Abstract
Background
Neurosurgical resection and whole-brain radiation therapy (WBRT) are accepted treatments for single and oligometastatic cancer to the brain. To avoid the decline in neurocognitive function (NCF) linked to WBRT, the authors conducted a prospective, multicenter, phase 2 study to determine whether surgery and carmustine wafers (CW), while deferring WBRT, could preserve NCF and achieve local control (LC).
Methods
NCF and LC were measured in 59 patients who underwent resection and received CW for a single (83%) or dominant (oligometastatic, 2 to 3 lesions) metastasis and received stereotactic radiosurgery (SRS) for tiny nodules not treated with resection plus CW. Preservation of NCF was defined as an improvement or a decline ≤1 standard deviation from baseline in 3 domains: memory, executive function, and fine motor skills, evaluated at 2-month intervals.
Results
Significant improvements in executive function and memory occurred throughout the 1-year follow-up. Preservation or improvement of NCF occurred in all 3 domains for the majority of patients at each of the 2-month intervals. NCF declined in only 1 patient. The chemowafers were well tolerated, and serious adverse events were reversible. There was local recurrence in 28% of the patients at 1-year follow-up.
Conclusions
Patients with brain metastases had improvements in their cognitive trajectory, especially memory and executive function, after treatment with resection plus CW. The rate of LC (78%) was comparable to historic rates of surgery with WBRT and superior to reports of WBRT alone. For patients who undergo resection for symptomatic or large-volume metastasis or for tissue diagnosis, the addition of CW can be considered as an option.
Keywords: brain metastases, carmustine wafers, neurocognitive function, stereotactic radiosurgery, whole brain radiotherapy
Introduction
Brain metastases affect 25% to 45% of patients with cancer.1–3 The rising incidence of metastasis to the brain threatens to limit gains made by new systemic treatments.4 Both the blood-tumor barrier and tumor-stromal interactions impede the delivery of systemic drugs to tumors in the brain.3–5
Based on the trials of Patchell and colleagues,6,7 neurosurgical resection and whole-brain radiation therapy (WBRT) are mainstays of current therapy.8–11 WBRT helps prevent local recurrence and new distant brain metastases,7,12–14 but it fails to improve the duration of functional independence or overall survival.13,14 WBRT carries risks of neurocognitive impairment, leukoencephalopathy, and brain atrophy.15–17 Thus, as patients with brain metastases live longer, increasing concern regarding neurocognitive function (NCF) has led to a paradigm shift to defer WBRT and treat locally with surgery and/or stereotactic radiosurgery (SRS) while monitoring for distant relapses.12,14,18,19
To enhance drug delivery, biodegradable carmustine polymer wafers (Gliadel; Eisai, Inc., Woodcliff Lake, NJ) were developed to be implanted into the surgical cavity of malignant gliomas.20–22 Preclinical, orthotopic models demonstrated prolonged survival and local control (LC) when carmustine wafers were used to treat brain metastases.23,24 In the only published clinical trial that used carmustine wafers to treat brain metastases, we demonstrated excellent LC when carmustine wafers were combined with resection and WBRT.25 Given the known NCF risks linked to WBRT,12,17–19 we designed the current study to evaluate the LC rate with resection and chemowafers while deferring WBRT. We hypothesized that NCF would be preserved or improved as we monitored the “cognitive trajectory” after surgery.26
Materials and Methods
This prospective, single-arm, phase 2 trial (NCT00525590) involved 12 US centers between December 2007 and December 2010. The institutional review board at each site approved the protocol, following Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided informed consent. A data safety committee monitored the study.
Eligible patients had 1 to 3 brain metastases, including patients with a single resectable metastasis or with 2 metastases that were resectable as 1 cavity (distance apart, <1 cm); age >18 years; a Karnofsky performance status (KPS) ≥70; and a recursive partitioning analysis (RPA) classification of 1 (35%) or 2 (65%).1,27 The most common histology (41%) was nonsmall cell lung cancer. Exclusion criteria included: small cell lung cancer, thyroid cancer, lymphoma, germ cell cancer, or primary central nervous system (CNS) tumor; prior cranial irradiation or exposure to carmustine; leptomeningeal disease; surgically targeted metastasis in brainstem or posterior fossa; pregnancy; unstable medical or psychiatric status; and receipt of investigational drugs or bevacizumab within 30 days or of anticoagulants within 7 days of entry.
