs nonskull base) on morbidity was performed using the chi-square test. P values < .05 were considered statistically significant. Analyses were done with STATA statistical software (version 12; Stata Corporation, College Station, Tex).
Results
In total, there were 261 patients from 19 studies. Patient characteristics are listed in Table 2. We determined that this study provided an appropriate representation of the previously published patients,6 because no significant differences were detected between data sets for the variables tested. The mean follow-up was 7.11 years. At last follow-up, 126 patients (48%) were reported as leading a completely normal life with no meningioma-associated morbidity or other comorbidity. There were 116 patients (44.5%) suffering from meningioma-associated morbidity, with 51 cases (19.5%) classified as mild and 65 (25%) classified as moderate/severe. The majority of patients with mild morbidity had minimal reductions in their Karnofsky performance status (KPS) or Glasgow Outcome Score (GOS) (KPS 90, 8.4%; KPS 80, 6.5%; and GOS 5 with deficit, 3.4%). The most frequently occurring morbidities in the moderate/severe category included visual deficit (6.9%), cranial nerve deficit (5.4%), seizure disorder (4.6%), GOS 4 (4.6%), and motor deficits (3.4%), such as hemiparesis and specific limb weakness (Fig. 2).
Table 2.
Patient Characteristics
| Characteristic | No. of Patients (n = 261) |
|---|---|
| Sex | |
| Male | 150 |
| Female | 111 |
| Age, y | |
| <3 | 13 |
| 3 to <12 | 90 |
| ≥12 | 158 |
| Location | |
| Supratentorial | 204 |
| Infratentorial | 33 |
| Spinal | 24 |
| Histology: WHO grade | |
| I | 200 |
| II | 41 |
| III | 20 |
| Neurofibromatosis | |
| Yes | 44 |
| No | 217 |
| Initial surgery | |
| Gross total resection | 204 |
| Subtotal resection | 57 |
| Upfront radiotherapy | |
| Yes | 27 |
| No | 234 |
| Relapse | |
| Yes | 48 |
| No | 213 |
Abbreviations: WHO, World Health Organization.
Figure 2.
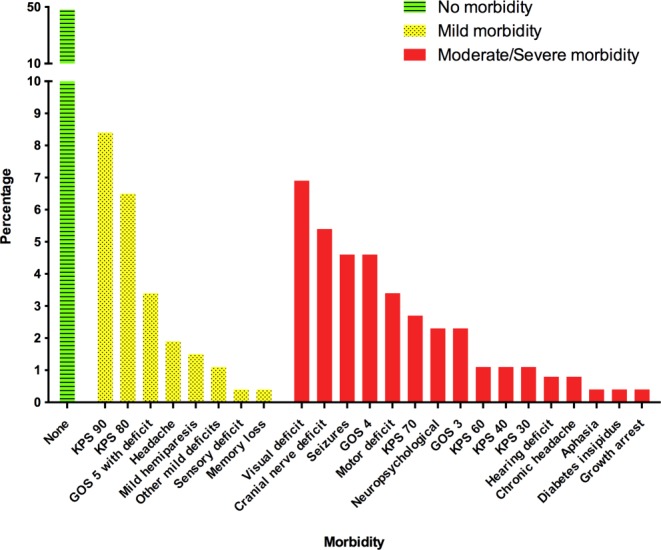
Morbidity is illustrated among survivors of childhood and adolescent meningioma. KPS indicates Karnofsky performance status; GOS, Glasgow Outcome Score.
Meningioma relapse was identified as the only independent risk factor for long-term morbidity on multivariate analysis (Table 3), with a 4-fold increased risk in patients who relapsed compared with patients who did not relapse (OR, 4.02; 95% confidence interval [CI], 2.11-7.65; P ≤ .001). On univariate analysis (Table 3), neurofibromatosis was associated with an increased risk of long-term morbidity (OR, 1.90; 95% CI, 1.04-3.48; P = .04). Subgroup analysis of intracranial tumor location revealed a significantly higher incidence of morbidity for survivors with skull base tumors (61%; n = 45 of 74 patients) versus those with nonskull base tumors (34%; n = 53 of 155 patients; P ≤ .001).
