Abstract
Nerve-mediated relaxation is necessary for the correct accomplishment of gastrointestinal (GI) motility. In the GI tract, NO and a purine are probably released by the same inhibitory motor neuron as inhibitory co-transmitters. The P2Y1 receptor has been recently identified as the receptor responsible for purinergic smooth muscle hyperpolarization and relaxation in the human gut. This finding has been confirmed in P2Y1-deficient mice where purinergic neurotransmission is absent and transit time impaired. However, the mechanisms responsible for nerve-mediated relaxation, including the identification of the purinergic neurotransmitter(s) itself, are still debatable. Possibly different mechanisms of nerve-mediated relaxation are present in the GI tract. Functional demonstration of purinergic neuromuscular transmission has not been correlated with structural studies. Labelling of purinergic neurons is still experimental and is not performed in routine pathology studies from human samples, even when possible neuromuscular impairment is suspected. Accordingly, the contribution of purinergic neurotransmission in neuromuscular diseases affecting GI motility is not known. In this review, we have focused on the physiological mechanisms responsible for nerve-mediated purinergic relaxation providing the functional basis for possible future clinical and pharmacological studies on GI motility targeting purine receptors.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| P2Y1 receptor | ATP |
| P2X receptors | Tetrodotoxin (TTX) |
| KCa2.3 channel | ODQ |
| SKCa channel | Apamin |
| SLC17A9 | IP3 |
| 5-HT4 receptor | MRS2179 |
| PDGFRα | MRS2500 |
| MRS2279 | |
| PPADS | |
| Suramin |
This Table lists key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,c,d,e).
Introduction
Purine receptors are classified into two families: receptors for adenosine (P1 receptors) and receptors for ATP and ADP (P2 receptors). P2 receptors are separated into two groups based upon their transduction mechanism. P2X receptors are ligand-gated ion channels and P2Y receptors are GPCRs. At present, seven P2X (P2X1–7) and eight P2Y (P2Y 1-2-4-6-11-12-13-14) receptor subtypes have been identified. Previous data using non-selective purinergic antagonists such as pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) or suramin already demonstrated a role for purine receptors in several functions of the gastrointestinal (GI) tract, including synaptic, neuromuscular transmission and secretion. However, due to the lack of selectivity of these antagonists (Hoyle et al., 1990; Vigne et al., 1998; Xue et al., 1999), it has, until recently, been impossible to identify the receptor(s) involved in purinergic neurotransmission. Newly developed antagonists of P2 receptors (Boyer et al., 1996; Camaioni et al., 1998; Cattaneo et al., 2004) have become important pharmacological tools for investigating the role of purines in GI function. In the present review, we will focus on the P2Y1 receptor, which is the receptor mainly involved in inhibitory neuromuscular transmission. The selectivity/potency of the pharmacological antagonists available might differ between species and it is noteworthy to point out important differences between frequently used laboratory animals and human tissue. Translational studies to move the research in purinergic neurotransmission from animal models to human samples have been a great part of the work of our laboratory for the last 10 years. Therefore, the aim of the present manuscript is to review the data available in the literature regarding the role of purine receptors and their pathways at the inhibitory neuromuscular junction.
Inhibitory junction potential (IJP)
In vitro, in intestinal preparations, electrical field stimulation (EFS) is usually employed to evoke tetrodotoxin (TTX)-sensitive action potentials in inhibitory motor neurons to release inhibitory neurotransmitters. EFS evokes an IJP in the smooth muscle cell, which is the electrophysiological basis for the mechanical relaxation or inhibition of the spontaneous contractions. It has been widely demonstrated that EFS induces the release of different neurotransmitters causing a fast IJP (IJPf) followed by a slow IJP (IJPs) (Crist et al., 1992; He and Goyal, 1993). Maybe with the exception of the human oesophageal body (EB) and the lower oesophageal sphincter (LES) (Lecea et al., 2011) (see below), this biphasic IJP is the most common electrophysiological response that can be recorded in different areas of the GI tract. Single pulses (or short trains of about 100 ms) induce an IJPf in human small intestine and colon (Figure 1) (Gallego et al., 2006; 2014). In other species, such as rodents and guinea pigs, the same stimulus causes an IJPf followed by an IJPs. A biphasic IJP can be recorded in human tissue using long trains of stimulation with high frequencies (usually about 5 Hz) (Figure 2) (Keef et al., 1993; Gallego et al., 2008a). It has been functionally demonstrated that vasointestinal polypeptide (VIP) is released in the mouse internal anal sphincter after long trains of EFS, leading to an ultraslow hyperpolarization and relaxation (Keef et al., 2013). It is not known whether VIP release can be measured in other areas of the GI tract under certain conditions of EFS. It is important to have all these differences in mind when muscle bath studies are performed, because different types of stimulation can cause the predominant release and/or post-junctional response of one or another neurotransmitter, whereas a different relative combination of neurotransmitters can be obtained by changing the frequencies of stimulation (Mañe et al., 2011).