Initially, the protocol included only patients with a single cerebral metastasis, based on the assumption that half of all metastases are single and, thus, are potentially treatable by surgical resection.6 However, with modern high-resolution magnetic resonance imaging (MRI) scans, additional asymptomatic micrometastases that are amenable to radiosurgery can be detected in up to 40% of patients.28 Therefore, the protocol was amended to include patients who had 1 to 3 MRI-detectable metastases for whom surgery was indicated for a dominant or symptomatic tumor, and SRS was an option for all remaining lesions. WBRT was withheld until patients developed recurrent disease.
All patients underwent resection of a dominant or single metastasis. Surgery was regarded as the best treatment option considering 1 or more of the following criteria: 1) tumor volume, 2) symptoms from mass effect or edema, or 3) need for histologic diagnosis. Advanced surgical techniques—including image guidance, microsurgery, tractography, and brain mapping—achieved gross total resection while minimizing the risk to surrounding brain (Fig. 1).
Figure 1.
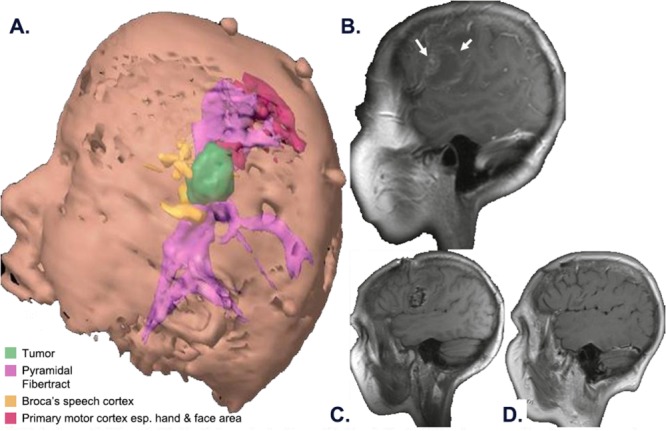
Preoperative and postoperative magnetic resonance images (MRIs) are shown. A patient with metastatic melanoma developed aphasia and facial weakness. (A) The 3-dimensional preoperative model reveals the tumor (green), the primary motor cortex (red) and arcuate fasciculus (purple), the Broca speech area (yellow), and the corticospinal tract (purple). (B) This preoperative sagittal MRI reveals a single hemorrhagic metastasis (arrows) in the left frontal lobe near the primary motor and the Broca speech areas. (C) This postoperative MRI reveals resection of the tumor and intracavitary placement of the carmustine wafers. Speech, motor, and neurocognitive function returned postoperatively. (D) A follow-up scan shows no tumor recurrence at 34 months.
After metastasis resection and histologic confirmation by frozen section, 1 to 8 carmustine wafers were used to line the tumor cavity. Water-tight dural closure, known to decrease complications,25 was used routinely. Ventricular openings <1 cm2 were sealed with resorbable hemostatic material.21 Chemowafers were avoided in the subarachnoid space, especially if the neurosurgeon encountered a major cerebral artery or infiltration of tumor. Corticosteroids and anticonvulsants were prescribed judiciously by the treating physician.21,25
Within 14 days before surgery, patients underwent baseline assessment, including history, physical examination, MRI, NCF, activities of daily living (ADL), KPS, and RPA classification.27 A list of concurrent medications was maintained. An MRI was obtained within 48 hours after surgery; then, follow-up MRI, NCF, ADL, KPS, RPA classification, and toxicity assessments were obtained at months 2, 4, 6, 9, and 12. Patients were followed for 12 months unless there was evidence of treatment failure (eg, local recurrence), neurologic decline because of CNS metastases, withdrawal from the study, or death.
The NCF assessment comprised 3 domains: 1) memory using the revised Hopkins Verbal Learning Test, 2) executive function using the Controlled Oral Word Association and Trailmaking Test B, and 3) fine motor coordination using the pegboard dominant and nondominant hand tests.29,30
Postsurgical imaging studies were reviewed for evidence of CNS recurrence.25 Local recurrences were defined as within 2 cm of the initial resection cavity. Distant CNS recurrences were defined as any of the following: >2 cm from the initial surgical site; or the contralateral side; or in the posterior fossa, spinal cord, or cerebrospinal fluid (carcinomatous meningitis). Adverse events (AEs) were graded for severity (mild, moderate, severe, or fatal) and attribution (unlikely, possibly, probably, or definitely related to CW use). Toxicities were graded using National Cancer Institute Common Toxicity Criteria, version 2.0.25
The trial design was based on statistical assumptions: a 35% rate of neurocognitive decline within 12 months, a 20% rate of local recurrence within 12 months, and a 15% drop-out rate. Accordingly, 50 to 58 patients were required for a 2-sided 95% confidence interval (CI).
