Abstract
Childhood and adolescence are crucial times for the development of a healthy skeletal and cardiovascular system. Disordered mineral and bone metabolism accompany chronic kidney disease (CKD) and present significant obstacles to optimal bone strength, final adult height, and cardiovascular health. Early increases in bone and plasma fibroblast growth factor 23 (FGF23) are associated with early defects in skeletal mineralization. Later in the course of CKD, secondary hyperparathyroidism—due to a combination of declining calcitriol values and phosphate retention—results in high turnover renal osteodystrophy while elevated levels of both phosphate and FGF23 contribute to cardiovascular disease. Treatment of hyperphosphatemia and secondary hyperparathyroidism improves high turnover bone disease but fails to correct defects in skeletal mineralization. Since overtreatment may result in adynamic bone disease, growth failure, hypercalcemia, and progression of cardiovascular calcifications, therapy must therefore be carefully titrated to maintain optimal serum biochemical parameters according to stage of CKD. Newer therapeutic agents and new treatment paradigms may effectively suppress serum PTH levels while limiting intestinal calcium absorption and skeletal FGF23 stimulation and may provide future therapeutic alternatives for children with CKD.
Keywords: osteodystrophy, growth, vascular calcification, parathyroid hormone (PTH, vitamin D, CKD Mineral and Bonde Disorder, Pediatrics
INTRODUCTION
Childhood and adolescence are a crucial time for developing a healthy skeletal and vascular system; alterations in bone modeling/remodeling or vascular biology in youth carry consequences that severely impact quality of life as well as life span. In childhood, chronic kidney disease (CKD) causes disordered regulation of mineral metabolism with subsequent alterations in bone modeling, remodeling, and growth. These alterations occur early in the course of CKD and are accompanied by the development of cardiovascular calcifications. Since growth failure and short stature are clinically apparent and concerning to patients, families, and physicians alike, optimization of growth and final adult height has been a focus of CKD management in children for decades. More recently, however, a growing awareness that cardiovascular calcifications accompany CKD, that cardiovascular disease is the leading cause of mortality in both adults and children with kidney disease, that a new hormone, FGF23, may be implicated in its progression, and that therapies designed to treat the skeletal consequences of CKD affect the progression of vascular pathology, has led to a reclassification of the mineral, skeletal, and vascular disease associated with progressive kidney failure. Together, these alterations are termed “CKD Mineral and Bone Disorder” (“CKD-MBD”)(1, 2).
The CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD that is manifested by either one or a combination of the following: 1) abnormalities of calcium, phosphorus, PTH, or vitamin D metabolism, 2) abnormalities in bone histology, linear growth, or strength, and 3) vascular or other soft tissue calcification. “Renal osteodystrophy” is the specific term used to describe the bone pathology that occurs as a complication of CKD and is therefore one aspect of the CKD-MBD. While the definitive evaluation of renal osteodystrophy requires a bone biopsy, this procedure is not routinely performed in the clinical setting. However, bone histomorphometry continues to be the gold standard for the assessment of three essential aspects of bone histology: turnover, mineralization, and volume (1, 2).
Due to new concepts and definitions of the mineral and bone alterations associated with CKD, this review outlines the current understanding of abnormal bone and mineral metabolism, present treatment strategies and their impacts on different aspects of this disorder.
ABNORMALITIES OF CALCIUM, PHOSPHORUS, FGF23, PTH, AND VITAMIN D METABOLISM
Pathogenesis of disordered mineral metabolism in CKD-MBD
Through signaling mechanisms between bone, kidney, and parathyroid glands, alterations in kidney function lead to changes in serum biochemical values and progressive skeletal and vascular disease. Early in the course of CKD, at a time when serum calcium and phosphorus levels are still within the normal range, changes in circulating bone and mineral hormones are evident. In CKD stages 2–3, levels of FGF23 rise in order to enhance urinary phosphate excretion. These increased values also suppress renal 1-alpha hydroxylase, resulting in reduced 1,25(OH)2vitamin D (calcitriol, 1,25(OH)2D) values and, subsequently, increased PTH levels, which also help to reduce blood phosphate levels. In advanced CKD, however, rising PTH levels cause a release of calcium and phosphorus from bone and, when compensatory mechanisms fail, severely impaired glomerular filtration rate (GFR) results in phosphate retention which itself directly suppresses 1α-hydroxylase activity (3). In late CKD, hypocalcemia (from decreased intestinal calcium absorption mediated by declining calcitriol levels), hyperphosphatemia, and low circulating 1,25(OH)2D values all combine to stimulate PTH secretion, thus acting as additional factors in the development of secondary hyperparathyroidism (2°HPT) (4).
As early as stage 2 CKD (GFR between 60 and 90 ml/1.73m2/min), circulating levels of FGF23 begin to rise in adults and children (5–7), likely due to a combination of factors including chronically elevated phosphorus load (3, 8), decreased renal excretion (9) and increased osteocyte FGF23 production (10). Early increases in FGF23, occurring while circulating levels of calcium, phosphorus, PTH, and 1,25(OH)2D levels are within the normal range, may be the first sign of altered osteocyte function in CKD in pediatric patients (7, 9, 10). Serum values of FGF-23 increase as CKD progresses and are markedly elevated in individuals with end-stage kidney disease (11). The effects of increasing FGF23 levels on mineral ion metabolism are multiple and include the induction of renal phosphate excretion by reducing expression levels of the renal sodium-dependent phosphate cotransporters, NaPi2a and NaPi2c and the suppression of circulating 1,25(OH)2D levels by inhibiting renal 1α-hydroxylase activity (12) and simulating 24-hydroxylase—the enzyme responsible for the metabolism of the biologically active 1,25(OH)2D (13). FGF23 may also regulate PTH secretion; in vitro and in vivo experiments indicate that FGF23, by activating MAPK pathways in the parathyroid gland, also directly suppress PTH release, independent of the action of FGF23 on vitamin D metabolism (14, 15).