Table 3.
Univariate and Multivariate Ordinal Logistic Regression Results for Individual Patient Data: Morbidity According to Prognostic Factor
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Prognostic Factor | OR | 95% CI | P | OR | 95% CI | P |
| Age, y | ||||||
| <3 | 1 | 1 | ||||
| 3–<12 | 0.51 | 0.15–1.67 | .27 | 0.44 | 0.13–1.51 | .19 |
| ≥12 | 0.68 | 0.21–2.16 | .51 | 0.60 | 0.18–2.00 | .41 |
| Sex | ||||||
| Female | 1 | 1 | ||||
| Male | 1.22 | 0.76–1.96 | .42 | 1.16 | 0.70–1.92 | .55 |
| Relapse | ||||||
| No | 1 | 1 | ||||
| Yes | 4.32 | 2.34–7.97 | < .001 | 4.02 | 2.11–7.65 | < .001 |
| WHO grade | ||||||
| I | 1 | 1 | ||||
| II | 1.54 | 0.84–2.85 | .17 | 1.58 | 0.82–3.03 | .17 |
| III | 0.73 | 0.28–1.87 | .51 | 0.69 | 0.25–1.91 | .48 |
| Neurofibromatosis | ||||||
| No | 1 | 1 | ||||
| Yes | 1.90 | 1.04–3.48 | .04 | 1.52 | 0.79–2.92 | .21 |
| Location | ||||||
| Supratentorial | 1 | 1 | ||||
| Infratentorial | 1.08 | 0.54–2.15 | .82 | 1.02 | 0.49–2.09 | .97 |
| Spinal | 1.81 | 0.86–3.79 | .12 | 1.23 | 0.55–2.75 | .61 |
Abbreviations: CI, confidence interval; OR, odds ratio; WHO, World Health Organization.
The timing at which the morbidity occurred was available for 70 of the 116 patients (60%) who suffered from meningioma-associated morbidity. In the majority (67%; n = 47 of 70 patients), the morbidity was present before surgery, occurring as a consequence of the meningioma with subsequent failure of recovery from symptoms after therapeutic intervention and at last follow-up. In 20% (n = 14 of 70 patients), the morbidity occurred as a direct result of treatment. Of these patients, cranial nerve injury after surgery for skull base meningioma accounted for 64% (n = 9 of 14 patients). The remainder (n = 5) were individual patients with growth arrest secondary to radiosurgery, cognitive impairment after radiotherapy, severe neurologic disability after surgery for recurrence, chronic headache, and new-onset seizure disorder. The small number of patients who suffered morbidity as a consequence of therapy precluded a meaningful statistical analysis of therapy as a risk factor. In 13% (n = 9 of 70 patients), morbidity was related to multiple craniospinal tumors in patients with neurofibromatosis.
Information regarding the presence of reversible postoperative deficits was available for 169 patients, and the occurrence rate was 22% (n = 38 of 169 patients). Reversible postoperative deficits comprised infections (n = 13), including meningitis and osteomyelitis; reversible motor deficits (n = 8); seizure disorders, which subsequently resolved (n = 7); intracranial hemorrhage/hematoma (n = 4); cerebrospinal fluid leak (n = 2); hydrocephalus (n = 2); pseudomeningocele (n = 1); and urinary retention (n = 1). When only considering the medical complications in these patients in addition to those that occurred in the 52 patients who died, the overall rate of medical complications was 8.1% (n = 18 of 221 patients) and included infections (n = 14), postoperative multiorgan failure (n = 3), and urinary retention (n = 1).