Figure 1.
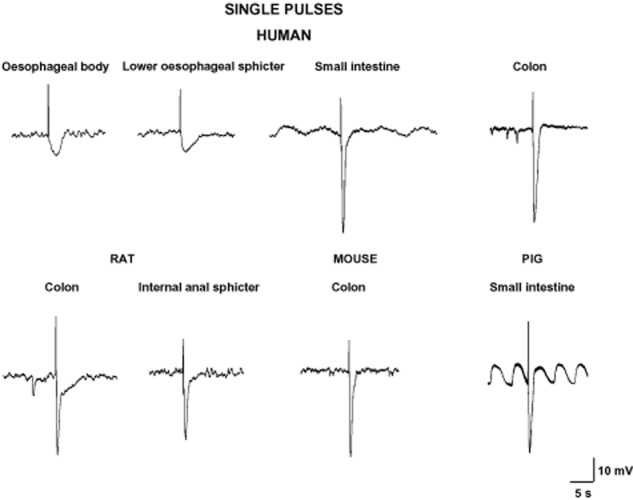
Single pulses or short trains elicit an IJPf in different areas of the GI tract. Note the absence of an IJPf in oesophageal tissues and the presence of spontaneous IJP in some tracings.
Figure 2.
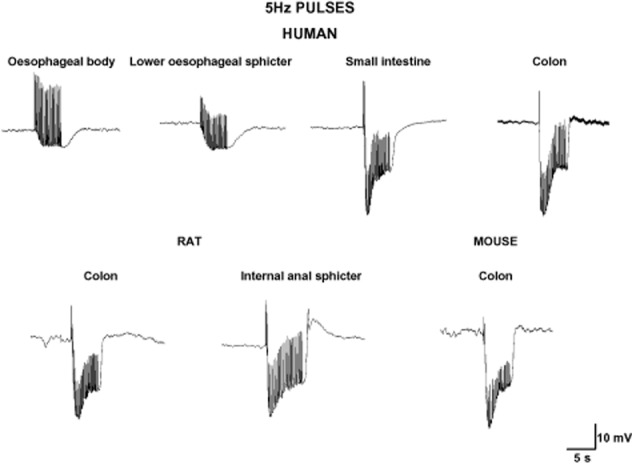
Pulses of 5 Hz for 5 s elicit a fast followed by a sustained hyperpolarization in different areas of the GI tract. Note the absence of an IJPf in the human oesophagus (oesophageal body and lower oesophageal sphincter).