In CKD, reduced circulating 1,25(OH)2D, resulting from a combination of increased FGF23 levels and declining renal mass, contributes to 2°HPT and parathyroid gland hyperplasia in a number of ways: through decreased intestinal calcium absorption, decreased VDR expression and function, and thus lack of PTH gene suppression, and reduced calcium sensing receptor (CaSR) expression.. Indeed, 1,25(OH)2D has been shown to suppress PTH gene transcription, both in vitro (bovine parathyroid cell culture) and in vivo (intact rats) (16, 17). Similarly, 25(OH)vitamin D (25(OH)D) deficiency, which is prevalent in patients with CKD due to decreased outdoor (sunlight) exposure; CKD dietary restrictions (particularly of dairy products) (18), decreased skin synthesis of vitamin D3 in response to sunlight compared with individuals with normal kidney function (19, 20), proteinuria and increased catabolism through increased 24-hydroxylase activitly (13), also contribute to the development of 2°HPT—both directly and through limiting substrate for the formation of 1,25(OH)2D. Recent evidence demonstrates that 1α-hydroxylase is present in the parathyroid glands; thus, 25(OH)D is converted not only in the kidney, but also inside the gland to 1,25(OH)2 D3, suppressing PTH (21). 25(OH)D administration suppresses PTH synthesis even when parathyroid gland 1α-hydroxylase is inhibited, indicating that 25(OH)D may contribute to PTH suppression, independent of its conversion to 1,25(OH)2 D3 (21). Indeed, supplementation with ergocalciferol has been shown to decrease serum PTH levels in patients with CKD (22, 23) and to delay the onset of 2°HPT in children with pre-dialysis CKD (24).
Phosphorus retention and hyperphosphatemia are also important factors in the pathogenesis of 2°HPT, but only in late stages of CKD. The development of 2°HPT is prevented in experimental animals with CKD when dietary phosphorus intake is lowered in proportion to the GFR (25). Dietary phosphate restriction can also reduce previously elevated serum PTH levels in patients with moderate renal failure (26). Phosphorus retention and hyperphosphatemia directly and indirectly promote the secretion of PTH. Hyperphosphatemia lowers blood ionized calcium levels as free calcium ions complex with excess inorganic phosphate; the ensuing hypocalcemia stimulates PTH release. Phosphorus also enhances the secretion of FGF23, thereby impairing renal 1α-hydroxylase activity, which diminishes the conversion of 25(OH)D to 1,25(OH)2D3 (8). Finally, phosphorus can directly enhance PTH synthesis by decreasing cytosolic phospholipase A2 (normally increased by CaSR activation), leading to a decrease in arachidonic acid production with a subsequent increase in PTH secretion (27). Hypophosphatemia also decreases PTH mRNA transcript stability in vitro (28), suggesting that phosphorous itself affects serum PTH levels, probably by increasing the stability of the PTH mRNA transcript.
Finally, alterations in parathyroid gland CaSR expression also occur in 2°HPT and may, in turn, contribute to parathyroid gland hyperplasia. The CaSR is a seven transmembrane G protein-coupled receptor with a large extracellular N-terminus, which binds acidic amino acids and divalent cations (29). Low extracellular calcium levels result in decreased calcium binding to the receptor, a conformational relaxation of the receptor and a resultant increase in PTH secretion (30), while activation of the receptor by high levels of serum calcium decreases PTH secretion (31, 32). The expression of the CaSR is reduced by 30% to 70% as judged by immunohistochemical methods in hyperplastic parathyroid tissue obtained from human subjects with renal failure (33). CaSR gene transcription is regulated by vitamin D through two distinct vitamin D response elements in the gene’s promoter region (34); thus, alterations in vitamin D metabolism in renal failure could account for changes in calcium sensing by the parathyroid glands and vitamin D may act upstream of the CaSR in preventing parathyroid cell hyperplasia (35). Decreased expression and activity of CaSR has been linked to decreased responsiveness in PTH secretion due to altered calcium levels (36). This decreased expression of the CaSR results in an insensitivity to serum calcium levels with subsequent uncontrolled secretion of PTH. Increased stimulation of the CaSR by calcimimetics has been shown to decrease PTH cell proliferation, implicating the CaSR as a regulator of cell proliferation, as well as PTH secretion (37).
RENAL OSTEODYSTROPHY: ABNORMALITIES IN BONE TURNOVER, MINERALIZATION, VOLUME, LINEAR GROWTH, OR STRENGTH
Evaluation of skeletal histology provides both a method for understanding the pathophysiology of renal bone disease and a guide to its proper management. The routine assessment of bone histology is not performed in the clinical setting; however, current recommendations from the National Kidney Foundation (KDOQI Guidelines) suggest that a bone biopsy should be considered in all patients with CKD who have fractures with minimal trauma (pathological fractures), suspected aluminum bone disease, or persistent hypercalcemia despite serum PTH levels between 400 to 600 pg/ml (Level of Evidence: Opinion) (38). After double tetracycline labeling, bone tissue is obtained from the iliac crest on an outpatient basis with minimal morbidity (39). As recently recommended by the Kidney Disease Improving Global Outcomes (KDIGO) workgroup, three areas of bone histology are examined: bone turnover, mineralization and volume, all of which may be altered in patients with chronic kidney disease (1, 2).
Bone Turnover
Traditionally, renal osteodystrophy has been classified primarily on alterations in bone turnover. The primary lesion of renal osteodystrophy in children is one of high bone turnover due to its association with elevated circulating PTH values. PTH activates the PTH/PTHrP receptor on osteocytes and osteoblasts, suppresses of osteocytic sclerostin (an inhibitor of Wnt signaling-mediated bone turnover) expression, and thereby increases cellular activity of both osteoblasts and osteoclasts (40–42), resulting in increased resorption of mineral and matrix along both the trabecular surface and within the Haversian canals of cortical bone. Bone formation rates remain normal in early (stages 2 and 3) CKD (7) (Figure 1) at a time when skeletal expression of sclerostin is increasing (42). In late stages of CKD, skeletal sclerostin expression decreases (42) and bone turnover increases (7) due to the development of 2°HPT. Long-term exposure to high serum PTH levels results in markedly increased bone turnover which lead to peri-trabecular fibrous changes in bones; thus, this lesion is often termed “osteitis fibrosa cystica” (43). Biochemically, increased bone turnover is associated with elevated circulating values of PTH and alkaline phosphate and by a decrease in circulating sclerostin concentrations (44). Elevated bone turnover is evident in patients with moderate to advanced stages of CKD (45), increasing in prevalence as GFR declines (45), and is nearly universal in untreated children at the initiation of dialysis (46).
Figure 1.
Percentage of pediatric patients with abnormalities in bone turnover (solid) and skeletal mineralization (open) and in circulating PTH (striped) and FGF23 (polka dot) values according to CKD stage.
A state of low-turnover bone disease (adynamic renal osteodystrophy) also occurs in children treated with maintenance dialysis and, although it has not been demonstrated in children with earlier stages of CKD (47), it is also highly prevalent in adults with pre-dialysis CKD (48). Adynamic bone is commonly associated with oversuppression of serum PTH levels due to excess treatment with vitamin D and calcium salts but has also been described in conjunction with elevated PTH levels in pediatric dialysis patients (49) and in the absence of vitamin D sterol therapy in adults with early CKD (48). This disorder is characterized by normal osteoid volume, an absence of fibrosis, and a reduced bone formation rate, as indicated by a reduced or absent double tetracycline label on bone histology (50). A paucity of osteoblasts and osteoclasts is present (51). Adynamic bone has also been associated with low alkaline phosphatase levels, high serum calcium levels, and vascular calcification (52, 53). In addition to the increased risk for fractures that is observed in adults with adyamic bone, adynamic bone in children treated with dialysis is associated with an increased severity of growth retardation (54, 55).