Discussion
Meningioma is the most frequently occurring CNS tumor in adults,56 and several studies have reported an increased risk of morbidity in survivors.57–61 In contrast, child and adolescent meningioma is rare, with the majority of reports for this condition retrospective in nature and restricted to single cases and case series. Consequently, there is a paucity of literature regarding long-term outcomes in survivors. To our knowledge, the current individual patient meta-analysis represents the largest study to date of morbidity specific to survivors of child and adolescent meningioma.
This study reveals that, although the majority of children and adolescents with meningioma have no or only mild residual morbidity after treatment, 25% of survivors have moderate/severe morbidity. This compares favorably with adult meningioma survivors, in which 67% have been reported to suffer from long-term neurologic sequelae.57 The lower incidence revealed in our study may be explained by the increased ability of children to undergo neurologic recovery compared with adults62 and/or the increased risk of comorbidities associated with age and lifestyle in adults. The incidence of moderate/severe morbidity identified in this study is similar to that reported in a previous study of long-term neurologic morbidity in a heterogeneous group of patients with low-grade childhood brain tumors who underwent surgery alone; in that study, 35% of patients had persistent moderate or severe neurologic deficits at last follow-up.63 Historically, the focus of survivorship has been related primarily to the sequelae of chemotherapy and radiotherapy. Because aggressive surgical resection is the recommended optimal therapeutic strategy for child and adolescent meningioma,6 our study further highlights the importance of monitoring and time-appropriate intervention for late effects in survivors of childhood brain tumors for whom surgery is the mainstay of treatment.
Although we have demonstrated an appreciable morbidity profile in survivors of child and adolescent meningioma, morbidity attributable to therapy was low. The rate of clinically detected, serious medical complications after resection of child and adolescent meningioma was low (8.1%) and was comparable to the reported incidence in adults (6.8%).64 In adults, a wide spectrum of serious medical complications has been reported after meningioma surgery64; however, in our analyses, infection was the primary cause of a serious postsurgical medical complication (78%; n = 14 of 18). Given this finding, appropriate antibiotic prophylaxis should be instigated perioperatively,65 and immediate presumptive treatment with broad-spectrum antibiotics should be undertaken if there is any clinical suspicion of infection after surgery for child and adolescent meningioma. For patients in whom timing at which the morbidity occurred was available, morbidity predominantly occurred as a direct consequence of the meningioma, was present before surgery, and the patient failed to recover from the symptoms. One possible explanation may be that the majority of meningiomas have an indolent growth pattern over a period of years, with consequent prolonged pressure exerted by the tumor on intracranial structures resulting in irreversible neurologic deficits before diagnosis. Indeed, in adults with untreated, radiologically suspected, WHO grade I meningiomas, neurocognitive deficits and reductions in health-related quality of life have been demonstrated, suggesting that these effects occur as a result of the tumor itself or as a consequence of tumor-related edema.60 These findings, in combination with the previously demonstrated low perioperative mortality (3%; n = 20 of 664 patients),6 support the recommendation for initial aggressive surgical management for child and adolescent meningioma.
However, we have identified several caveats for which a more cautious surgical approach should be considered. The first is for meningioma located at the skull base. Subgroup analysis revealed a significantly higher incidence of morbidity among patients with skull base tumors overall; and it is noteworthy that, of the 14 patients who had therapy-related morbidity, 9 developed a cranial nerve deficit secondary to surgical excision of a skull base meningioma. The proximity of the cranial nerves to the skull base, poor accessibility, more complex approach, and dural attachments that cannot be sacrificed increase the complexity and duration of surgery for skull base meningioma and, ultimately, the ability to perform gross total resection.26 For these patients, the risk of significant long-term neurologic morbidity needs to be carefully considered against the benefit of aggressive surgery to achieve gross total resection. If the risk of morbidity precludes gross total resection for skull base meningioma, then it may be more beneficial to perform a subtotal resection and to reserve repeat surgery for tumor progression.
