Abstract
Aim
To assess the feasibility of administering Patient Reported Outcomes Measures (PROMs) in patients treated with ablation for cardiac arrhythmias, and to conduct the first stage of development and testing of a new PROM tool.
Methods and results
A new tool was developed by a multidisciplinary team and tested alongside an adaptation of the patient perception of arrhythmia questionnaire (PPAQ) and EQ-5D-5L in a multicentre retrospective audit involving 791 consecutive cardiac arrhythmia patients treated with catheter ablation at three UK centres over 13 months. Data were recorded in the National Cardiac Rhythm Management Database, part of the National Institute for Cardiovascular Outcomes Research. The response rate was 71.9% (n = 569). Patients reported significant improvements across all outcomes and impacts, with reductions in symptoms of 51.7% (heart racing), 33.9% (fatigue) 31.8% (heart flutters), 43.5% (dizziness), 38.6% (breathlessness), 44.2% (chest pressure), 33.1% (trouble concentrating), 15.9% (headache), 28.3% (neck pressure), and 23.4% (fainting) (P < 0.001). The mean number of social days affected reduced by 7.49 days/month (P < 0.001); mean work/school days affected/month reduced by 6.26 (P < 0.001); mean GP/hospital visits reduced by 1.36 days/month (P < 0.001). The procedure met patient expectations in 72% of responders.
Conclusions
The high response rate suggests that the use of PROMs in this patient group is feasible, with rates equalling those of the National PROMs Programme. The results showed that patients experienced significant improvements in their quality of life following ablation, while feedback allowed the tools to be improved. Further work is required to validate these tools; however, the findings suggest that PROMs could be useful in the audit of ablation techniques.
Keywords: Patient reported outcome measures, PROMs, Cardiac ablation, Arrhythmia, Quality of life
What's new?
A Patient Report Outcome Measures for use in cardiac arrhythmia patients undergoing radiofrequency ablation treatment.
Collecting Patient-Reported Outcomes in patients treated with ablation techniques for cardiac arrhythmias is a feasible way of measuring procedural success.
The majority of responders were positive about the outcome of their ablation, with over 72% of patients reporting that the procedure had met their expectations.
Introduction
The effectiveness of medical interventions has traditionally been measured from a clinical perspective, looking at factors including complication and mortality rates, with clinical improvements assessed by healthcare staff. However, emphasis is increasingly being placed on gaining patients' own perceptions of treatment success1,2 using methods such as Patient Reported Outcome Measures (PROMs). Patient Reported Outcome Measures are questionnaires which are administered both before and after a procedure or treatment to measure changes in a patient's' opinion of their health status following an intervention. A PROM tool may be generic, such as EQ5D,3 which assesses general health status and may be used in any population, or disease-specific, assessing the impact of a particular condition or disease on a patient. Disease-specific measures are often used to identify clinically important treatment related changes to an individual's health and wellbeing, while generic tools can compare outcomes across different treatment and patient groups. As they have different strengths, both types may be administered during a health outcomes assessment. Patient Reported Outcome Measures have the potential to drive changes within the National Health Service (NHS),4 supporting the provision of patient-orientated care with benefits including reduced cost and improved safety and quality outcomes.5,6
The field of cardiac ablation is an ideal area for the use of PROMs as these procedures are often aimed at improving or abolishing specific symptoms. Estimated to affect over 1 million people in the UK alone,7 cardiac arrhythmias are associated with features including nausea, fatigue, heart racing, breathlessness, and chest pain. Symptoms may be difficult to quantify clinically but have significant adverse effects on health and the quality of life.8–10 Many patients report suffering a lack of independence and for many, symptoms lead to a loss of time at work and impaired ability to carry out normal daily routines. Furthermore, anti-arrhythmic drugs themselves can cause unpleasant side effects,11 including worsening arrhythmia, dizziness, and photosensitivity. Currently, no disease-specific tools have been validated in the UK to assess the patient-reported outcomes of ablation procedures in patients with non-specific cardiac arrhythmias; therefore, the full impact of these conditions remains unclear.
The purpose of this study was to develop a short disease-specific tool and adapt the longer patient perception of arrhythmia questionnaire (PPAQ) developed by Wood et al.12 for use in a UK population of patients treated with ablation for a symptomatic arrhythmia. Furthermore, this study aimed to pilot these together with the EQ-5D-5L in a multicentre audit to explore the feasibility of obtaining PROM data in this population. The secondary aims were to identify weaknesses in the tools and administration process to improve the methods and further develop the questionnaires.