Mineralization
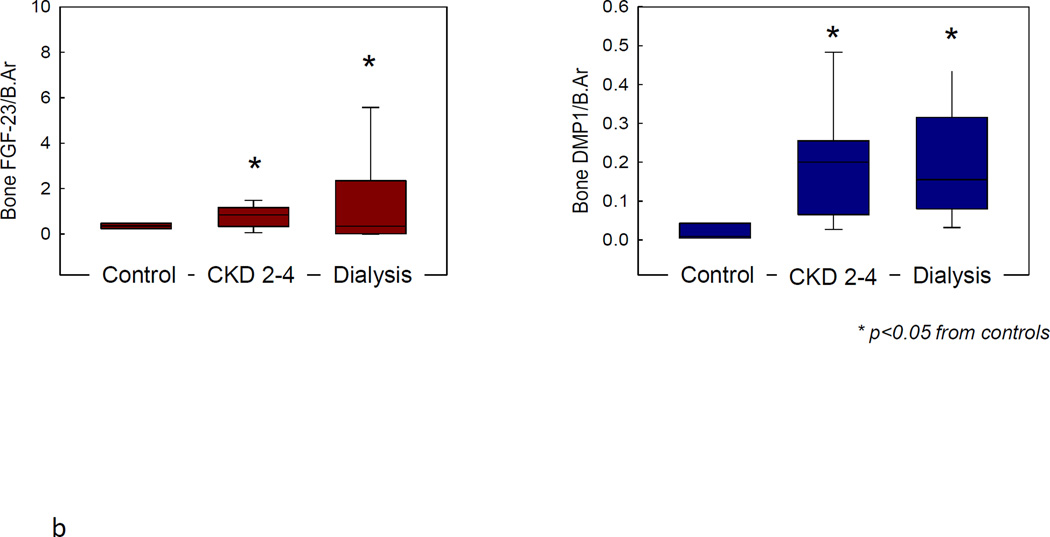
Alterations in skeletal mineralization are prevalent in children with CKD (45, 56). Increases in unmineralized bone (osteoid) in conjuction with delayed rates of mineral deposition occur in early stages of CKD while bone turnover are within the normal range; their prevalence increases as GFR declines, are apparent in the majority of patients with end-stage kidney disease, and remain prevalent after correction of 2°HPT (43, 57). Defective mineralization that is associated with high turnover bone disease is termed “mixed lesion”; when associated with low to normal bone turnover, it is referred to as “osteomalacia” (1, 2). While the clinical implications of defective mineralization in patients with CKD remain to be established, increased fracture rates, bone deformities, and growth retardation—common features of children with rickets (58, 59)—are common in the pediatric CKD population (46) and these lesions persist despite adequate suppression of 2°HPT (60). Interestingly, the skeletal expression of FGF23 and of dentin matrix protein 1 (DMP1), the osteocyte-derived repressor of skeletal FGF23 expression, are dysregulated in patients with all stages of CKD (10) (Figure 2) and this dysregulation is associated with aberrant skeletal mineralization; however, the effects of different therapies for the treatment of renal osteodystrophy on expression of these osteocytic proteins remains unknown.
Figure 2.
a. Skeletal expression of FGF23 in normal control (A) and in a patient with early CKD (B). Skeletal expression of DMP1 in normal control (C) and early CKD (D).
b. Skeletal expression of FGF23 and DMP1 are increased in all stages of CKD relative to control.
Volume
Since PTH is an anabolic steroid at the level of trabecular bone, high levels of serum PTH are typically associated with increases in bone volume, trabecular volume, and trabecular width (39, 57, 61, 62). Thus, children with CKD typically have normal or high bone volume as assessed by bone histomorphometry. Those treated with corticosteroids, however, may display loss of bone volume, termed “osteoporosis”. The impact of osteoporosis in childhood may not always be immediately apparent; however, sub-optimal peak bone mass accretion in adolescence is associated with an increased risk of osteoporosis, hip fractures, and mortality in adulthood (63).
Linear Growth
Linear growth is a unique feature of childhood, occurring through the modeling of new bone by skeletal accretion and longitudinal growth in the growth plate. One third of the total growth occurs during the first two years of life and is driven primarily by nutrition (64). Later childhood is marked by a lesser, although constant, growth rate (5–7 cm/year), and is dependent primarily on the actions of growth and thyroid hormones. At the onset of puberty, estrogen and testosterone induce a second increase of growth velocity. During growth, the epiphyseal cartilage goes through a process of progressive maturation, and when no additional epiphyseal cartilage remains to provide further long bone growth, bone fusion occurs between the shaft and the epiphysis, ending the linear growth process (65).
Growth retardation is the hallmark of CKD in children. Protein and calorie malnutrition, metabolic acidosis, end-organ growth hormone resistance, and renal bone disease are the factors most commonly implicated in growth failure (66). Malnutrition may be the primary contributor to poor growth in children less than 24 months of age; indeed, data from the International Pediatric Peritoneal Dialysis Network (IPPN) Registry have demonstrated that optimization of feedings, preferably with the placement of gastrostomy-tubes—optimizes growth in the first two years of life (67). However, despite correction of malnutrition, acidosis, and anemia; normalization of serum calcium and phosphorus levels; and vitamin D sterol therapy replacement, the majority of older children with CKD continue to grow poorly. Growth failure worsens as renal function declines; the average height of children with even mild CKD (GFR 50–70 ml/minute/1.73 m2) is 1 standard deviation (SDS) below the average for healthy children. Moderate CKD (GFR 25–49 ml/minute/1.73 m2) is associated with a height SDS of −1.5, and, at the time of initiation of dialysis, the mean height SDS is −1.8 (68). Secondary hyperparathyroidism contributes to this persistent growth retardation, although optimal target values for PTH in children in all stages of CKD remain controversial. In children with moderate CKD, some data indicate that normal growth velocity is achieved when PTH levels are maintained within the normal range (69) while others have demonstrated a linear correlation between growth and PTH levels in the same patient population—those with the highest PTH values maintaining the highest rates of growth (70). In children treated with maintenance dialysis, adynamic bone disease and growth failure have been associated with PTH levels around 100 pg/ml (1st generation assay) (54) while data from the IPPN Registry suggest that optimal growth velocity in peritoneal dialysis patients are associated with PTH concentrations below 500 pg/ml (71), although methodological problems with the PTH determinations were present in this latter study. Alterations in the growth hormone/insulin-like growth factor (IGF) pathway axis and their receptors also contribute to growth retardation; indeed, despite normal or elevated circulating levels of growth hormone, decreased growth hormone receptor expression results in end-organ resistance and increased levels of IGF binding proteins, in the presence of normal IGF1 levels, results in decreased bioactivity of IGF1 (72–74).