Pharmacological evidence that the P2Y1 receptor mediates the IJPf
In the vast majority of laboratory animals (mouse, rat and guinea pig) and in human GI tissue, the IJPf is largely insensitive to NOS inhibitors and partially sensitive to suramin and PPADs (Xue et al., 1999). MRS2179, a selective P2Y1 receptor antagonist (Boyer et al., 1996; Gao et al., 2006), has been used to study neuromuscular interaction in the GI tract. MRS2179 was effective at blocking both the IJPf (Figure 3) and the non-nitrergic mechanical relaxation in different tissues and areas of the GI tract, including the human small intestine and colon (Table 1). The potency of MRS2179 varies between species; the IC50 is usually 1 μM in guinea pig, pig and human tissue. However, higher concentrations of this antagonist are needed in rodents (data from colon and internal anal sphincter) to inhibit the IJPf and the purinergic mechanical relaxation (Table 1). Due to the lack of complete blockade in rodents, it was postulated that other P2Y receptor subtypes might participate in the purinergic inhibitory neurotransmission. The development of two new P2Y1 antagonists, MRS2279 and MRS2500 (Cattaneo et al., 2004), with higher selectivity and potency for the P2Y1 receptor has opened up the possibility for further research. We have recently shown that the rank order of potency of P2Y1 antagonists is MRS2500 > MRS2279 > MRS2179 both in rat and in human colonic tissue (Figure 3) (Grasa et al., 2009; Gallego et al., 2011). For example, 20 μM of MRS2179 is needed to inhibit about 50% of the IJPf in the rat colon, but 1 μM of MRS2500 completely blocks the IJPf in this tissue. Comparatively, the IC50 to inhibit the IJPf in the human colon is about 1 μM for MRS2179 and about 70 nM for MRS2500. It would be important to study these newly available antagonists in other tissues where MRS2179 was not able to completely block the IJPf or the non-nitrergic relaxation (Table 1). According to these pharmacological studies, it is reasonable to conclude that the P2 receptor responsible for the fast component of the IJP and the EFS-induced relaxation is the P2Y1 receptor. An important exception is the dog’s small intestine where the IJPf seems to be at least in part sensitive to NOS inhibitors and consequently is nitrergic (Christinck et al., 1991a; Stark et al., 1991). However, an interaction between purinergic and nitrergic neurotransmission has been postulated in this species (Xue et al., 2000), but data using more selective P2Y1 antagonists are not available. Differences in pharmacological potency between species and different mechanisms of inhibitory neurotransmission indicate the importance of the animal model when performing translational studies.
Figure 3.
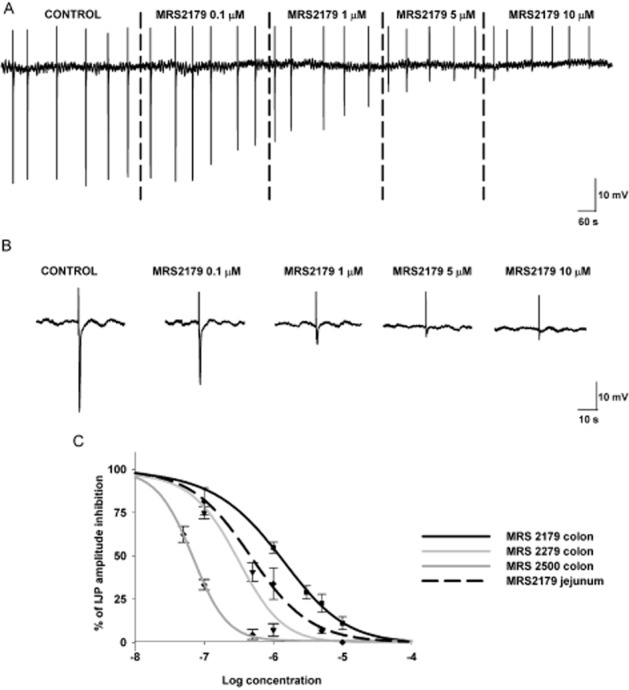
Inhibitory junction potentials are concentration-dependently inhibited by P2Y1 receptor antagonists. Tracings are from human jejunum and data from human jejunum and colon.
Table 1.