Methods
Questionnaires
The cardiac ablation PROM tool comprised three individual questionnaires used as a single tool. The first, a short arrhythmia-specific questionnaire, was developed following literature reviews and research, with input from a multidisciplinary team of cardiac consultants, arrhythmia nurses, clinical advisors, and research scientists. This tool explored patients' treatment expectations prior to the procedure and whether these were subsequently met. It also considered the number of ablations received and investigated unexpected complications arising from the procedure. The second element of the tool comprised of a modification of the arrhythmia-specific PPAQ questionnaire developed by Wood et al.12 This was originally developed using techniques including formative research, exploratory factor analysis, expert review, and pilot study. Following adaptations for use in a UK population, this updated tool included seven domains covering severity of symptoms, regularity and length of adverse features, impact on social life and normal routine, arrhythmia-related visits to GP/hospital, and impact on life.
The third element was the generic EQ-5D-5L questionnaire, a validated tool consisting of five domains assessing mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each domain includes five levels of response ranging from no problems to severe problems. It also has a visual analogue scale (VAS) inviting patients to rate their health on a scale of 0–100 with 0 being the worst health imaginable and 100 being the best imaginable. Supporting documentation, including the cover letter and consent form, was also developed, including Welsh translations. A contact name and telephone number were provided for patients' queries.
Study design
The tools were designed to be completed by patients before the procedure and again at a minimum of eight weeks post-procedure. However, due to time constraints of the pilot, a retrospective study design was used, whereby patients were sent pre- and post-procedure questionnaires at the same time and asked to complete the pre-procedure questionnaires from memory. Reminders containing copies of the documentation were sent out 16–29 days after the first contact to non-responders.
Participants
A cohort of 800 patients who had received radiofrequency ablation therapy for a cardiac arrhythmia prior to the start of the study on 1 November 2010 from three treatment centres were retrospectively identified for inclusion. Participants were required to (i) have received a cardiac ablation a minimum of 8 weeks prior to the study; (ii) be aged ≥17 at the time of the study; and (iii) have had their details entered onto the Central Cardiac Audit Database (CCAD), part of the National Institute for Cardiovascular Outcomes Research. Consecutive patients were enrolled from each site: Queen Elizabeth Hospital, University Hospitals Birmingham (400 patients); The Freeman Hospital, Newcastle Upon Tyne NHS Trust (200 patients), and University Hospital of Wales, Cardiff & Vale University Health Board (200 patients). Three patients from Birmingham and six from Newcastle were subsequently removed from the study as they were determined to be duplicate patients who had moved house or been retreated, or were identified as deceased; this left 791 patients in the study group.
Data collection, storage, and analysis
Responses were sent to Cedar; an NHS organization independent of the treatment clinics, for data entry and logged onto the CCAD with 15% of entries checked for accuracy. The CCAD is a clinical audit facility which collects information on cardiac procedures within the UK. It is used as a confidential data collection point allowing comparative reporting between treatment centres, data quality reporting, and mortality tracking, with all UK ablation centres highly encouraged to submit procedural data. A specially designed extension to the CCAD was developed to allow the linkage of PROMs returns to existing clinical data while retaining patient anonymity. Clinical data and PROMs data were stored separately to prevent clinical staff accessing individual identifiable PROM data at any stage.
Data were analysed using SPSS (IBM SPSS Statistics Version 21) and a 5% significance level was used. For statistical analysis, missing values were excluded on a pairwise basis. EQ-5D questionnaires were scored using the EQ-5D-5L Crosswalk index value calculator.13 Differences between pre- and post-procedure nominal variables (e.g. presence or absence of symptoms) were analysed using McNemar's test. Differences between pre- and post-procedure means of continuous data were analysed using a paired-sample t-tests, where the appropriateness of parametric tests was in question, a related-sample Wilcoxon signed-rank test was used.
Results
Responses
An initial response rate of between 45% and 50% was achieved from each centre following initial mailing, rising to a rate of 70–75% from each centre after reminders had been sent, with a final total of 569 analysable responses (71.9%). Three responses were received after the study end date and were excluded from analysis. In 12 cases, a narrative response was received without a completed questionnaire with reasons cited for non-completion including death, ill health, and on-going treatment. One letter was returned to the sender.