Second, caution should be undertaken for patients who have neurofibromatosis. Meningiomas are the second most frequently occurring tumors in patients with neurofibromatosis type 2,66 whereas they reportedly occur at the same incidence as the general population in patients with neurofibromatosis type 1.67 Several studies have demonstrated that a large proportion of meningiomas in patients with neurofibromatosis type 2 exhibit minimal or saltatory growth on serial imaging.68,69 Both types of neurofibromatosis predispose patients to multiple craniospinal tumors, and the natural history of the disease has to be balanced against the risk of additional long-term morbidity with aggressive surgical management. Our results have indicated an increase in meningioma-associated morbidity among patients with neurofibromatosis, with the effect persisting at a reduced level after adjustment for covariates. Our findings add further support to the recommendation that surgery should be reserved for symptom-producing tumors,69 with the overall neurosurgical goal of achieving symptom control rather than radical resection in patients with neurofibromatosis.
Our study demonstrated a significant increase in morbidity for patients who relapsed. This may occur because of the cumulative effect of pressure on intracranial structures by recurrent/progressive tumor and/or repeated therapeutic intervention resulting in irreversible neurologic deficits. In our analyses, only 3 patients developed morbidity as a result of repeat surgery after a relapse, and 2 of those patients had meningiomas located at the skull base. Patients with meningioma relapse are challenging, because this likely signifies recalcitrant disease with potential for a higher proliferative index.70 Given our findings and taking into consideration the aforementioned caveats, we advocate aggressive surgical management for children and adolescents with meningioma relapse.
This study has several limitations, the most notable being that it is retrospective. In addition, there is no standardized classification system for morbidity, which has resulted in heterogeneous recording and reporting by different authors. Furthermore, morbidity was reported as an ancillary finding within the majority of studies for child and adolescent meningioma. Indeed, only 21 of the 41 studies (51%) that were eligible for entry reported morbidity data. Moreover, examination of long-term sequelae, such as neurocognitive and health-related quality of life assessment, was not undertaken. To our knowledge, only 1 detailed case report exists documenting time-dependent recovery of motor and cognitive functions in a boy aged 6 years who had a right frontal lobe atypical meningioma.71 The paucity of data regarding these outcome measures is in contrast to adult meningioma survivors, in which several studies have demonstrated that significant numbers of patients experience impaired cognition and worse health-related quality of life compared with healthy matched controls.58–61 Because child and adolescent meningioma is rare, the number of patients available for study is small, which may explain the lack of statistical significance for several variables associated with morbidity. The small number of patients who suffered morbidity as a consequence of therapy precluded meaningful statistical analysis for therapy as a risk factor. For the same reason, we were unable to distinguish between the different types of neurofibromatoses. A feasible approach to overcome the limitations of this analysis and definitively assess the extent of morbidity and risk factors pertaining to long-term sequelae in survivors of child and adolescent meningioma would be to establish a central registry with standardized international reporting of morbidity data.
In conclusion, in contrast to adults, the majority of survivors of child and adolescent meningioma had no or only mild long-term morbidity. However, 25% of patients had moderate/severe long-term neurologic morbidity. Given that the risk of morbidity is significantly increased in patients with relapsed disease and that, in the majority of patients, morbidity occurs as a consequence of the tumor itself, we conclude that aggressive surgery to achieve gross total resection is warranted. However, for children and adolescents with neurofibromatosis and for those with meningioma of the skull base, a more cautious surgical approach should be observed.
Funding Support
Rishi S. Kotecha is supported by the Princess Margaret Hospital Foundation. Nicholas G. Gottardo is supported by the John Lillie Cancer Research Fellowship and the Raine Clinical Fellowship.
Conflict of Interest Disclosures
Rishi S. Kotecha and Nicholas G. Gottardo have received grants from Pfizer unrelated to the current work.