Effect of P2Y1 antagonists on inhibitory junction potential and mechanical relaxation
| Inhibitory junction potential | ||||
|---|---|---|---|---|
| Area of the GI tract | Species | Drug | Inhibition | Reference |
| Human tissue | ||||
| Colon | MRS2179 | |||
| Longitudinal | IC50: 1.31 μM | Gallego et al. (2006) | ||
| Circular | IC50: 1.21 μM | |||
| Colon | MRS2279 | IC50: 0.28 μM | Gallego et al. (2011) | |
| Circular | MRS2500 | IC50: 71 nM | ||
| Jejunum | MRS2179 | IC50: 0.55 μM | Gallego et al. (2014) | |
| Circular | ||||
| Laboratory animals* | ||||
| Ileum (circular) | Guinea pig (FS) | MRS2179 | IC50: 0.2 μM | Wang et al. (2007) |
| Ileum (circular) | Pig (EFS) | MRS2179 | IC50: 0.7 μM | Gallego et al. (2008b) |
| Caecum | Mouse (EFS) | MRS2179 | 10 μM: about 25% | Zizzo et al. (2007) |
| Caecum | Mouse (EFS) | MRS2179 | IC50: 8.8 μM | Gil et al. (2013) |
| MRS2500 | IC50: 20.1 nM | |||
| Colon | Mouse (EFS) | MRS2179 | 10 μM: about 80% inhibition | Zhang et al. (2010) |
| Colon | Rat (EFS) | MRS2179 | IC50: 13.1 μM | Grasa et al. (2009) |
| MRS2279 | IC50: 17.8 nM | |||
| MRS2500 | IC50: 14.0 nM | |||
| Internal anal sphincter | Mouse (EFS) | MRS2179 | 10 μM: about 50% | McDonnell et al. (2008) |
| Internal anal sphincter | Rat (EFS) and nicotinic-induced release | MRS2500 | 1 μM: 100% | Opazo et al. (2011) |
| Mechanical activity | ||||
|---|---|---|---|---|
| Area of the GI tract | Species | Drug | Inhibition | Reference |
| Human tissue | ||||
| Colon (circular) | MRS2179 | IC50: 0.87 μM | Gallego et al. (2006) | |
| Colon (circular) | MRS2179 | 10 μM: 100% inhibition purinergic latency | Auli et al. (2008) | |
| Ileum (longitudinal and circular) | MRS2179 | 10 μM: about 100% inhibition | Undi et al. (2009) | |
| Colon (circular) | MRS2279 | IC50: 0.26 μM | Gallego et al. (2011) | |
| MRS2500 | IC50: 88 nM | |||
| Jejunum and ileum (circular) | MRS2179 | 10 μM: about 100% inhibition | Gallego et al. (2014) | |
| Laboratory animals* | ||||
| Ileum (longitudinal) | Rat (mesenteric electrical stimulation) | MRS2179 | 10 μM: No effect | Kadowaki et al. (2003) |
| Jejunum (circular) | Mouse (EFS) | MRS2179 | 1 μM: From 100% to 60% | De Man et al. (2003) |
| Ileum (circular) | Pig (EFS) | MRS2179 | 10 μM: 60 to 80% | Gallego et al. (2008b) |
| Colon | Rat (EFS) | MRS2179 | IC50: 3.5 μM | Grasa et al. (2009) |
| MRS2279 | IC50: 43.9 nM | |||
| MRS2500 | IC50: 16.5 nM | |||
| Internal anal sphincter | Mouse (EFS: single stimuli) | MRS2179 | 10 μM: about 50% inhibition | McDonnell et al. (2008) |
| Internal anal sphincter | Rat (EFS) and nicotinic-induced release | MRS2500 | 1 μM: 100% inhibition | Opazo et al. (2011) |
| Internal anal sphincter | Sheep (EFS) | MRS2179 | 10 μM: about 35% inhibition | Acheson et al. (2009) |
Purinergic IJP and EFS-induced relaxation is absent in P2Y1-deficient mice.
EFS, electrical field stimulation; FS, focal electrical stimulation (ganglia or interganglionic fibre tracts).
Purinergic and nitrergic co-transmission
There is no structural or functional evidence about the presence of two or more different types of inhibitory motor neurons in the enteric nervous system. The most probable mechanism is a co-transmission process, that is, the same neuron releases at least two transmitters (Burnstock, 1976). Therefore, it is assumed that a purine and NO are released from the same neuron. Inhibition of the IJPf by P2Y1 receptor antagonists reveals the IJPs, which is then sensitive to NOS and GC inhibitors such as L-NNA and ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) respectively (Gallego et al., 2008a; Gil et al., 2012). Accordingly, the IJPs is NO-mediated and its effect is due to the stimulation of soluble GC, which produces cGMP. These results suggest parallel pathways of co-transmission between purines and NO, although a prejunctional interaction between both pathways is not definitively discarded (Van Crombruggen et al., 2007).