Fifteen telephone enquiries were received regarding the questionnaires. In five cases, this was to inform the team that patients were unable to complete the questionnaire due to ill health (two patients), death (two patients), and emigration (one patient). One enquiry was received from a patient requiring advice regarding ongoing health issues; this patient was referred to a contact at the relevant treatment centre. Other enquiries regarded issues such as questions clarification and concerns including whether other peoples' opinions (e.g. clinicians/family members) should be taken into account.
Data
The mean age of the 569 respondents was 57.8 years, and 315 (55%) were males (Table 1). Responses were received from patients with ten different arrhythmia substrates although the majority (70.3%) fell into just three categories (flutter, atrial fibrillation, and atrio-ventricular nodal re-entrant tachycardia).
Table 1.
Patient data by substrate
| Substrate | Number (%) | Mean age (range) | Male (%) |
|---|---|---|---|
| Atrial flutter—common (c/Flutter) | 143 (25.2) | 63.1 (26–88) | 105 (73.4) |
| AF | 131 (23.0) | 59.6 (28–79) | 95 (72.5) |
| AVNRT | 126 (22.1) | 50.1 (18–86) | 42 (33.3) |
| Complete AVN | 42 (7.4) | 74.7 (50–88) | 18 (42.9) |
| Overt accessory pathway (oAP) | 34 (6.0) | 47.1 (18–81) | 14 (41.2) |
| Atrial tachycardia (AT) | 28 (5.0) | 59.6 (17–85) | 9 (32.1) |
| Ventricular tachycardia (VT) | 20 (3.5) | 57.2 (22–80) | 12 (60.0) |
| Atypical flutter (u/Flutter) | 18 (3.2) | 57.1 (27–78) | 7 (38.9) |
| Concealed AP (cAP) | 15 (2.6) | 36.4 (17–55) | 7 (46.7) |
| Ventricular Ectopics (VE) | 12 (2.1) | 53.2 (26–75) | 7 (58.3) |
| Total | 569 (100) | 57.8 (17–88) | 315 (55) |
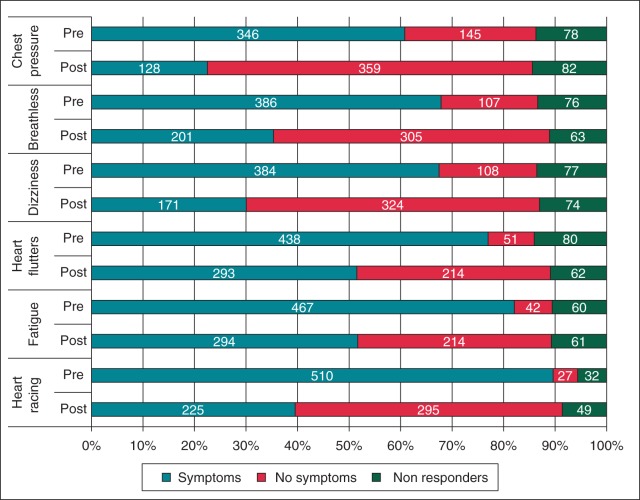
Responding patients reported an improvement across all symptoms investigated by the questionnaires (Figure 1) (symptoms reported by <60% of patients pre-procedure; headache, trouble concentrating, neck pounding, and fainting, not illustrated). There was a statistically significant difference in the proportion of patients reporting symptoms (mild, moderate, and severe combined) before the procedure compared with after the procedure (P < 0.001 for all 10 symptoms). There was also a reduction in the severity for those who remained symptomatic. The largest difference in symptom prevalence between the pre- and post-procedure reported symptoms was in heart racing [pre-procedure 510 patients (89.6%), post-procedure 225 patients (39.5%)] and the smallest was in headache [pre-procedure 216 patients (38.0%), post-procedure 154 patients (27.1%)]. Table 2 further illustrates changes in patient symptoms within the three largest arrhythmia substrate groups. Within these groups, atrio-ventricular nodal re-entrant tachycardia (AVNRT) patients were most likely to report a complete abolition of symptoms. However, the picture for symptom improvement was more mixed across the substrates, and with the exception of chest pressure, over 50% of patients from each group reported an improvement in symptom severity.