Bone Structure and Strength
Bone deformities are common in uremic children due to altered skeletal remodeling. Epiphyseal widening, particularly around wrists, ankles, and the costochondral junctions (rachitic rosary) is common in infants. Slipped epiphyses, genu valgum, femoral and wrist deformities are most common in preadolescent children with long-standing CKD (43, 46). Avascular necrosis of the femoral head and pathologic fractures of the extremities and chest wall due to osteoporosis and bone deformities may occur with minimal trauma. In addition, vertebral crush fractures contribute to significant morbidity in this population. The initial management of skeletal deformities requires the normalization of serum calcium, phosphorus, and PTH levels. Surgical correction is often also necessary but should be performed only after correction of biochemical abnormalities.
VASCULAR CALCIFICATION
The etiology of vascular disease in CKD is multifactorial and includes traditional risk factors, such as hyperlipidemia, and inflammation, as well as alterations in mineral metabolism specific to CKD; thus, treatment is also multifaceted. Children with CKD develop cardiovascular disease in the absence of established risk factors as diabetes, obesity, and smoking. In contrast to the calcifications of atherosclerotic plaques in the vascular intima that develop with age in individuals with normal kidney function, vascular calcification in the uremic milieu develops primarily in the vascular media. Hypercalcemia, hyperphosphatemia, elevated levels of the calcium x phosphorus product, treatment with high doses of calcium salts and vitamin D sterols have all been implicated in the progression of the burden of extra-skeletal calcification in both adults and children (75–81). However, 40% of adult patients with stage 3 CKD, without these risk factors, show evidence of calcification (82) and changes in carotid artery wall thickness are apparent in children as early as CKD stage 2 (7, 78), suggesting that factors unique to CKD and independent of circulating mineral content contribute to vascular disease.
Although the mechanisms of the development of vascular calcification remain to be fully elucidated, osteoblasts and vascular smooth muscle cells have a common mesenchymal origin; Core binding factor-1 (Cbfa1) is thought to trigger mesenchymal cell to osteoblast transformation. Mice deficient in Cbfa1 fail to mineralize bone (83) and arteries obtained from patients undergoing renal transplantation show increased levels of the protein (84). Upregulation of the sodium-dependent phosphate transporter PIT-1 likely contributes to the increased calcification (85). Furthermore upregulation of pro-mineralization factors such as osteopontin, bone sialoprotein, osteonectin, alkaline phosphatase, type I collagen, and bone morphogenic protein-2 (BMP-2) is potentiated by the uremic milieu (86–89), while expression of calcification inhibitors, such as fetuin A and matrix gla protein, is suppressed (90–92). Klotho, the co-receptor of FGF23, is also expressed in vascular tissue and decreases in vascular Klotho have linked to loss of vascular elasticity (93). Interestingly, CKD has been associated with decreased circulating levels of soluble Klotho and with reduced arterial Klotho expression (94). Levels of circulating FGF23 may also contribute, as in both the pediatric and adult populations these values have been associated with cardiovascular calcification (95, 96).
TREATMENT
The goal of therapy for childhood CKD-MBD is to normalize mineral metabolism with the aim of improving growth, reducing bone deformities and fragility, and minimizing the progression of extra-skeletal calcification. Biochemical markers of serum calcium, phosphorus, and PTH are primarily used to guide therapy; current therapeutic agents are targeted to maintain values in the normal range for stage of CKD.
Phosphorus Control
As a result of phosphorus retention, patients with advanced pre-dialysis CKD (4) and those undergoing treatment with traditional maintenance dialysis regimens (i.e. thrice weekly hemodialysis or nightly peritoneal dialysis) often require dietary phosphate restriction as well as treatment with phosphate-binding agents to maintain serum phosphorus levels in the normal range. Daily hemodialysis has been shown to normalize serum phosphorus levels, improve growth, and decrease phosphate binder useage (97, 98). Thus, in select patient populations and dialysis centers which are able to perform daily hemodialysis, the use of phosphate binding medications may be decreased or entirely avoided. Hypophosphatemia is one potential side effect of daily hemodialysis, however, and may have important implications for the growing skeletion (99).
Current recommendations suggest that phosphate-binding agents be initiated when circulating phosphate concentrations rise above the normal range for age (38). Due to increased rates of growth, higher circulating phosphate concentrations are required in infants and young children than in older children. Calcium-containing salts are recommended as the mainstay in phosphorus binding therapy in CKD stages 3 through 5 when serum phosphate concentrations rise above the normal range. While several calcium salts— including calcium carbonate, calcium acetate, and calcium citrate—are widely used, calcium carbonate is currently the most commonly used compound (38). In recent years, however, increasing concern of progressive vascular calcifications associated with a positive calcium balance and hypercalcemia has led to current recommendations suggesting that total calcium intake from calcium-based phosphate binders should not exceed twice the recommended DRI for dietary calcium (38). Thus, the use of newer calcium-free phosphate binders also plays a key role in maintaining normo-phosphatemia in dialyzed children. One of these compounds, sevelamer, when compared to calcium-containing salts, has been shown to halt the progression of vascular calcification and decrease mortality rates in adult patients with CKD stages 3 and 4 and in adult dialysis patients (100–102). Sevelamer is also both safe and effective in pediatric patients treated with maintenance dialysis (60, 62, 103). Lanthanum carbonate, another non-calcium containing, but metal-containing (lanthanum), phosphorus binding agent is also effective in reducing serum phosphorous levels with fewer hypercalcemic episodes than calcium carbonate (104–106); however, plasma lanthanum levels increase over time and bone lanthanum content increases during therapy and remains elevated at least a year after discontination of treatment (107). Further evidence from rodents indicates that lanthanum progressively accumulates in the liver, bone, and growth plate (108). Thus, athough an effective phosphate binder, lanthanum has not been approved for use in children as the long-term consequences of its accumulation in multiple tissues remains unknown.