Lack of IJPf in P2Y1 knockout (KO) mice
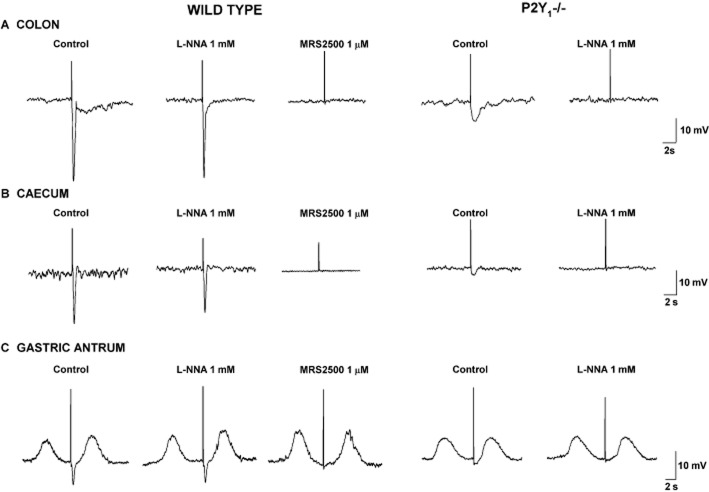
P2Y1 KO mice are excellent biological tools to investigate the involvement of P2Y1 receptors in purinergic neuromuscular transmission. Simultaneously, two groups published similar results showing that the IJPf is absent in the colon of P2Y1 KO mice (Gallego et al., 2012; Hwang et al., 2012). Interestingly, experiments were independently performed and the concordance in the results was noteworthy (King, 2012). It is important to note that P2Y1 KO mice exhibit preserved and functional nitrergic neurotransmission. The absence of IJPf in P2Y1 KO mice is not restricted to the colon, it is also observed in other GI tissues such as the stomach and caecum (Figure 4) (Gil et al., 2013). This experimental approach validates the pharmacological approach obtained in GI tissue from animals and humans and was considered substantial progress in the understanding of purinergic neuromuscular transmission in the gut (Goyal et al., 2013).
Figure 4.
Representative tracings summarizing studies in knockout mice. MRS2500-sensitive IJPf recorded in the colon (A), caecum (B) and gastric antrum (C) in wild-type animals are absent in P2Y1−/− mice. In P2Y1−/− animals, the IJPs is totally L-NNA-sensitive.
Purinergic response rundown
Single or short train pulses elicit IJPf that shows a reduction in amplitude when a second pulse (test pulse) is applied at different short time intervals after the first conditioning pulse (Gallego et al., 2008a). This mechanism has been previously denominated as IJP rundown in animal studies (King, 1994; Matsuyama et al., 2002) and can be clearly visualized when a 1 Hz pulse is applied (Figure 5). In human intestinal tissues, IJP rundown occurs in both the colon and small intestine. In other species such as rodents, the IJP rundown is also present (Mañe et al., 2011), but apparently less pronounced than in human tissue. The mechanism responsible for the IJP rundown is still not known and both pre- and post-junctional mechanisms might contribute to the decrease of the IJPf. In the hamster proximal colon, NO release might cause the IJPf rundown acting prejunctionally (Matsuyama et al., 2002), but this is not the case in human colon as the IJP rundown is still present after NOS blockade (Gallego et al., 2008a). It is possible that other purine receptors such as adenosine receptors might cause inhibition of purine release, but they are still not indentified. Post-junctional desensitization of the P2Y1 receptor (see the section Intracellular pathways in smooth muscle cells) is another possibility to consider in future studies.
Figure 5.