Figure 1.
Number of patients reporting symptoms before and after ablation.
Table 2.
Symptom change by substrate
| Heart racing | Fatigue | Heart flutter | Dizziness | Breathlessness | Chest pressure | |
|---|---|---|---|---|---|---|
| Percentage change in the number of patients reporting any symptoms following ablation (change in the number of patients) | ||||||
| All substrates | −53.9% (256) | −34.3% (149) | −33.1% (136) | −54.5% (192) | −47.3% (168) | −59.5% (185) |
| AF | −47.8% (54) | −36.5% (38) | −36.2% (38) | −54.3% (44) | −40.5% (34) | −49.2% (31) |
| AVNRT | −64.6% (73) | −48.4% (46) | −25.8% (24) | −66.7% (56) | −63.8% (51) | −80.9% (72) |
| C Flutter | −50.9% (59) | −31.0% (36) | −41.6% (42) | −42.4% (36) | −43.4% (43) | −45.3% (29) |
| Percentage of patients reporting an improvement in symptom severity following ablation (no. of patients) | ||||||
| All substrates | 81.27% (408) | 61.1% (291) | 62.2% (305) | 61.1% (208) | 56.3% (259) | 55.8% (251) |
| AF | 75.2% (91) | 65.5% (74) | 68.1% (77) | 76.1% (60) | 51.2% (65) | 39.0% (41) |
| AVNRT | 95.6% (109) | 63.6% (68) | 62.2% (67) | 68.2% (73) | 68.2 (73) | 83.0% (88) |
| C Flutter | 77.2% (95) | 67.5% (83) | 68.7% (79) | 56.5% (65) | 57.3% (67) | 39.8% (45) |
Every symptom studied showed a reduction in the proportion of patients reporting both severe and moderate symptoms (not combined). Again heart racing showed the largest improvement after treatment (Figure 2) with patients reporting severe and moderate heart racing reducing from 342 (63.7%) and 134 (25.0%) to 43 (8.3%) and 69 (13.3%), respectively. The smallest reduction was reported for headache with severe and moderate headaches reducing from 39 (8.5%) and 63 (13.8%) to 14 (2.8%) and 41 (8.3%), respectively.
Figure 2.
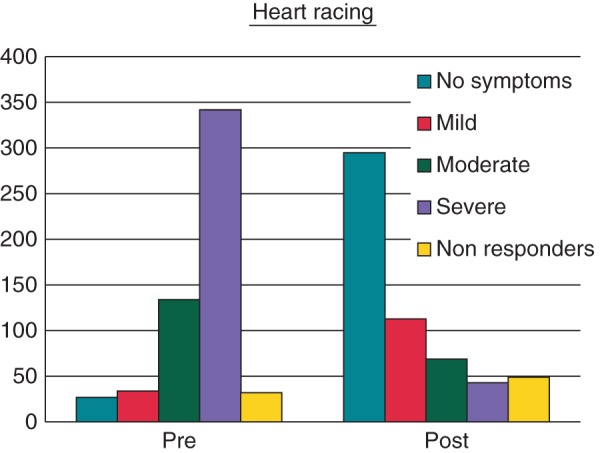
Patient reported heart-racing severity before and after ablation.
The responses showed a large decrease in both frequency of arrhythmia episodes and length of episode in those patients who still suffered arrhythmia following ablation. Pre-ablation, 15 patients (2.6%) “Never” suffered episodes of arrhythmia, rising to 270 patients (47.5%) following the procedure (Figure 3). The length of episode was significantly reduced, with patients experiencing average episodes lasting ≥1 h reducing from 292 (51.3%) pre-ablation to 29 patients (5.1%) post-ablation (Figure 4).
Figure 3.
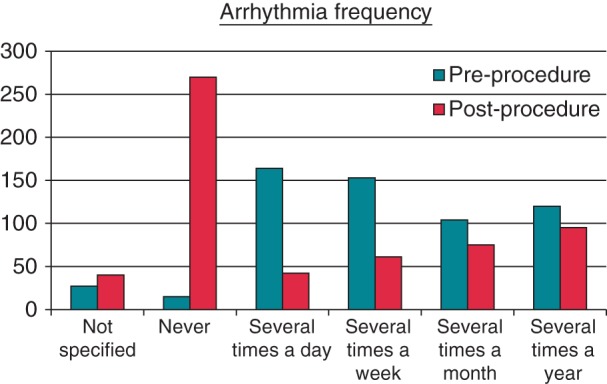
Arrhythmia frequency (how often do you get fast/irregular heartbeats?).