NCF endpoints included standardized score changes (from baseline) for each visit, including time and severity of neurocognitive change. Each NCF domain had an average standardized Z-score, and changes from each patient’s own baseline were analyzed using the t test for paired data (with 95% CIs). Preservation of NCF was defined as a decrease of ≤1 standard deviation (SD), any improvement, or no change (0 SD); and worsening was defined as a decrease >1 SD. Deterioration in NCF was defined as deterioration from baseline in at least 2 domains. For each domain, deterioration occurred if a Z-score average decreased from baseline by ≥3 SD on 2 consecutive visits or at the last follow-up visit. For a missing NCF evaluation, if the reason was “neurologic,” then a score of −5.00 was imputed.
Other endpoints included the rates of local and distal tumor recurrence; the time to recurrence; and the correlation of recurrence with mass effect, cognitive functioning, and clinical symptoms. The time to recurrence was analyzed using the Kaplan-Meier method.31 Univariate and multivariate Cox proportional hazard models for the time to local, distant, and overall recurrence were produced using 11 predictive factors (baseline tumor volume, age, RPA classification, KPS, NCF score, resected tumor volume, number of implanted wafers, number of metastatic tumors, primary cancer type, and radiation sensitivity).
Results
Eighty-two patients were screened, 69 were enrolled, and 59 received treatment (Fig. 2, Table 1). Patients were followed for a median of 7.7 months. Fourteen of 59 patients (24%) completed the 12-month assessment period; 5 (36%) initially had an RPA classification of 1, and 9 (64%) initially had an RPA classification of 2. Five patients were excluded from efficacy analyses because of protocol violations after wafer implantation.
Figure 2.
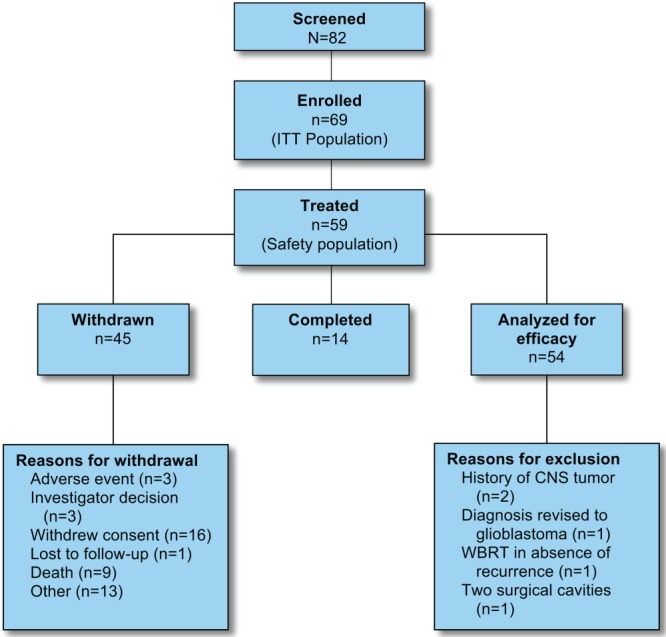
Participant flow is illustrated. CNS indicates central nervous system; WBRT, whole-brain radiation therapy.
Table 1.
Patient Demographics and Cancer History: Safety Population (n = 59)
| Variable | No. of Patients (%) |
|---|---|
| Sex | |
| Men | 26 (44) |
| Women | 33 (56) |
| Age: Median [range], y | 63 [37–81] |
| Race | |
| White | 55 (93) |
| Black or African American | 1 (2) |
| Asian | 2 (3) |
| American Indian or Alaska Native | 1 (2) |
| RPA classification (n = 54) | |
| Class 1 | 19 (35) |
| Class 2 | 35 (65) |
| Type of primary cancer | |
| NSCLC | 24 (41) |
| Melanoma | 15 (25) |
| Breast | 7 (12) |
| Renal | 4 (7) |
| Other | 7 (12) |
| Unknown | 2 (3) |
| Primary cancer status | |
| Newly diagnosed, no prior therapy | 18 (31) |
| Previously diagnosed, off therapy ≥30 d | 36 (61) |
| Previously diagnosed, receiving systemic chemotherapy | 5 (8) |
| No. of brain lesions on MRI | |
| 1 | 49 (83) |
| 2 | 6 (10) |
| 3 | 4 (7) |
Abbreviations: MRI, magnetic resonance imaging; NSCLC, non–small cell lung cancer; RPA, recursive partitioning analysis.
Preservation or improvement of NCF was observed in all 3 domains for the majority of patients throughout the study (month 2, 26 of 46 patients [57%]; month 4, 24 of 37 patients [65%]; month 6, 17 of 26 patients [65%]; month 9, 14 of 22 patients [64%]; and month 12, 9 of 14 patients [64%]). In 1 patient (2%), NCF deterioration was observed in the fine motor coordination domain at months 2 and 4 and in the memory domain at month 4 (last follow-up visit).