Although phosphate-binding medications are currently recommended only when circulating phosphate concentrations rise above the normal range, a phenomenon that typically occurs in CKD stage 4 and in dialysis patients, changing concepts with regards to the pathogenesis of 2°HPT has called into question whether phosphate binding agents should be initiated in early stages of CKD, before the onset of overt hyperphosphatemia. Indeed, increased values of FGF23 are in CKD stage 2, before serum PTH or phosphate concentrations begin to rise (45, 109). Increased circulating levels of FGF23 have been linked to the development of 2°HPT (110); indeed, short-term studies in the adult pre-dialysis CKD population have demonstrated that the early use of calcium-free (although not of calcium containing) phosphate binders is able to prevent the rise in FGF23, delaying the onset of 2°HPT (111–113). These data, combined with growing evidence linking elevated FGF23 concentrations to left ventricular hypertrophy (114, 115), vascular calcification (95, 96), progressive renal disease (116, 117), and increased mortality rates (118), raise the question as to whether phosphate binders should be initiated earlier in the course of CKD, before serum phosphate concentrations are overtly elevated. In children, however, FGF23 has also been linked to IGF1 levels (5), to nutritional status (119), and to height (45); thus, studies are warranted to assess the safety and efficacy of therapeutic strategies aimed at preventing a rise in FGF23 in the pediatric population.
Secondary Hyperparathyroidism
25(OH) vitamin D deficiency is common and its correction has been shown to delay the onset of 2°HPT in children with CKD (120). Thus, assessment and repletion is recommended for all children in stages 2 to 4 CKD with elevated PTH levels (38). After repletion of 25(OH) vitamin D stores, therapy with active vitamin D sterols recommended in order to suppress PTH levels to withing target range for stage of CKD. Calcitriol and alfacalcidol are two forms of active vitamin D sterol widely used in children, having been shown to be effective in suppressing PTH and improving growth in children with CKD when given in either daily or intermittent doses (70, 121). However, hypercalcemia has been linked to their administration, particularly when given with calcium-containing phosphate binders. Thus, vitamin D analogues have been developed to maximize affinity for parathyroid tissue, while minimizing effects on intestinal calcium and phosphorus absorption. Three new vitamin D analogues are available for use in patients with CKD: 22-oxacalcitriol (OCT) in Japan, as well as 19-nor-1,25-dihydroxyvitamin D2 (paracalcitol) and 1α-hydroxyvitamin D2 (doxercalciferol) in the USA. Oral forms of paricalcitol (70) and doxercalciferol are approved for CKD 3 through 5 in adults, though not yet in children. Doxercalciferol and paricalcitol are effective in lowering PTH (60, 122) and doxercalciferol has been shown to be as efficacious as calcitriol in controlling the skeletal lesions of 2°HPT but with greater suppressive effect on osteoclastogenesis in pediatric patients treated with maintenance dialysis (56). Unfortunately, all forms of active vitamin D have been associated with progressive increases in circulating FGF23 concentrations; whether these increased values are associated with long-term adverse cardiac outcomes remains to be determined.
Calcimimetic agents act as allosteric activators of the calcium sensing receptor (CaSR) and are also available for the treatment of 2°HPT in the adult dialysis population. By increasing the sensitivity of the CaSR, these small organic molecules are able to reduce PTH levels, decrease the calcium-phosphorus product, and may provide a medical means of halting the progression of parathyroid gland hyperplasia (123). Cinacalcet has shown to be effective in the control of 2°HPT in adult patients treated with maintenance dialysis (124) and in severe cases of persistent 2°HPT and hypercalcemia after successful renal transplantation (125); however, these agents have not been approved for use in children, and, due to the presence of the CaSR on the growth plate (126), studies are required to confirm their safety and efficacy in young patients.
Growth
Despite correction of acidosis and optimization of nutrition, many children with CKD continue to grow poorly. Daily hemodialysis, in addition to improving control on phosphate metabolism, has been shown to improve growth in pre-pubertal children (98). Recombinant growth hormone therapy may also be indicated in those whose height is below the 5th percentile for age and who have open epiphyseal plates. Skeletal x-rays to evaluate bone age are indicated prior to hormone initiation. Skeletal deformities such as active rickets or slipped capital femoral epiphyses should be allowed to heal prior to initiation of therapy. Furthermore, since growth hormone therapy may worsen 2°HPT, serum PTH levels must be well controlled prior to start of therapy and should be routinely monitored during treatment (38). Of note, the use of recombinant growth hormone has been linked to increases in circulating FGF23 values (127). Moreover, the clinical response to recombinant growth hormone differs according to stage of CKD, children with stage 5 CKD displaying a less robust growth response than those with less severe kidney disease (128, 129). Thus, many factors, including growth potential, degree of kidney failure, optimal dialysis prescription (98), concomitant morbidities, and control of renal osteodystrophy, should be considered prior to the initiation of growth hormone.
Vascular Disease
Since cardiovascular disease is the leading cause of death in both adults and children with CKD, the prevention of its progression is crucial to the management of the CKD-MBD. Unfortunately, the evaluation of vascular disease in children is challenging. Coronary electron beam tomography (EBCT) has been used to evaluate cardiovascular calcifications but is fraught with technical limitations in pediatric patients and, while ultrasound of carotid intimal-medial wall thickness is a sensitive technique, it is not widely available and observer experise is critical (77). Echocardiogram has been shown to be effective in predicting cardiovascular disease in CKD (130) and should be used routinely.
Lipid lower agents are effective therapy in reducing cardiac mortality in adults with pre-dialysis CKD (131) and in those with stable renal allografts (132), although no benefit from their use has been established in adult patients treated with maintenance dialysis (133) or in any subset of the pediatric CKD population. However, normalization of mineral metabolism by the use of phosphate binders and avoidance of hypercalcemia has been associated with improved cardiac indices in pediatric dialysis patients (78). To prevent progressive vascular disease, current recommendations suggest that total calcium intake should be limited to less than twice the daily recommended intake for age and that biochemical parameters should be routinely monitored and phosphate binder and vitamin D sterol dosages adjusted. Although circulating values of FGF23 have received increasing attention as potential mediators of cardiovascular disease (114), randomized trials are needed to assess whether interventions aimed at decreasing FGF23 values are associated with improved outcomes in these groups as well as in both the adult and pediatric CKD populations.
SUMMARY
In summary, abnormalities in bone and cardiovascular physiology are linked to abnormalities in mineral metabolism in pediatric patients with CKD. These abnormalities occur early in the course of CKD and progress with declining kidney function and current guidelines focus on maintaining indices of mineral metabolism within ranges specific to CKD stage. The identification of FGF23 as a critical regulator of phosphate and vitamin D metabolism and in the pathogenesis of CKD-MBD challenges current treatment guidelines; however, prospective randomized trials are needed in order to assess whether therapies targeted at controlling FGF23 values are warranted.
ACKNOWLEDGEMENTS
This work was supported in part by USPHS grants DK-67563, DK-35423, DK-51081, DK-073039, DK-080984and UL1-RR-033176 and funds from the Casey Lee Ball Foundation.