References
- Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Kuehni CE, Rueegg CS, Michel G, et al. Cohort profile: the Swiss Childhood Cancer Survivor Study. Int J Epidemiol. 2012;41:1553–1564. doi: 10.1093/ije/dyr142. [DOI] [PubMed] [Google Scholar]
- Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MM, Lancashire ER, Winter DL, et al. The British Childhood Cancer Survivor Study: objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50:1018–1025. doi: 10.1002/pbc.21335. [DOI] [PubMed] [Google Scholar]
- Shaw AK, Morrison HI, Speechley KN, et al. The late effects study: design and subject representativeness of a Canadian, multi-centre study of late effects of childhood cancer. Chronic Dis Can. 2004;25:119–126. [PubMed] [Google Scholar]
- Kotecha RS, Pascoe EM, Rushing EJ, et al. Meningiomas in children and adolescents: a meta-analysis of individual patient data. Lancet Oncol. 2011;12:1229–1239. doi: 10.1016/S1470-2045(11)70275-3. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Ferrante L, Acqui M, Artico M, Mastronardi L, Rocchi G, Fortuna A. Cerebral meningiomas in children. Childs Nerv Syst. 1989;5:83–86. doi: 10.1007/BF00571115. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen M, Scheie D, Muller T, Lundar T, Helseth E. Neurosurgical treatment of meningiomas in children and young adults. Childs Nerv Syst. 2001;17:719–723. doi: 10.1007/s00381-001-0516-5. [DOI] [PubMed] [Google Scholar]
- Turgut M, Ozcan OE, Bertan V. Meningiomas in childhood and adolescence: a report of 13 cases and review of the literature. Br J Neurosurg. 1997;11:501–507. doi: 10.1080/02688699745655. [DOI] [PubMed] [Google Scholar]
- Choux M, Lena G, Genitori L, Azambuja N, Cunha C, Dechambenoit G. Meningiomas in children. In: Schmidek HH, editor. Meningiomas and Their Surgical Management. Philadelphia, PA: WB Saunders; 1991. pp. 93–102. [Google Scholar]
- Im SH, Wang KC, Kim SK, et al. Childhood meningioma: unusual location, atypical radiological findings, and favorable treatment outcome. Childs Nerv Syst. 2001;17:656–662. doi: 10.1007/s003810100507. [DOI] [PubMed] [Google Scholar]
- Zwerdling T, Dothage J. Meningiomas in children and adolescents. J Pediatr Hematol Oncol. 2002;24:199–204. doi: 10.1097/00043426-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Rochat P, Johannesen HH, Gjerris F. Long-term follow up of children with meningiomas in Denmark: 1935 to 1984. J Neurosurg. 2004;100:179–182. doi: 10.3171/ped.2004.100.2.0179. [DOI] [PubMed] [Google Scholar]
- Tufan K, Dogulu F, Kurt G, Emmez H, Ceviker N, Baykaner MK. Intracranial meningiomas of childhood and adolescence. Pediatr Neurosurg. 2005;41:1–7. doi: 10.1159/000084858. [DOI] [PubMed] [Google Scholar]
- Baumgartner JE, Sorenson JM. Meningioma in the pediatric population. J Neurooncol. 1996;29:223–228. doi: 10.1007/BF00165652. [DOI] [PubMed] [Google Scholar]
- Alexiou GA, Mpairamidis E, Psarros A, Sfakianos G, Prodromou N. Intracranial meningiomas in children: report of 8 cases. Pediatr Neurosurg. 2008;44:373–375. doi: 10.1159/000149903. [DOI] [PubMed] [Google Scholar]
- Menon G, Nair S, Sudhir J, Rao BR, Mathew A, Bahuleyan B. Childhood and adolescent meningiomas: a report of 38 cases and review of literature. Acta Neurochir (Wien) 2009;151:239–244. doi: 10.1007/s00701-009-0206-8. discussion 244. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang R, Mao Y, Wang Y. Childhood and juvenile meningiomas. Childs Nerv Syst. 2009;25:1571–1580. doi: 10.1007/s00381-009-0964-x. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao J. Intracranial meningiomas of childhood and adolescence: report of 34 cases with follow-up. Childs Nerv Syst. 2009;25:1411–1417. doi: 10.1007/s00381-009-0949-9. [DOI] [PubMed] [Google Scholar]