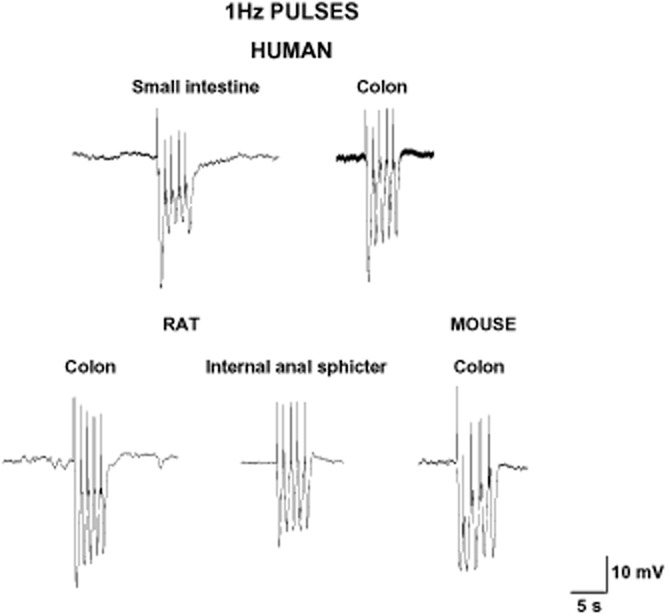
Pulse of 1 Hz for 5 s reveal the presence of an IJPf rundown. The first IJPf has a bigger amplitude compared with the following responses.
Inhibitory neural tone
EFS is the most common experimental procedure to induce in vitro neurotransmitter/s release. This is due to the fact that the electrical stimulus is repetitive, transient and usually independent of presynaptic inputs to inhibitory motor neurons. Interestingly, some tissues develop an inhibitory neural tone in vitro caused by ‘spontaneous’ release of inhibitory neurotransmitters not associated to classical EFS-induced junction potential. The neural tone is caused by action potentials in inhibitory neurons releasing both NO and a purine as co-transmitters. Accordingly, in tissues with endogenous neural activity, inhibitory neurotransmitters can be randomly released from nerve endings even in the absence of EFS. The post-junction electrophysiological consequences of an inhibitory neural tone are (i) neural-mediated hyperpolarized membrane potentials in smooth muscle cells and (ii) the appearance of spontaneous IJP (Figures 1 and 6). When the tissue is incubated with the neural blocker TTX, the membrane potential depolarizes, the tissue contracts and spontaneous IJP are inhibited (Gil et al., 2010). Interestingly, spontaneous IJP are absolutely insensitive to L-NNA, they are apamin sensitive (Spencer et al., 1998; Powell et al., 2001) and are inhibited by P2Y1 receptor antagonists (Gil et al., 2010). It is also well known that smooth muscle cells depolarize and tone increases after incubation in L-NNA. These results could be explained by the process of co-transmission: NO being responsible for the level of the membrane potential in smooth muscle cells and a purine, through P2Y1 receptors, for the spontaneous IJP. Thus, when an inhibitory neural tone is present, the muscular tone or spontaneous contractility of tissues incubated with L-NNA usually increases due to smooth muscle depolarization; whereas this does not occur after P2Y1 receptor blockade. In fact, when P2Y1 receptors are blocked, a decrease in spontaneous motility might occur (Gil et al., 2010). Spontaneous IJP can be recorded in the colon of wild-type (WT) mice and are MRS2500-sensitive (mediated by P2Y1 receptors). This pharmacological result is confirmed by the absence of spontaneous IJP in tracings obtained from P2Y1 KO mice (Figure 6) that presented a preserved and functional nitrergic inhibitory neural tone (Gallego et al., 2012).
Figure 6.
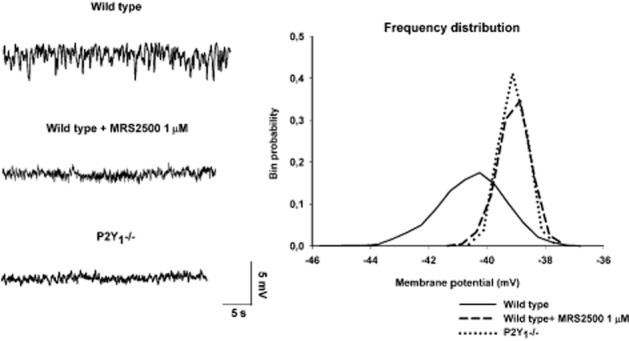
Spontaneous IJP are MRS2500 sensitive in wild-type mice and completely absent in P2Y1 KO mice. Frequency distribution of the RMP fully supports these results. In the frequency distribution of recordings from wild-type animals, ongoing sIJP create a tail towards the most negative values. This tail does not appear in the frequency distribution obtained from tissue incubated with MRS2500 or from P2Y1 KO mice. L-NNA-treated tissue (not shown) depolarizes smooth muscle cells without changing the internal frequency distribution, which is consistent with the presence of spontaneous IJP.