References
- 1.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;(Suppl):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Portale AA, Booth BE, Halloran BP, Morris RC., Jr Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J.Clin.Invest. 1984;73:1580–1589. doi: 10.1172/JCI111365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 5.Bacchetta J, Dubourg L, Harambat J, Ranchin B, bou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J.Clin.Endocrinol.Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 6.van Husen M, Fischer AK, Lehnhardt A, Klaassen I, Moller K, Muller-Wiefel DE, Kemper MJ. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010;78:200–206. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 7.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Juppner H, Salusky IB. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J.Clin.Endocrinol.Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 9.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB. Relationship between plasma FGF-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J.Clin.Endocrinol.Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J.Bone Miner.Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 13.Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro O, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J.Clin.Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J.Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 16.Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J.Clin.Invest. 1986;78:1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc.Natl.Acad.Sci.U.S.A. 1985;82:4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn JW, Koppel MH, Brickman AS, Massry SG. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973;3:264–272. doi: 10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D and the kidney. Kidney Int. 1987;32:912–929. doi: 10.1038/ki.1987.295. [DOI] [PubMed] [Google Scholar]
- 20.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 21.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–659. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 22.Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 23.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr.Pract. 2008;14:10–17. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroff R, Wan M, Rees L. Can vitamin D slow down the progression of chronic kidney disease? Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-2071-y. [DOI] [PubMed] [Google Scholar]
- 25.Slatopolsky E, Caglar S, Pennell JP, Taggart DD, Canterbury JM, Reiss E, Bricker NS. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971;50:492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llach F, Massry SG. On the mechanism of secondary hyperparathyroidism in moderate renal insufficiency. J Clin Endocrinol Metab. 1985;61:601–606. doi: 10.1210/jcem-61-4-601. [DOI] [PubMed] [Google Scholar]
- 27.Almaden Y, Canalejo A, Ballesteros E, Anon G, Canadillas S, Rodriguez M. Regulation of arachidonic acid production by intracellular calcium in parathyroid cells: effect of extracellular phosphate. J.Am.Soc.Nephrol. 2002;13:693–698. doi: 10.1681/ASN.V133693. [DOI] [PubMed] [Google Scholar]
- 28.Silver J, Kilav R, Sela-Brown A, Naveh-Many T. Molecular mechanisms of secondary hyperparathyroidism. Pediatr.Nephrol. 2000;14:626–628. doi: 10.1007/s004670000355. [DOI] [PubMed] [Google Scholar]
- 29.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 30.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Juppner H. A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: implications for PTH measurements in renal failure. J.Clin.Endocrinol.Metab. 1999;84:4287–4290. doi: 10.1210/jcem.84.11.6236. [DOI] [PubMed] [Google Scholar]
- 31.Freichel M, Zink-Lorenz A, Holloschi A, Hafner M, Flockerzi V, Raue F. Expression of a calcium-sensing receptor in a human medullary thyroid carcinoma cell line and its contribution to calcitonin secretion. Endocrinology. 1996;137:3842–3848. doi: 10.1210/endo.137.9.8756555. [DOI] [PubMed] [Google Scholar]
- 32.Kirkwood JR, Ozonoff MB, Steinbach HL. Epiphyseal displacement after metaphyseal fracture in renal osteodystrophy. Am.J.Roentgenol.Radium.Ther.Nucl.Med. 1972;115:547–554. doi: 10.2214/ajr.115.3.547. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Salvago M, Villar-Rodriguez JL, Palma-Alvarez A, Beato-Moreno A, Galera-Davidson H. Decreased expression of calcium receptor in parathyroid tissue in patients with hyperparathyroidism secondary to chronic renal failure. Endocr.Pathol. 2003;14:61–70. doi: 10.1385/ep:14:1:61. [DOI] [PubMed] [Google Scholar]
- 34.Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J.Biol.Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc.Natl.Acad.Sci.U.S.A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AJ, Zhong M, Ritter C, Brown EM, Slatopolsky E. Loss of calcium responsiveness in cultured bovine parathyroid cells is associated with decreased calcium receptor expression. Biochem.Biophys.Res.Commun. 1995;212:861–867. doi: 10.1006/bbrc.1995.2048. [DOI] [PubMed] [Google Scholar]
- 37.Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J.Clin.Invest. 1997;100:2977–2983. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Kidney F. K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am.J.Kidney Dis. 2005;46:S1–S121. [PubMed] [Google Scholar]
- 39.Salusky IB, Coburn JW, Brill J, Foley J, Slatopolsky E, Fine RN, Goodman WG. Bone disease in pediatric patients undergoing dialysis with CAPD or CCPD. Kidney Int. 1988;33:975–982. doi: 10.1038/ki.1988.96. [DOI] [PubMed] [Google Scholar]
- 40.Atkins D, Peacock M. A comparison of the effects of the calcitonins, steroid hormones and thyroid hormones on the response of bone to parathyroid hormone in tissue culture. J.Endocrinol. 1975;64:573–583. [PubMed] [Google Scholar]
- 41.Lee K, Deeds JD, Bond AT, Juppner H, bou-Samra AB, Segre GV. In situ localization of PTH/PTHrP receptor mRNA in the bone of fetal and young rats. Bone. 1993;14:341–345. doi: 10.1016/8756-3282(93)90162-4. [DOI] [PubMed] [Google Scholar]
- 42.Sabbagh Y, Graciolli FG, O’Brien S, Tang W, Dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 43.Malluche H, Faugere MC. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int. 1990;38:193–211. doi: 10.1038/ki.1990.187. [DOI] [PubMed] [Google Scholar]
- 44.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Juppner H, Salusky IB. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW. Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int. 2003;63:266–275. doi: 10.1046/j.1523-1755.2003.00727.x. [DOI] [PubMed] [Google Scholar]
- 47.Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358–363. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lobao R, Carvalho AB, Cuppari L, Ventura R, Lazaretti-Castro M, Jorgetti V, Vieira JG, Cendoroglo M, Draibe SA. High prevalence of low bone mineral density in pre-dialysis chronic kidney disease patients: bone histomorphometric analysis. Clin Nephrol. 2004;62:432–439. doi: 10.5414/cnp62432. [DOI] [PubMed] [Google Scholar]
- 49.Goodman WG, Ramirez JA, Belin TR, Chon Y, Gales B, Segre GV, Salusky IB. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46:1160–1166. doi: 10.1038/ki.1994.380. [DOI] [PubMed] [Google Scholar]