- Hung PC, Wang HS, Chou ML, Sun PC, Huang SC. Intracranial meningiomas in childhood. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1994;35:495–501. [PubMed] [Google Scholar]
- Mandai K, Tamaki N, Kurata H, Eguchi T. The clinical analysis of pediatric meningioma: 5 cases [article in Japanese] No Shinkei Geka. 1997;25:131–136. [PubMed] [Google Scholar]
- Santos MV, Furlanetti L, Valera ET, Brassesco MS, Tone LG, de Oliveira RS. Pediatric meningiomas: a single-center experience with 15 consecutive cases and review of the literature. Childs Nerv Syst. 2012;28:1887–1896. doi: 10.1007/s00381-012-1823-8. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Jiang CC, Zhao L, Gong Y, Hu J, Chen H. Clinical features and treatment of World Health Organization grade II and III meningiomas in childhood: report of 23 cases. J Neurosurg Pediatr. 2012;10:423–433. doi: 10.3171/2012.7.PEDS12179. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Zeng XW, Zhang BY, et al. Spinal meningioma in childhood: clinical features and treatment. Childs Nerv Syst. 2012;28:129–136. doi: 10.1007/s00381-011-1570-2. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Neidert MC, Grotzer MA, Krayenbuhl N, Bozinov O. Surgical resection of pediatric skull base meningiomas. Childs Nerv Syst. 2013;29:83–87. doi: 10.1007/s00381-012-1906-6. [DOI] [PubMed] [Google Scholar]
- Maranhao-Filho P, Campos JC, Lima MA. Intracranial meningiomas in children: ten-year experience. Pediatr Neurol. 2008;39:415–417. doi: 10.1016/j.pediatrneurol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Teixidor P, Guillen A, Cruz O, Costa JM. Intracranial meningiomas in children: report of 10 cases [article in Spanish] Neurocirugia (Astur) 2008;19:434–439. [PubMed] [Google Scholar]
- Davidson GS, Hope JK. Meningeal tumors of childhood. Cancer. 1989;63:1205–1210. doi: 10.1002/1097-0142(19890315)63:6<1205::aid-cncr2820630627>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Perilongo G, Sutton LN, Goldwein JW, et al. Childhood meningiomas. Experience in the modern imaging era. Pediatr Neurosurg. 1992;18:16–23. doi: 10.1159/000120637. [DOI] [PubMed] [Google Scholar]
- Glasier CM, Husain MM, Chadduck W, Boop FA. Meningiomas in children: MR and histopathologic findings. AJNR Am J Neuroradiol. 1993;14:237–241. [PMC free article] [PubMed] [Google Scholar]
- Darling CF, Byrd SE, Reyes-Mugica M, et al. MR of pediatric intracranial meningiomas. AJNR Am J Neuroradiol. 1994;15:435–444. [PMC free article] [PubMed] [Google Scholar]
- Germano IM, Edwards MS, Davis RL, Schiffer D. Intracranial meningiomas of the first two decades of life. J Neurosurg. 1994;80:447–453. doi: 10.3171/jns.1994.80.3.0447. [DOI] [PubMed] [Google Scholar]
- Sheikh BY, Siqueira E, Dayel F. Meningioma in children: a report of nine cases and a review of the literature. Surg Neurol. 1996;45:328–335. doi: 10.1016/0090-3019(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Mallucci CL, Parkes SE, Barber P, et al. Paediatric meningeal tumours. Childs Nerv Syst. 1996;12:582–588. doi: 10.1007/BF00261651. discussion 589. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Kim SS, Kim IO, et al. MRI of primary meningeal tumours in children. Neuroradiology. 1999;41:512–516. doi: 10.1007/s002340050794. [DOI] [PubMed] [Google Scholar]