For the individual domains, the cumulative rates of preservation and worsening of NCF were determined. In the memory domain, 37 of 54 patients (69%) had preservation of NCF (including 26 of 54 patients [48%] who had improvement), 8 of 54 patients (15%) had worsening NCF, and 9 patients were not assessed. In the executive function domain, 39 of 54 patients (72%) had preservation of NCF (including 23 of 54 patients [43%] who had improvement), 4 of 54 patients (7%) had worsening NCF, and 11 patients were not assessed. In the fine motor coordination domain, 34 of 54 patients (63%) had preservation of NCF (including 27 of 54 patients [50%] who had improvement), 11 of 54 patients (20%) had worsening NCF, and 9 patients were not assessed. Mean changes in Z-scores are provided in Figure 3.
Figure 3.
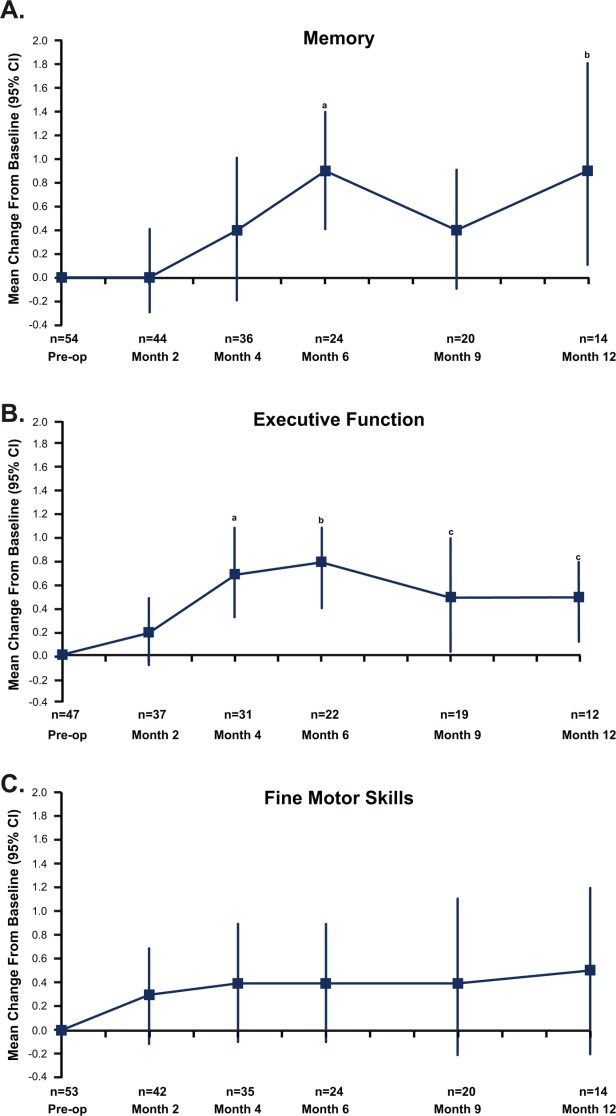
Improvement in neurocognitive function was measured after surgical resection and carmustine wafer placement for brain metastasis using average, standardized Z-scores (indicated as the mean change with 95% confidence interval [CI]). Statistically significant changes were noted in (A) memory (a, P = .001; b, P = .029) and (B) executive function (a, P = .001; b, P = .0007; c [left], P = .041; c [right], P = .018). (C) Fine motor skills.
Fifty of our 54 patients had postbaseline MRI assessments. Local recurrences (Fig. 4A) developed in 14 of 50 evaluable patients (28%). The recurrence rate varied with histology: patients with melanoma had the lowest recurrence rate (7%; 1 of 15 patients), those with nonsmall cell lung cancer had a moderate rate (24%; 5 of 21 patients), and patients with breast cancer had the highest rate (57%; 4 of 7 patients). These differences were not statistically significant. Univariate predictors of the time to local recurrence were: 1) baseline total tumor volume (hazard ratio [HR], 1.043; P = .009) and 2) the number of wafers implanted (HR, 1.366; P = .040), which is also a function of tumor volume. Significant multivariate predictors of the time to local recurrence were: 1) baseline total tumor volume (HR, 1.095; P = .001), 2) primary cancer status (previously diagnosed and receiving systemic chemotherapy vs newly diagnosed and no prior therapy; HR, 22.506; P = .013), 3) baseline KPS (HR, 0.922; P = .031), and 4) baseline NCF score (HR, 1.534; P = .002).
Figure 4.