- 50.Salusky IB, Coburn JW, Foley J, Nelson P, Fine RN. Effects of oral calcium carbonate on control of serum phosphorus and changes in plasma aluminum levels after discontinuation of aluminum-containing gels in children receiving dialysis. J.Pediatr. 1986;108:767–770. doi: 10.1016/s0022-3476(86)81064-2. [DOI] [PubMed] [Google Scholar]
- 51.Sherrard DJ. Renal osteodystrophy. Semin.Nephrol. 1986;6:56–67. [PubMed] [Google Scholar]
- 52.Ott SM, Maloney NA, Klein GL, Alfrey AC, Ament ME, Coburn JW, Sherrard DJ. Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann.Intern.Med. 1983;98:910–914. doi: 10.7326/0003-4819-98-6-910. [DOI] [PubMed] [Google Scholar]
- 53.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J.Am.Soc.Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 54.Kuizon BD, Goodman WG, Juppner H, Boechat I, Nelson P, Gales B, Salusky IB. Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney Int. 1998;53:205–211. doi: 10.1046/j.1523-1755.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- 55.Kuizon BD, Salusky IB. Intermittent calcitriol therapy and growth in children with chronic renal failure. Miner.Electrolyte Metab. 1998;24:290–295. doi: 10.1159/000057384. [DOI] [PubMed] [Google Scholar]
- 56.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Juppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2010 doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 57.Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, Goodman WG. Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int. 1994;45:253–258. doi: 10.1038/ki.1994.31. [DOI] [PubMed] [Google Scholar]
- 58.Munns CF, Rauch F, Travers R, Glorieux FH. Three children with lower limb fractures and a mineralization defect: a novel bone fragility disorder? Bone. 2004;35:1023–1028. doi: 10.1016/j.bone.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 59.consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat.Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 60.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Juppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79:112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 61.Salusky IB, Goodman WG, Kuizon BD, Lavigne JR, Zahranik RJ, Gales B, Wang HJ, Elashoff RM, Juppner H. Similar predictive value of bone turnover using first- and second-generation immunometric PTH assays in pediatric patients treated with peritoneal dialysis. Kidney Int. 2003;63:1801–1808. doi: 10.1046/j.1523-1755.2003.00915.x. [DOI] [PubMed] [Google Scholar]
- 62.Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Juppner H. Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J.Am.Soc.Nephrol. 2005;16:2501–2508. doi: 10.1681/ASN.2004100885. [DOI] [PubMed] [Google Scholar]
- 63.Ho AY, Kung AW. Determinants of peak bone mineral density and bone area in young women. J.Bone Miner.Metab. 2005;23:470–475. doi: 10.1007/s00774-005-0630-7. [DOI] [PubMed] [Google Scholar]
- 64.Mahesh S, Kaskel F. Growth hormone axis in chronic kidney disease. Pediatr Nephrol. 2008;23:41–48. doi: 10.1007/s00467-007-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyton A, Hall J. Pituitary hormones and their control by the hypothalamus. In: Guyton A, editor. Textbook of medical physiology. Philadelphia: Elsevier Saunders; 2006. pp. 918–929. [Google Scholar]
- 66.Tonshoff B, Schaefer F, Mehls O. Disturbance of growth hormone--insulin-like growth factor axis in uraemia. Implications for recombinant human growth hormone treatment. Pediatr.Nephrol. 1990;4:654–662. doi: 10.1007/BF00858645. [DOI] [PubMed] [Google Scholar]
- 67.Rees L, Azocar M, Borzych D, Watson AR, Buscher A, Edefonti A, Bilge I, Askenazi D, Leozappa G, Gonzales C, van Hoeck K, Secker D, Zurowska A, Ronnholm K, Bouts AH, Stewart H, Ariceta G, Ranchin B, Warady BA, Schaefer F. Growth in very young children undergoing chronic peritoneal dialysis. J Am Soc Nephrol. 2011;22:2303–2312. doi: 10.1681/ASN.2010020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2008 Annual Report. 2008 [Google Scholar]
- 69.Waller SC, Ridout D, Cantor T, Rees L. Parathyroid hormone and growth in children with chronic renal failure. Kidney Int. 2005;67:2338–2345. doi: 10.1111/j.1523-1755.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt CP, Ardissino G, Testa S, Claris-Appiani A, Mehls O. Growth in children with chronic renal failure on intermittent versus daily calcitriol. Pediatr.Nephrol. 2003;18:440–444. doi: 10.1007/s00467-003-1091-7. [DOI] [PubMed] [Google Scholar]
- 71.Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, Zambrano P, Ahlenstiel T, Bakkaloglu SA, Spizzirri AP, Lopez L, Ozaltin F, Printza N, Hari P, Klaus G, Bak M, Vogel A, Ariceta G, Yap HK, Warady BA, Schaefer F. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int. 2010;78:1295–1304. doi: 10.1038/ki.2010.316. [DOI] [PubMed] [Google Scholar]
- 72.Tonshoff B, Cronin MJ, Reichert M, Haffner D, Wingen AM, Blum WF, Mehls O. Reduced concentration of serum growth hormone (GH)-binding protein in children with chronic renal failure: correlation with GH insensitivity. The European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. The German Study Group for Growth Hormone Treatment in Chronic Renal Failure. J.Clin.Endocrinol.Metab. 1997;82:1007–1013. doi: 10.1210/jcem.82.4.3893. [DOI] [PubMed] [Google Scholar]
- 73.Tonshoff B, Blum WF, Mehls O. Derangements of the somatotropic hormone axis in chronic renal failure. Kidney Int. 1997;58(Suppl):S106–S113. [PubMed] [Google Scholar]
- 74.Powell DR, Durham SK, Liu F, Baker BK, Lee PD, Watkins SL, Campbell PG, Brewer ED, Hintz RL, Hogg RJ. The insulin-like growth factor axis and growth in children with chronic renal failure: a report of the Southwest Pediatric Nephrology Study Group. J.Clin.Endocrinol.Metab. 1998;83:1654–1661. doi: 10.1210/jcem.83.5.4755. [DOI] [PubMed] [Google Scholar]
- 75.Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int. 1990;38:931–936. doi: 10.1038/ki.1990.293. [DOI] [PubMed] [Google Scholar]
- 76.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N.Engl.J.Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 77.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 78.Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16:1494–1500. doi: 10.1681/ASN.2004110932. [DOI] [PubMed] [Google Scholar]
- 79.Chavers BM, Li S, Collins AJ, Herzog CA. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 2002;62:648–653. doi: 10.1046/j.1523-1755.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- 80.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 81.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J.Am.Soc.Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am.J.Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 83.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 84.Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 85.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ.Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am.J.Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 87.Bostrom K. Insights into the mechanism of vascular calcification. Am.J.Cardiol. 2001;88:20E–22E. doi: 10.1016/s0002-9149(01)01718-0. [DOI] [PubMed] [Google Scholar]