Figure 4.
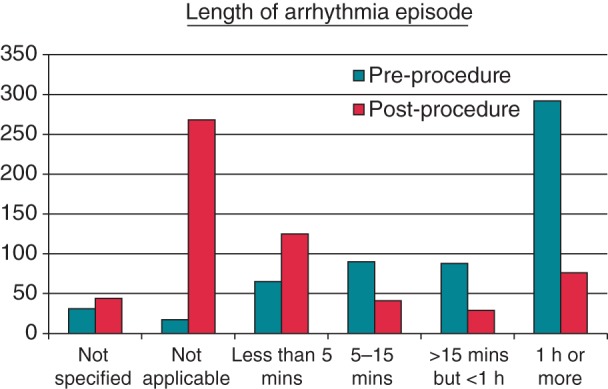
Episode length (how long do your episodes of fast/irregular heartbeat usually last?).
Changes in the impact of symptoms on functional ability
The PROM tool investigated the number of days' impact on social activities, days missed at work/school/college, and number of GP/hospital visits in the 30 days prior to the procedure and the 30 days prior to receiving the questionnaires post procedure. There was a reduction in the mean score after ablation in all the three impact parameters for the total participant group (P < 0.001) and across all 10 substrates (Table 3). For social days affected, statistically significant differences (P < 0.05) were observed in 9 of 10 substrates; for both work/school days affected and GP/hospital visits, statistically significant differences (P < 0.05) were observed in 6 of 10 substrates (Table 3).
Table 3.
Change in the impact following ablation
| Substrate | Social days affected |
Work/school days affected |
GP/hospital visits |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Reduction | 95% CI | P value | n | Reduction | 95% CI | P value | n | Reduction | 95% CI | P value | |
| AT | 23 | 7.96 | 2.62–13.30 | 0.005 | 21 | 3.86 | −0.23–7.95 | 0.063 | 19 | 0.90 | 0.34–1.45 | 0.003 |
| AF | 110 | 6.47 | 4.31–8.64 | <0.001 | 106 | 5.89 | 2.72–9.05 | <0.001 | 109 | 0.81 | 0.49–1.13 | <0.001 |
| AVN | 29 | 8.48 | 3.48–13.48 | 0.002 | 19 | 11.90 | 5.68–18.11 | 0.001 | 24 | 0.92 | −1.63–3.46 | 0.464 |
| AVNRT | 103 | 7.79 | 5.57–10.00 | <0.001 | 102 | 5.96 | 3.85–8.07 | <0.001 | 107 | 0.92 | 0.52–1.32 | <0.001 |
| cAP | 12 | 7.00 | −0.32–14.32 | 0.059 | 11 | 4.27 | −1.76–10.31 | 0.146 | 12 | 1.25 | 0.01–2.50 | 0.049 |
| oAP | 26 | 5.12 | 1.33–8.90 | 0.01 | 25 | 3.96 | −0.27–8.19 | 0.065 | 28 | 4.86 | −1.85–11.57 | 0.149 |
| u/Flutter | 15 | 7.67 | 1.90–13.44 | 0.013 | 13 | 6.69 | 0.70–12.68 | 0.031 | 14 | 3.07 | −1.15–7.29 | 0.14 |
| c/Flutter | 117 | 8.29 | 5.99–10.60 | <0.001 | 111 | 7.15 | 4.86–9.45 | <0.001 | 120 | 1.33 | 0.39–2.26 | 0.006 |
| VE | 11 | 9.36 | 2.69–16.04 | 0.011 | 10 | 10.30 | 2.38–18.22 | 0.016 | 11 | 3.36 | 0.49–6.23 | 0.026 |
| VT | 16 | 6.94 | 0.81–13.06 | 0.029 | 16 | 2.94 | −5.41–11.29 | 0.465 | 16 | 0.63 | −0.51–1.76 | 0.258 |
| Total | 462 | 7.49 | 6.42–8.55 | <0.001 | 434 | 6.26 | 5.04–7.47 | <0.001 | 460 | 1.36 | 0.85–1.87 | <0.001 |
Reduction here denotes reduction in the average number of days affected/visits reported before and after ablation.