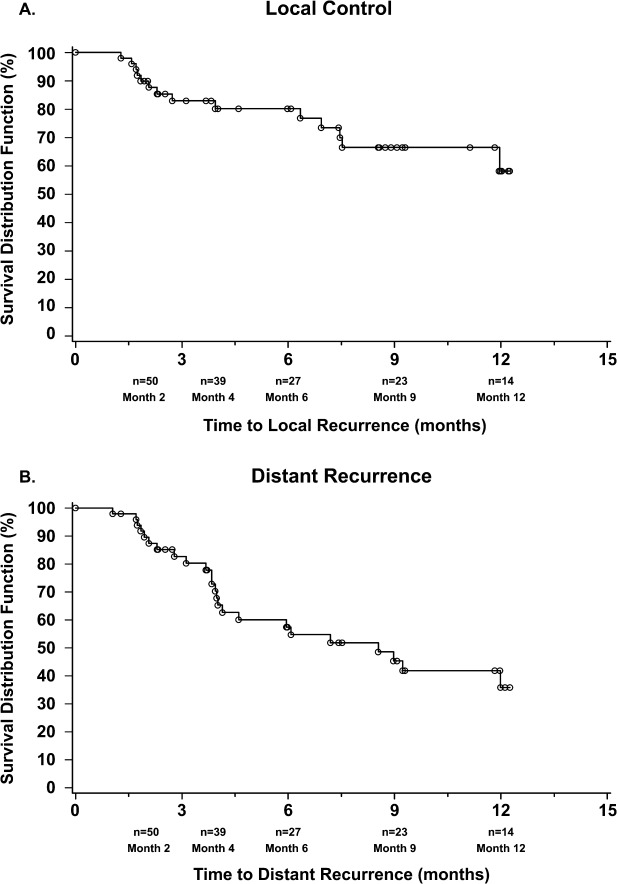
These are Kaplan-Meier curves for (A) local recurrence and (B) distant recurrence. The median time to distant recurrence was 8.5 months.
Distant recurrences (Fig. 4B) developed in 24 of 50 patients (48%) and consisted of diverse histologies, including nonsmall cell lung cancer (33%; 7 of 21 patients), breast cancer (57%; 4 of 7 patients), and melanoma (53%; 8 of 15 patients). Over half of the distant recurrences (17 of 24 recurrences) developed by month 4. Recurrences in these patients were treated with SRS or WBRT, and they achieved favorable oncologic control without neurologic-related death.
The median number of wafers inserted was 5 (range, 1.5-8.0 wafers), corresponding to a dose of 38.5 mg carmustine. Eight patients with large tumors received the maximum dose of 8 wafers (61.6 mg carmustine). Six patients had the wafers removed because of a serious AE (SAE); in 2 of those patients, the investigator believed that the SAE was possibly, or probably, related to the chemowafers, and these patients remained in the study.
The AEs reflected the morbidity of the primary cancer and the side effects of systemic therapy (eg, chemotherapy, radiation therapy, paraneoplastic syndromes, etc) (Table 2). SAEs (including deaths) were reported for 40 of 59 patients. Six patients had a total of 10 SAEs that were considered possibly related to the chemowafers (Table 3). None were fatal, and all resolved with appropriate surgical or medical management; 2 SAEs (a chemowafer-related infection and a wound infection) required removal of the wafers, and 1 SAE (the aforementioned chemowafer-related infection) had permanent sequelae. Nine patients died during the study, including 1 neurologic death and 8 deaths related to the extracranial, primary cancer.
Table 2.
Incidence of Treatment-Emergent Adverse Events in ≥5% of Patients: Treated Population (n = 59)
| Variable | No. of Patients (%) |
|---|---|
| No. with ≥1 treatment-emergent adverse event | 53 (90) |
| Hematologic disorders | 5 (9) |
| Anemia | 3 (5) |
| Cardiac disorders | 5 (9) |
| Bradycardia | 3 (5) |
| Eye disorders | 5 (9) |
| Vision blurred | 3 (5) |
| Gastrointestinal disorders | 21 (36) |
| Constipation | 9 (15) |
| Nausea | 9 (15) |
| Vomiting | 4 (7) |
| General disorders and administration site conditions | 25 (42) |
| Asthenia | 7 (12) |
| Fatigue | 13 (22) |
| Edema peripheral | 6 (10) |
| Pyrexia | 5 (9) |
| Infections | 15 (25) |
| Candidiasis | 3 (5) |
| Oral candidiasis | 3 (5) |
| Metabolic disorders | 23 (39) |
| Anorexia | 3 (5) |
| Dehydration | 4 (7) |
| Failure to thrive | 3 (5) |
| Hyperglycemia | 17 (29) |
| Musculoskeletal disorders | 10 (17) |
| Muscular weakness | 6 (10) |
| Neoplasms: Benign, malignant, and unspecified | 5 (9) |
| Malignant neoplasm progression | 3 (5) |
| Neurologic disorders | 33 (56) |
| Aphasia | 4 (7) |
| Seizure | 6 (10) |
| Headache | 13 (22) |
| Lethargy | 3 (5) |
| Psychiatric disorders | 12 (20) |
| Anxiety | 4 (7) |
| Confusional state | 3 (5) |
| Insomnia | 3 (5) |
| Respiratory disorders | 16 (27) |
| Dyspnea | 6 (10) |
| Cutaneous disorders | 11 (19) |
| Drug-induced rash | 4 (7) |
| Vascular disorders | 10 (17) |
| Deep vein thrombosis | 6 (10) |
| Hypertension | 4 (7) |
Table 3.