- Amirjamshidi A, Mehrazin M, Abbassioun K. Meningiomas of the central nervous system occurring below the age of 17: report of 24 cases not associated with neurofibromatosis and review of literature. Childs Nerv Syst. 2000;16:406–416. doi: 10.1007/s003819900205. [DOI] [PubMed] [Google Scholar]
- Demirtas E, Ersahin Y, Yilmaz F, Mutluer S, Veral A. Intracranial meningeal tumours in childhood: a clinicopathologic study including MIB-1 immunohistochemistry. Pathol Res Pract. 2000;196:151–158. doi: 10.1016/S0344-0338(00)80095-3. [DOI] [PubMed] [Google Scholar]
- Perry A, Giannini C, Raghavan R, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60:994–1003. doi: 10.1093/jnen/60.10.994. [DOI] [PubMed] [Google Scholar]
- Rushing EJ, Olsen C, Mena H, et al. Central nervous system meningiomas in the first two decades of life: a clinicopathological analysis of 87 patients. J Neurosurg. 2005;103:489–495. doi: 10.3171/ped.2005.103.6.0489. [DOI] [PubMed] [Google Scholar]
- Greene S, Nair N, Ojemann JG, Ellenbogen RG, Avellino AM. Meningiomas in children. Pediatr Neurosurg. 2008;44:9–13. doi: 10.1159/000110656. [DOI] [PubMed] [Google Scholar]
- Arivazhagan A, Devi BI, Kolluri SV, Abraham RG, Sampath S, Chandramouli BA. Pediatric intracranial meningiomas—do they differ from their counterparts in adults? Pediatr Neurosurg. 2008;44:43–48. doi: 10.1159/000110661. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li F, Zhu S, Liu M, Wu C. Clinical features and treatment of meningiomas in children: report of 12 cases and literature review. Pediatr Neurosurg. 2008;44:112–117. doi: 10.1159/000113112. [DOI] [PubMed] [Google Scholar]
- Mehta N, Bhagwati S, Parulekar G. Meningiomas in children: a study of 18 cases. J Pediatr Neurosci. 2009;4:61–65. doi: 10.4103/1817-1745.57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhdar F, Arkha Y, El Ouahabi A, et al. Intracranial meningioma in children: different from adult forms? A series of 21 cases. Neurochirurgie. 2010;56:309–314. doi: 10.1016/j.neuchi.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Vij M, Mehrotra A, Jaiswal AK, Srivastava AK, Behari S. A clinicopathological and neuroradiological study of paediatric meningioma from a single centre. J Clin Neurosci. 2011;18:1084–1089. doi: 10.1016/j.jocn.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Pinto PS, Huisman TA, Ahn E, et al. Magnetic resonance imaging features of meningiomas in children and young adults: a retrospective analysis. J Neuroradiol. 2012;39:218–226. doi: 10.1016/j.neurad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Thuijs NB, Uitdehaag BM, Van Ouwerkerk WJ, van der Valk P, Vandertop WP, Peerdeman SM. Pediatric meningiomas in the Netherlands 1974–2010: a descriptive epidemiological case study. Childs Nerv Syst. 2012;28:1009–1015. doi: 10.1007/s00381-012-1759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante L, Acqui M, Artico M, Mastronardi L, Fortuna A. Paediatric intracranial meningiomas. Br J Neurosurg. 1989;3:189–196. doi: 10.3109/02688698909002794. [DOI] [PubMed] [Google Scholar]
- Caroli E, Russillo M, Ferrante L. Intracranial meningiomas in children: report of 27 new cases and critical analysis of 440 cases reported in the literature. J Child Neurol. 2006;21:31–36. doi: 10.1177/08830738060210010801. [DOI] [PubMed] [Google Scholar]
- Schut L, Canady AI, Sutton LN, Bruce DA. Meningeal tumors in children. 1983. Pediatr Neurosurg. 1994;20:207–212. doi: 10.1159/000120790. discussion 213. [DOI] [PubMed] [Google Scholar]