Complementary roles for ATP and NO
Experimental data suggest that NO might mediate sustained inhibition and relaxation, whereas activation of P2Y1 receptors probably causes phasic relaxation (Spencer et al., 1998; Gil et al., 2012). According to this hypothesis, (i) NO causes a sustained hyperpolarization, that is, the slow component of the IJP and continuous hyperpolarization when an inhibitory tone is present; (ii) no nitrergic desensitization occurs; otherwise, it would be impossible to constantly inhibit the motility; and (iii) NOS inhibitors cause a marked increase in tone and spontaneous motility when an inhibitory neural tone is present. In contrast, purinergic neurotransmission mediates phasic relaxation because (i) it causes a prominent but transient hyperpolarization; (ii) spontaneous IJP are recorded in a discontinuous manner; (iii) IJPf has a rundown; and (iv) blockade of P2Y1 receptors does not increase spontaneous contractility. Therefore, both neurotransmitters have complementary physiological functions (Gallego et al., 2008a; Gil et al., 2012; Mañe et al., 2011) (Table 2). The inhibitory electrophysiological and mechanical responses are only abolished when both pathways are inhibited, (Gallego et al., 2006; 2014) (Figure 7).
Table 2.
Mechanical and electrophysiological responses of inhibition of nitrergic and purinergic pathways
| Inhibition of | ||
|---|---|---|
| Nitrergic neurotransmission | Purinergic neurotransmission1 | |
| Membrane potential2 | Depolarization | No effect |
| Spontaneous motility2 | Increase | No effect/Decrease3 |
| Spontaneous IJP2 | No effect | Inhibition |
| EFS-induced IJP | Inhibition of the slow component | Inhibition of the fast component |
| EFS-induced relaxation4 | Partial reversion | No effect/Partial reversion |
Based upon previous data using inhibitors of P2Y1 receptors (Gallego et al., 2006; 2008a; 2011; Grasa et al., 2009) and P2Y1 KO mice (Gallego et al., 2012).
These criteria should be used if an inhibitory neural tone is present in the preparation (Gil et al., 2010).
A decrease in spontaneous motility might be expected if ATP is limiting pre-/post-junctional NO effect.
EFS-induced relaxations might be reversed by P2Y1 antagonists/NOS inhibitors depending upon the frequency of EFS.
Figure 7.
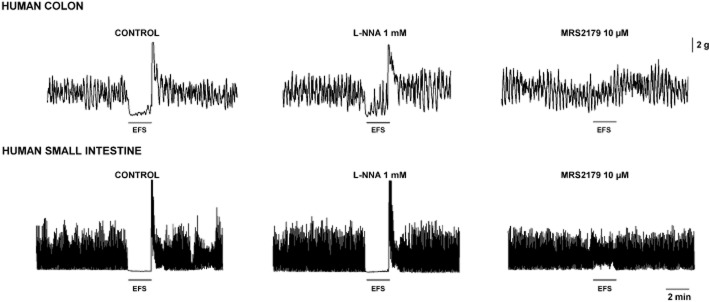
Both L-NNA and MRS2179 are necessary to inhibit (5 Hz, supramaximal voltage) EFS-induced relaxation in the human small and large intestine.