Serious Adverse Events
| Patient No. | Serious Adverse Event | Severity | Related to Wafers? | Outcome |
|---|---|---|---|---|
| 1 | Intracranial hypotension | Severe | Probably | Resolved |
| Soft tissue necrosis | Severe | Probably | Resolved | |
| 2 | Chemowafer-related infection | Severe | Probably | Resolved with sequelae (wafers removed) |
| 3 | Wound infection | Severe | Probably | Resolved (wafers removed) |
| 4 | Asthenia | Moderate | Possibly | Resolved |
| Dehydration | Moderate | Possibly | Resolved | |
| Convulsion | Moderate | Possibly | Resolved | |
| 5 | Brain abscess | Severe | Possibly | Resolved |
| 6 | Mental status change | Moderate | Possibly | Resolved |
| Convulsion | Severe | Possibly | Resolved |
Discussion
Our findings add to the growing body of evidence supporting intensive local and multifocal treatment for brain metastases12,30,32–34 with sparing of the surrounding white matter fibers, vessels, and myelin in an effort to preserve NCF.35 Neurocognitive testing provides a quantitative tool for monitoring the effects of therapy and developing tailored therapies to enhance functional well being.36 This study is significant because it demonstrates that: 1) the “cognitive trajectory” after craniotomy is one of preserved or improved NCF for the majority of patients, and 2) NCF can be improved or preserved while achieving acceptable rates of LC with a strategy of resection plus chemowafer, while deferring WBRT. Such an approach may be particularly appropriate when surgery is clinically indicated for a larger tumor (2-3 cm in greatest dimension), a symptomatic metastasis, or a lesion requiring tissue diagnosis.37
Brain metastases represent an increasing health priority because of the prolonged survival of cancer patients, the inability of some novel cancer therapies to reach the CNS, and enhanced detection using advanced neuroimaging.2,28 Thus, as the outlook for meaningful survival has increased with improved systemic therapy, the goals of brain metastasis therapy are shifting from short-term palliation to prolonged control of intracerebral tumor growth while maintaining/improving neurologic and functional status and avoiding the potential toxicity of WBRT.12,18,19,32,33,37,38 Indeed, results from the European Organization for Research and Treatment of Cancer (EORTC) 22952-26001 trial (surgery/SRS then WBRT or surveillance) support withholding the upfront use of WBRT, thereby improving functional outcomes, without compromising survival.12,32
The current results suggest that deferring WBRT while using upfront surgery and carmustine wafers can preserve NCF and provide acceptable local cancer control rates, similar to surgery and WBRT.7,12 We observed a global NCF decline in only 1 patient (2%), which is markedly superior to WBRT.12,29,30,32 NCF was preserved in all 3 domains in 65% of patients. In addition, NCF improvements were observed in >40% of patients for each individual domain, especially executive function and memory. The improvement may be caused by a reduction of perilesional vasogenic edema or the neuroplasticity of white matter fiber tracts. These findings compare favorably to a recent study of SRS with/without WBRT, in which memory and learning declined in 24% of patients who underwent SRS alone and in 52% of those who underwent SRS with WBRT.30
For patients with brain metastases, NCF scores correlate with tumor volume and predict survival32; >90% of patients with brain metastases demonstrate impairment of ≥1 neurocognitive tests at baseline,32,36 which can be improved with surgery.37 The current study demonstrates a sustained improvement in NCF after surgery and indicates that carmustine chemowafers do not adversely affect NCF.