- 88.Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 89.Chen NX, O’Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 90.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J.Biol.Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 91.Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J.Thromb.Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 92.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J.Clin.Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim K, Lu TS, Molostvov G, Lee C, Lam F, Zehnder D, Hsiao LL. Vascular Klotho Deficiency Potentiates the Development of Human Artery Calcification and Mediates Resistance to FGF-23. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 95.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol.Dial.Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 96.Srivaths PR, Goldstein SL, Silverstein DM, Krishnamurthy R, Brewer ED. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 97.Tom A, McCauley L, Bell L, Rodd C, Espinosa P, Yu G, Yu J, Girardin C, Sharma A. Growth during maintenance hemodialysis: impact of enhanced nutrition and clearance. J.Pediatr. 1999;134:464–471. doi: 10.1016/s0022-3476(99)70205-2. [DOI] [PubMed] [Google Scholar]
- 98.Fischbach M, Terzic J, Menouer S, Dheu C, Soskin S, Helmstetter A, Burger MC. Intensified and daily hemodialysis in children might improve statural growth. Pediatr.Nephrol. 2006;21:1746–1752. doi: 10.1007/s00467-006-0226-z. [DOI] [PubMed] [Google Scholar]
- 99.Hothi DK, Harvey E, Piva E, Keating L, Secker D, Geary DF. Calcium and phosphate balance in adolescents on home nocturnal haemodialysis. Pediatr Nephrol. 2006;21:835–841. doi: 10.1007/s00467-006-0048-z. [DOI] [PubMed] [Google Scholar]
- 100.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 101.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 102.Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487–493. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 103.Mahdavi H, Kuizon BD, Gales B, Wang HJ, Elashoff RM, Salusky IB. Sevelamer hydrochloride: an effective phosphate binder in dialyzed children. Pediatr.Nephrol. 2003;18:1260–1264. doi: 10.1007/s00467-003-1298-7. [DOI] [PubMed] [Google Scholar]
- 104.D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A, Coen G, Sulowicz W, Ferreira A, Torres A, Curic S, Popovic M, Dimkovic N, De Broe ME. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int. 2003;(Suppl):S73–S78. doi: 10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- 105.Hutchison AJ, Speake M, Al-Baaj F. Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol.Dial.Transplant. 2004;19:1902–1906. doi: 10.1093/ndt/gfh282. [DOI] [PubMed] [Google Scholar]
- 106.Finn WF, Joy MS, Hladik G. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin.Nephrol. 2004;62:193–201. doi: 10.5414/cnp62193. [DOI] [PubMed] [Google Scholar]
- 107.Spasovski GB, Sikole A, Gelev S, Masin-Spasovska J, Freemont T, Webster I, Gill M, Jones C, De Broe ME, D’Haese PC. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol.Dial.Transplant. 2006;21:2217–2224. doi: 10.1093/ndt/gfl146. [DOI] [PubMed] [Google Scholar]
- 108.Slatopolsky E, Liapis H, Finch J. Progressive accumulation of lanthanum in the liver of normal and uremic rats. Kidney Int. 2005;68:2809–2813. doi: 10.1111/j.1523-1755.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 109.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011 doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am.J.Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 111.Oliveira RB, Cancela AL, Graciolli FG, dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moyses RM. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin.J.Am.Soc.Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol.Dial.Transplant. 2010 doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–2571. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 114.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J.Am.Soc.Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 117.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N.Engl.J.Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bacchetta J, Cochat P, Salusky IB, Wesseling-Perry K. Uric acid and IGF1 as possible determinants of FGF23 metabolism in children with normal renal function. Pediatr Nephrol. 2012 doi: 10.1007/s00467-012-2110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Wells D, Aitkenhead H, Manickavasagar B, van’t Hoff W, Rees L. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol. 2012;7:216–223. doi: 10.2215/CJN.04760511. [DOI] [PubMed] [Google Scholar]
- 121.Waller S, Ledermann S, Trompeter R, van’t HW, Ridout D, Rees L. Catch-up growth with normal parathyroid hormone levels in chronic renal failure. Pediatr.Nephrol. 2003;18:1236–1241. doi: 10.1007/s00467-003-1284-0. [DOI] [PubMed] [Google Scholar]
- 122.Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am.J.Kidney Dis. 2004;43:877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Wada M, Nagano N. Control of parathyroid cell growth by calcimimetics. Nephrol.Dial.Transplant. 2003;3(18 Suppl):iii13–iii17. doi: 10.1093/ndt/gfg1004. [DOI] [PubMed] [Google Scholar]
- 124.Martin KJ, Juppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary LC, Guo MD, Turner SA, Bushinsky DA. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int. 2005;68:1236–1243. doi: 10.1111/j.1523-1755.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 125.Serra AL, Schwarz AA, Wick FH, Marti HP, Wuthrich RP. Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrol.Dial.Transplant. 2005;20:1315–1319. doi: 10.1093/ndt/gfh925. [DOI] [PubMed] [Google Scholar]
- 126.Chang W, Tu C, Bajra R, Komuves L, Miller S, Strewler G, Shoback D. Calcium sensing in cultured chondrogenic RCJ3.1C5.18 cells. Endocrinology. 1999;140:1911–1919. doi: 10.1210/endo.140.4.6639. [DOI] [PubMed] [Google Scholar]
- 127.Gardner J, Ashraf A, You Z, McCormick K. Changes in plasma FGF23 in growth hormone deficient children during rhGH therapy. J Pediatr Endocrinol Metab. 2011;24:645–650. doi: 10.1515/jpem.2011.301. [DOI] [PubMed] [Google Scholar]
- 128.Fine RN, Kohaut EC, Brown D, Perlman AJ. Growth after recombinant human growth hormone treatment in children with chronic renal failure: report of a multicenter randomized double-blind placebo-controlled study. Genentech Cooperative Study Group. J.Pediatr. 1994;124:374–382. doi: 10.1016/s0022-3476(94)70358-2. [DOI] [PubMed] [Google Scholar]
- 129.Schaefer F, Wuhl E, Haffner D, Mehls O. Stimulation of growth by recombinant human growth hormone in children undergoing peritoneal or hemodialysis treatment. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. Adv.Perit.Dial. 1994;10:321–326. [PubMed] [Google Scholar]
- 130.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: role of calcium-phosphorus metabolism. J.Am.Soc.Nephrol. 2005;16:2796–2803. doi: 10.1681/ASN.2005030291. [DOI] [PubMed] [Google Scholar]
- 131.Tonelli M, Keech A, Shepherd J, Sacks F, Tonkin A, Packard C, Pfeffer M, Simes J, Isles C, Furberg C, West M, Craven T, Curhan G. Effect of pravastatin in people with diabetes and chronic kidney disease. J.Am.Soc.Nephrol. 2005;16:3748–3754. doi: 10.1681/ASN.2005070779. [DOI] [PubMed] [Google Scholar]
- 132.Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen-Riska C, Neumayer HH, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am.J.Transplant. 2005;5:2929–2936. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 133.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N.Engl.J.Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]