The largest improvements in days affected (either social or work/school) were observed in atrio-ventricular nodal, AVN [a reduction of 8.48 social days affected (P = 0.002); 11.90 reduction in work/school days affected (P = 0.001)] and VE substrates [a reduction of 9.36 social days affected (P = 0.011); a reduction of 10.30 work/school days affected (P = 0.016)]; VE also accounted for the highest significant improvement in number of GP/hospital visits (3.36 reduction (P = 0.026)).
EQ5D data
The complete index values for both pre- and post-procedure questionnaires were available for 498 patients; these showed improvements in general health in 349 patients (70.1%). No change was observed in 97 (19.5%) patients, while 52 patients (10.4%) reported a worsening in general health. EQ5D VAS score had paired sample pre- and post-procedure responses for 530 patients. Here, improvements were observed in 399 patients (75.3%), with no change in 85 patients (16.1%) and poorer post-procedure general health reported in 46 patients (8.7%). The EQ5D VAS score showed a statistically significant difference pre- and post-procedure (19.09 mean improvement; P < 0.005); at a substrate level a statistically significant improvement was also seen for all substrates, except ventricular tachycardia (VT, P > 0.61); AT mean improvement 9.2 (P = 0.002); AF mean improvement 21.09 (P = <0.005); AVN mean improvement 18.31 (P ≤ 0.005); AVNRT mean improvement 17 (P ≤ 0.005); concealed accessory pathway (cAP) mean improvement 19.36 (P = 0.001); oAP mean improvement 12.5 (P = 0.012) u/Flutter mean improvement 27.29 (P < 0.005); c/Flutter mean improvement 19.69 (P < 0.005); VE mean improvement 27.73 (P = 0.003).
Unexpected complications
A total of 213 unexpected post-procedural complications were reported by 190 (33.4%) patients. These included clinical complications such as three cases of cardiac tamponade (incidence rate 0.5%), two transient ischaemic attacks (0.4%), and six strokes (1.1%). Excessive bruising was reported by 75 patients (13.2%), while “Other complications” including headache, bleeding at the entry site, tachycardia, chest pain, and dizziness were reported by 79 patients (13.9%), with one femoral pseudoaneurysm reported. Extended or re-hospitalizations for reasons including infections, pneumonia, dyspnoea, pulmonary embolism, and myocardial infarction were reported by 46 patients (8.1%). The majority of responders were positive about the outcome of their ablation, with 413 of 545 patients (75.8%) reporting that the procedure had met their expectations. Analysis of this outcome for the three most prevalent substrate groups revealed that expectations were met in 72.4, 88.6, and 72.8% of AF, AVNRT, and c/Flutter patients, respectively.
Non-responder data
Analysis of non-responder data using CCAD records found that non-responders were significantly more likely to have had a completely successful procedure (P < 0.024) (acute success assessed by the clinician). This finding is suggestive that the PROMs may underestimate the clinical benefit. The length of time between the procedure and receiving the PROM was also significant (P < 0.019), with patients who sent the questionnaires at <9 months post-procedure more likely to reply than those who sent it at ≥9 months. Age was also a significant factor, with non-responders likely to be younger than responders (P < 0.001). Gender, occurrence of complications, and urgency of treatment (elective vs. emergency) did not show any significant differences in response rates.
Issues and weaknesses
Responses highlighted weaknesses in some areas of the questionnaires. These included a lack of clarity with wording of some questions, e.g. for patients who had undergone a previous procedure “Please state how many you have had” did not specify whether to include the current procedure. Some patients indicated more than one answer for some questions as it was not specified that only one answer should be chosen. The questionnaires also attempted to capture the number of work days (or equivalent) affected by arrhythmia, and this question was aimed at all patients. However, it was ignored by a number of retired or unemployed patients who noted that they felt this did not apply to them; some other questions were also left blank when patients felt they were not applicable.