The 28% local recurrence rate reported here is comparable to the previously reported rates for SRS alone32 and for surgery with WBRT7 and is generally superior to the rates reported from studies of surgery alone (without wafer).7,32 The addition of WBRT to SRS increases LC rates13,32 up to 97% for low-volume metastases.39 Previously, WBRT combined with surgery plus chemowafers produced complete (100%) LC.25 Because local recurrence rates are a function of both tumor volume and surgical technique,40,41 the extent to which local efficacy is attributable to advances in neurosurgical technology40,41 versus the effect of the carmustine wafer42 cannot be determined without a controlled comparative trial.
There were 9 deaths during the 12-month follow-up period, and 5 of those deaths occurred >300 days after surgery. It is noteworthy that only 1 patient (2%) died because of neurologic disease progression. In the EORTC study of surgery/SRS plus WBRT, neurologic death occurred in 28% of patients who received WBRT compared with 44% of patients who were managed with surveillance, although overall survival was unchanged.32 The low neurologic mortality rate compares favorably with patients who underwent SRS alone, reported as 12%.33
The toxicity and AEs reported here are largely because of the systemic cancer and concurrent therapies. Because the chemowafers release carmustine locally, there is no detectable level of chemotherapy in the bloodstream, avoiding many of the systemic toxicities.20–22 It is known that chemowafers carry neurosurgical risks of cerebral edema, wound dehiscence, or seizures,20,21 which are reduced by the judicious use of steroids, water-tight dural closures, and anticonvulsant medications.20,21,25 In our prior study of chemowafers, surgery, and WBRT, we observed seizures in 2 patients (8%), but no other toxicities.25
The current study has limitations. First, this was not a randomized, controlled trial. The specific benefits/toxicities of surgery, chemotherapy, SRS, and avoidance of WBRT were compared with historic controls. Placebo-controlled trials using chemowafers, which have proven efficacy in high-grade gliomas,20,22 are problematic to design for single and oligometastatic brain cancer, for which there are multiple existing options (eg, surgery alone, SRS alone, resection plus SRS, resection plus WBRT) and deeply held biases.18 Second, single-arm studies of NCF in patients with brain metastases are difficult to interpret because of numerous confounding variables, including selection bias.43 In the current study, there was a selection bias, because our patients had an RPA classification of 1 or 2, a favorable KPS, and a prognosis that favored undergoing craniotomy. Nevertheless, each patient acted as their own control, so an immediate postoperative and sustained improvement in NCF is noteworthy and novel. Current approaches to the treatment of brain metastases must balance local and distant control in the CNS with neurocognitive preservation. The current study demonstrates that the strategy of neurosurgical resection plus chemowafers provides local cancer control comparable to that provided by radiation therapy. Furthermore, the results indicate a benefit in NCF from withholding WBRT and treating limited brain metastases in a targeted manner. The range of options for patients with brain metastases also will be increased as the novel, pathway-specific, targeted agents44 are evaluated. The preserved and often improved NCF observed using resection and local carmustine wafers, as well as the known pharmacokinetics of carmustine,20,21,42 suggest that this strategy can be used without injury to white matter fiber tracts that affect NCF.45
Funding Support
This work was supported by a research grant from Eisai Inc. MedVal Scientific Information Services, LLC, provided editorial support for article preparation, funded by Eisai Inc. Dr. Ewend’s time was supported by the Weatherspoon Eminent Distinguished Professorship in Neurosurgery. Eisai provided funds to the Moffitt Cancer Center to conduct the clinical trial and support Dr. Brem as principal investigator, including authorship of the manuscript. Funding to support open access for this article was provided by Arbor Pharmaceuticals
Conflict of Interest Disclosures
Dr. Meyers has received compensation as a consultant to Eisai Inc. Dr. Palmer is an employee of Eisai Inc. Dr. Booth-Jones received a grant, consulting fees, and travel support from Eisai Inc. Dr. Ewend has received research funding from Eisai Inc. and from Northwest Biotherapeutics. Dr Ewend’s wife has acted as an unpaid consultant to Sanofi Oncology, Wyeth=Pfizer, Genentech, Bristol-Myers Smith, Novartis, Amgen, and GlaxoSmithKline.