- Hope JK, Armstrong DA, Babyn PS, et al. Primary meningeal tumors in children: correlation of clinical and CT findings with histologic type and prognosis. AJNR Am J Neuroradiol. 1992;13:1353–1364. [PMC free article] [PubMed] [Google Scholar]
- Erdincler P, Lena G, Sarioglu AC, Kuday C, Choux M. Intracranial meningiomas in children: review of 29 cases. Surg Neurol. 1998;49:136–140. doi: 10.1016/s0090-3019(97)00343-1. discussion 140–131. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landriel Ibanez FA, Hem S, Ajler P, et al. A new classification of complications in neurosurgery. World Neurosurg. 2011;75:709–715. doi: 10.1016/j.wneu.2010.11.010. discussion 604–611. [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14:658–666. doi: 10.1093/neuonc/nos013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuizen D, Klein M, Stalpers LJ, Leenstra S, Heimans JJ, Reijneveld JC. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84:271–278. doi: 10.1007/s11060-007-9366-7. [DOI] [PubMed] [Google Scholar]
- Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80:910–915. doi: 10.1136/jnnp.2007.138925. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuizen D, Ambachtsheer N, Heimans JJ, Reijneveld JC, Peerdeman SM, Klein M. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113:433–440. doi: 10.1007/s11060-013-1132-4. [DOI] [PubMed] [Google Scholar]
- Miao Y, Lu X, Qiu Y, Jiang J, Lin Y. A multivariate analysis of prognostic factors for health-related quality of life in patients with surgically managed meningioma. J Clin Neurosci. 2010;17:446–449. doi: 10.1016/j.jocn.2009.07.111. [DOI] [PubMed] [Google Scholar]
- Niedzwecki CM, Marwitz JH, Ketchum JM, Cifu DX, Dillard CM, Monasterio EA. Traumatic brain injury: a comparison of inpatient functional outcomes between children and adults. J Head Trauma Rehabil. 2008;23:209–219. doi: 10.1097/01.HTR.0000327253.61751.29. [DOI] [PubMed] [Google Scholar]
- Sonderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Muller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003;21:1347–1351. doi: 10.1200/JCO.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Rutkowski MJ, Shangari G, et al. Risk factors for the development of serious medical complications after resection of meningiomas. Clinical article. J Neurosurg. 2011;114:697–704. doi: 10.3171/2010.6.JNS091974. [DOI] [PubMed] [Google Scholar]
- Barker FG., 2nd Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2007;60:887–894. doi: 10.1227/01.NEU.0000255425.31797.23. discussion 887–894. [DOI] [PubMed] [Google Scholar]
- Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neurooncol. 2010;99:341–347. doi: 10.1007/s11060-010-0339-x. [DOI] [PubMed] [Google Scholar]
- Goutagny S, Bah AB, Henin D, et al. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: clinical, radiological, and molecular features. Neuro Oncol. 2012;14:1090–1096. doi: 10.1093/neuonc/nos129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks MS, Butman JA, Kim HJ, et al. Long-term natural history of neurofibromatosis type 2-associated intracranial tumors. J Neurosurg. 2012;117:109–117. doi: 10.3171/2012.3.JNS111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno A, Fujimaki T, Sasaki T, et al. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol. 1996;91:504–510. doi: 10.1007/s004010050458. [DOI] [PubMed] [Google Scholar]
- Carlsson G, Hufnagel M, Jansen O, Claviez A, Nabavi A. Rapid recovery of motor and cognitive functions after resection of a right frontal lobe meningioma in a child. Childs Nerv Syst. 2010;26:105–111. doi: 10.1007/s00381-009-0984-6. [DOI] [PubMed] [Google Scholar]