Comments added to questionnaires provided useful feedback. For example, a number of patients wrote that although their symptoms were unchanged since the procedure, they had been able to reduce or stop their medication; others noted that they suffered co-morbidities. Some patients commented that they were grateful for the opportunity to feedback on the procedure, with some adding remarks, including “I am very grateful for the operation that up to now has totally cured my symptoms. The doctor and team were fantastic and very professional. They made me feel very safe whilst in their care and I am extremely grateful for their help” and “I would like to say how impressed I was with both the quality of care I received and the kindness shown to me both during and after my cardiac cryoablation. The skill and professionalism of all the staff was greatly appreciated”.
Discussion
Findings
The findings of the study are encouraging, and the high response rate suggests that collection of PROMs in this patient group is feasible. The improvement in symptom occurrence and severity, reduction in the impact on work and social activities, and reduction in the number of GP/hospital visits indicate that the procedure has a considerable positive impact on the quality of life for the majority of patients. As well as the impact of symptoms on patients' functional abilities, the pre-ablation responses illustrate the wider implications regarding service use and work days lost. In general, the results of the newly developed tools showed high correlation with the EQ5D. However, the newly developed tools showed increased sensitivity to issues such as changes in patient symptoms and concerns, and provided useful data to compare between arrhythmia substrates. It was also able to identify complications and assess patient expectation.
Study limitations
As pre- and post-procedure questionnaires were sent out together, the volume of questionnaires may have deterred some responders. However, as the primary aim was to test acceptability and understanding of the questionnaires and the feasibility of gaining responses, this limitation was felt to be acceptable. Furthermore, as this study was not conducted in real time, some patients were completing the questionnaires up to 16 months post-procedure. This led to reliance on patient memory, and some struggled to recall how they felt prior to the procedure, while others may have given inaccurate answers, potentially biased by treatment outcomes for example, leading to erroneous data. Some patients received additional treatment during the time period or may have suffered from other health problems making it difficult to differentiate between arrhythmia-associated complications and non-related symptoms. In some cases, patients stated that they experienced an initial improvement in symptoms following the ablation, followed by a subsequent deterioration. This may have been due to a natural disease progression which would not have been picked up by the process if it had been administered in a traditional way. As with any PROMs it can be argued that the non-responders may be those who benefitted least (or most) from the procedure, again leading to skewed data.
Weakness of the tools
The weaknesses highlighted in the audit can largely be rectified by simple rewording of affected questions; however, some areas require more extensive work to clarify issues. This is the case in areas including how to address “normal routine” in patients who are retired or do not work, and how to encourage patients to complete all fields even when they feel a question does not apply to them.
Additional areas to consider for improving the questionnaire include the insertion of questions relating to medications used to identify changes in these following the procedure, and also information regarding co-morbidities. This will add depth of understanding to the questionnaires; particularly to the EQ5D which may show little change following a highly successful ablation procedure if a patient has unrelated medical problems. Using feedback from patients as part of an iterative process of questionnaire improvement is an important stage in the validation process.
Strengths of the study
The audit received a satisfactory response rate, suggesting that the length and content of the questionnaires were not unreasonable, even in the format used where both pre-and post-procedure questionnaires were received together. Feedback received from patients during telephone enquiries and via comments on the questionnaires was useful and gave an insight into areas requiring clarification: a useful mechanism to improve the tools. Patients were pleased to have the opportunity to comment on the treatment they had received.
High success rates for catheter ablation have been reported in the literature, with up to 99% success in some studies.14 The feedback received from patients in this study also suggests that ablation affords a high level of patient satisfaction, while giving data in a greater depth of detail. Overall, the results showed that cardiac ablation leads to a significant improvement in symptoms in the majority of patients; this supports its use as a technique for improving the quality of life in patients suffering from cardiac arrhythmias. Improvements seen in the EQ-5D VAS and Index scores (75.1 and 70.3% of patients reported an improvement, respectively) compare well with those observed in the National PROMs programme (during 2009–2010, groin, hernia, hip replacement, knee replacement, and varicose vein surgery saw VAS improvements of 39.1, 61.4, 50.8, and 39.8%, respectively, with index score improvements in 50.5, 86.7, 77.9, and 51.6%, respectively15).