References
- Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14:910–918. doi: 10.1093/neuonc/nos087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus EB. Neurosurgical management of metastases in the central nervous system. Nat Rev Clin Oncol. 2012;9:79–86. doi: 10.1038/nrclinonc.2011.179. [DOI] [PubMed] [Google Scholar]
- Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- Brem SS, Bierman PJ, Brem H, et al. Central nervous system cancers. J Natl Compr Canc Netw. 2011;9:352–400. doi: 10.6004/jnccn.2011.0036. [DOI] [PubMed] [Google Scholar]
- Mehta M. The dandelion effect: treat the whole lawn or weed selectively? J Clin Oncol. 2011;29:121–124. doi: 10.1200/JCO.2010.33.3294. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with 1 to 3 brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- Rades D, Panzner A, Dziggel L, Haatanen T, Lohynska R, Schild SE. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer. 2012;118:3852–3859. doi: 10.1002/cncr.26680. [DOI] [PubMed] [Google Scholar]
- Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- Shibamoto Y, Baba F, Oda K, et al. Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72:1168–1173. doi: 10.1016/j.ijrobp.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Monaco EA, III, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119:226–232. doi: 10.1002/cncr.27504. [DOI] [PubMed] [Google Scholar]
- Khan AJ, Dicker AP. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;31:11–13. doi: 10.1200/JCO.2012.46.0410. [DOI] [PubMed] [Google Scholar]
- Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment–from bench to bedside. Neuro Oncol. 2012;14(suppl 4):37–44. doi: 10.1093/neuonc/nos196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- Menei P, Metellus P, Parot-Schinkel E, et al. Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 2010;17:1740–1746. doi: 10.1245/s10434-010-1081-5. [DOI] [PubMed] [Google Scholar]
- Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Chemotherapy wafers for high grade glioma [serial online] Cochrane Database Syst Rev. 2011;(3):CD007294. doi: 10.1002/14651858.CD007294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewend MG, Williams JA, Tabassi K, et al. Local delivery of chemotherapy and concurrent external beam radiotherapy prolongs survival in metastatic brain tumor models. Cancer Res. 1996;56:5217–5223. [PubMed] [Google Scholar]
- Ewend MG, Sampath P, Williams JA, Tyler BM, Brem H. Local delivery of chemotherapy prolongs survival in experimental brain metastases from breast carcinoma. Neurosurgery. 1998;43:1185–1193. doi: 10.1097/00006123-199811000-00093. [DOI] [PubMed] [Google Scholar]
- Ewend MG, Brem S, Gilbert M, et al. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin Cancer Res. 2007;13:3637–3641. doi: 10.1158/1078-0432.CCR-06-2095. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens P, Karlsson B, Yeo TT, Chou N, Beute G. Detection of brain micrometastases by high-resolution stereotactic magnetic resonance imaging and its impact on the timing of and risk for distant recurrences. J Neurosurg. 2011;115:499–504. doi: 10.3171/2011.4.JNS101832. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of 1 to 3 cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush S, Elliott RE, Morsi A, et al. Incidence, timing, and treatment of new brain metastases after Gamma Knife surgery for limited brain disease: the case for reducing the use of whole-brain radiation therapy. J Neurosurg. 2011;115:37–48. doi: 10.3171/2011.2.JNS101724. [DOI] [PubMed] [Google Scholar]
- Chen X, Xiao J, Li X, et al. Fifty percent patients avoid whole brain radiotherapy: stereotactic radiotherapy for multiple brain metastases. A retrospective analysis of a single center. Clin Trans Oncol. 2012;14:599–605. doi: 10.1007/s12094-012-0849-4. [DOI] [PubMed] [Google Scholar]
- Nazem-Zadeh MR, Chapman CH, Lawrence TL, Tsien CI, Cao Y. Radiation therapy effects on white matter fiber tracts of the limbic circuit. Med Phys. 2012;39:5603–5613. doi: 10.1118/1.4745560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witgert ME, Meyers CA. Neurocognitive and quality of life measures in patients with metastatic brain disease. Neurosurg Clin North Am. 2011;22:79–85. doi: 10.1016/j.nec.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009;92:275–282. doi: 10.1007/s11060-009-9839-y. [DOI] [PubMed] [Google Scholar]
- Patel TR, Knisely JP, Chiang VL. Management of brain metastases: surgery, radiation, or both? Hematol Oncol Clin North Am. 2012;26:933–947. doi: 10.1016/j.hoc.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Shehata MK, Young B, Reid B, et al. Stereotactic radiosurgery of 468 brain metastases ≤2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009;110:730–736. doi: 10.3171/2008.8.JNS08448. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2010;113:181–189. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- Arifin DY, Lee KY, Wang CH, Smith KA. Role of convective flow in carmustine delivery to a brain tumor. Pharm Res. 2009;26:2289–2302. doi: 10.1007/s11095-009-9945-8. [DOI] [PubMed] [Google Scholar]
- Vordermark D. Avoiding bias in the prospective evaluation of patients with brain metastases. J Clin Oncol. 2007;25:4023–4025. doi: 10.1200/JCO.2007.12.4610. [DOI] [PubMed] [Google Scholar]
- Lim E, Lin NU. New insights and emerging therapies for breast cancer brain metastases. Oncology (Williston Park) 2012;26:652–659. [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]