Conclusion
This pilot study has provided a solid foundation on which to build a robust disease-specific PROM tool for cardiac ablation. The process was successful with high response and completion rates, supporting the feasibility of collecting PROMs in this patient group. Although this audit was only a preliminary part of the PROMs development procedure, the responses are a useful part of the validation process and facilitate improvements to the tools and method involved. The results of responses during these preliminary stages are very encouraging and illustrate the benefits of performing cardiac ablation in symptomatic patients. Further research is aimed at psychometric testing and validation of the tool. Cardiac arrhythmias have an adverse effect on the quality of life of many patients and successful treatment can be life-enhancing.
Further research
Following the success of this audit, further work into this area has been commissioned by the National Institute of Health and Care Excellence. Based on the responses received, the questionnaires have been updated and adapted into single pre- and post-procedure documents. Ethical approval has been given and a two-phase study is now underway (registered on the Clinical Trials website, reference: NCT01672528). This comprises of patient interviews to test, improve, and retest the questionnaires further and a postal survey phase involving up to 600 patients from three study centres as part of a prospective study. These patients will receive questionnaire pre-ablation, at 8–16 weeks, post-procedure and at 1 and 5 years post-procedure. The aim of this further study is to produce a validated PROMs tool for use in the UK to collect data from ablation patients. This will facilitate national measurement of the health change in patients following ablation therapy, and will allow statistical comparison between arrhythmia substrates and other subgroups.
Funding
Cedar is funded by the National Institute of Health and Care Excellence (NICE) to act as an External Assessment Centre (EAC) for the Medical Technologies Evaluation Programme. Funding to pay the Open Access publication charges for this article was provided by Cedar.
Acknowledgements
The authors thank Dr Mark Kelly (South East Wales Trials Unit, Cardiff University, Wales) for statistical support.
Conflict of interest: S.M. acts as an advisor to two companies which manufacture cardiac ablation equipment: Medtronic and Boston Scientific.
References
- 1.Darzi A. High quality care for all: NHS Next Stage Review final report http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_085828.pdf. (28 January 2013, accessed)
- 2.Department of Health. Equity and excellence: Liberating the NHS. 2010 July https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213823/dh_117794.pdf. (26 September 2013, accessed)
- 3.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 4.Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 5.Roehr B. Potential benefits of “well engaged patients” are akin to those from a blockbuster drug, say experts. BMJ. 2013;346:f886. doi: 10.1136/bmj.f886. [DOI] [PubMed] [Google Scholar]
- 6.Devlin N, Appleby J. Getting the Most Out of PROMs: Putting Health Outcomes at the Heart of NHS Decision-making. London: The Kings Fund; 2010. [Google Scholar]
- 7.NHS Choices. Heart Rhythm Problems http://www.nhs.uk/livewell/healthyhearts/pages/arrhythmias.aspx. (26 September 2013, accessed).
- 8.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 9.Wood KA, Wiener CL, Kayser-Jones J. Supraventricular tachycardia and the struggle to be believed. Eur J Cardiovasc Nurs. 2007;6:293–302. doi: 10.1016/j.ejcnurse.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrall G, Lane D, Carroll D, Lip G. Quality of life in patients with atrial fibrillation: a systematic review. AM J Med. 2006;119:448.e1–448.e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 11.Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation (Review) Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD005049.pub3. DOI:10.1002/14651858.CD005049.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Wood KA, Stewart AL, Drew BJ, Scheinman MM, Froëlicher ES. Development and initial psychometric evaluation of the patient perspective of arrhythmia questionnaire. Res Nurs Health. 2009;32:504–516. doi: 10.1002/nur.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EuroQol Group. EQ-5D-5L Value Sets http://www.euroqol.org/about-eq-5d/valuation-of-eq-5d/eq-5d-5l-value-sets.html. (11 September 2013 accessed)
- 14.O'Hara GE, Philippon F, Champagne J, Blier L, Molin F, Cote JM, et al. Catheter ablation for cardiac arrhythmias: a 14-year experience with 5330 consecutive patients at the Quebec Heart Institute, Laval Hospital. Can J Cardiol. 2007;23:67B–70B. doi: 10.1016/s0828-282x(07)71013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Health and Social Care Information Centre. 2012. Finalised Patient Reported Outcome Measures (PROMs) in England April 2010 to March 2011 Pre- and post-operative data https://catalogue.ic.nhs.uk/publications/hospital/proms/proms-eng-apr-10-mar-11-final/fina-prom-eng-apr-10-mar-11-pre-post-rep1.pdf. (27 September 2013, accessed)