Apamin versus P2Y1 receptor antagonists
Apamin, a small conductance calcium-activated potassium channel (SKCa) blocker, is a pharmacological tool that has been used to distinguish between the IJPf and the IJPs. The terminology ‘apamin sensitive vs. apamin resistant’ is frequently used to distinguish both IJP components (Zhang et al., 2010). sKCa currents are activated by P2Y receptor agonists and blocked by apamin in smooth muscle cells (Koh et al., 1997; Vogalis and Goyal, 1997). Apamin usually reduces the IJPf, showing that SKCa channels are responsible for the fast hyperpolarization. Therefore, both P2Y1 antagonist and apamin should have ‘similar’ effects. However, this is not totally true, and in some cases, important differences exist between P2Y1 antagonists and apamin. The reduction in the IJPf amplitude caused by apamin varies depending on the species. Apamin abolishes the IJP in the guinea pig and a major reduction is observed in other species such as rodents, pig or dog. In guinea pig, focal stimulation causes a ‘pure’ purinergic fast IJP, which is both MRS2179- and apamin-sensitive (Koh et al., 1997; Wang et al., 2007). These data show that in these species, smooth muscle hyperpolarization is largely mediated by SKCa activation. In contrast, in the mouse colon, apamin-sensitive and apamin-resistant IJPf (both of them are MRS2179-sensitive) have been recently reported. The difference between both IJPf might be the projection of the inhibitory motor neuron, that can be oral, aboral or circumferential (Zhang et al., 2010). Interestingly, in the human small intestine and colon, the reduction obtained with apamin in the IJPf is only about 25–30%, suggesting that the majority of the response is independent of SKCa channels or, alternatively, the SKCa channels involved are apamin-insensitive (Xue et al., 1999; Gallego et al., 2006). Moreover, when the IJPf and the IJPs are recorded, apamin reduces both components in human colonic tissue (Keef et al., 1993). Isolation of the nitrergic component with MRS2500 reveals an IJPs in the rat colon, which is nitrergic and partially inhibited by high concentrations of apamin (Gil et al., 2012). Furthermore, apamin usually increases spontaneous motility in the colon and also depolarizes smooth muscle cells. These results suggest that apamin might not be an appropriate pharmacological tool to distinguish purinergic from nitrergic neurotransmission.
The oesophagus and LES: the exception
Due to anatomical similarities, the opossum has been an animal model to study neuromuscular transmission in the oesophagus. In this area of the GI tract, the IJP is largely apamin-insensitive (Cayabyab and Daniel, 1996; Jury et al., 1996) and probably the contribution of SKCa channels is minor due to a major NO component (Christinck et al., 1991b). Moreover, different innervations have been reported in clasp (biphasic IJP) and sling fibres (monophasic IJP) in the mouse LES with different sensitivities to apamin (Zhang et al., 2008). In the pig, the IJP of the EB is NO-mediated (Lecea et al., 2012). In the human EB and LES (both clasp and sling fibres), the IJP and the corresponding relaxation are monophasic and mainly nitrergic (Figures 1 and 2) (Lecea et al., 2011).
Intracellular pathways in smooth muscle cells
P2Y1 receptors are GPCRs that activate PLC. The second messenger, IP3 (inositol 1,4,5-trisphosphate), causes the release of calcium from intracellular stores mainly located in the sarcoplasmic reticulum. This mechanism has been demonstrated in different subclasses of enteric neurons (Kimball et al., 1996; Christofi et al., 1997), enteric glial cells (Kimball and Mulholland, 1996) and smooth muscle of laboratory animals (Blottiere et al., 1996; Pacaud et al., 1996; Bayguinov et al., 2000; Kong et al., 2000). In colonic myocytes, a P2Y receptor agonist causes an increase in cytosolic ‘calcium puffs/sparks’ and increases spontaneous transient outward currents, which are both charybdotoxin and apamin sensitive (Bayguinov et al., 2000; Kong et al., 2000). Localized calcium release near the plasma membrane causes the electrical event responsible for purinergic hyperpolarization. Data from our laboratory show that ADPβS, a preferential P2Y1/12/13 receptor agonist, causes calcium transients both in enteric neurons (Gallego et al., 2008b) and in human cultured colonic smooth muscle cells (Martinez-Cutillas et al., 2011). In both cell types, the calcium rise is blocked by MRS2179, showing that P2Y1 receptors are specifically involved in the response. The increase in the concentration of cytosolic calcium and/or DAG activates PKC, a kinase that has been reported to be responsible for P2Y1 desensitization in platelets (Hardy et al., 2005) and endothelial cells (Rodriguez-Rodriguez et al., 2009). It is still not known if this pathway is responsible for the rundown of the IJPf. A recent paper suggests that the P2Y1 receptor is a GPCR not linked to PLC. In this study, activation of P2Y1 receptors in colonic myocytes causes a reduction in IP3 and postulates a new mechanism of action for the receptor leading to smooth muscle hyperpolarization and relaxation (MacMillan et al., 2012).
Role of P2Y1 receptors in motility
P2Y1 receptor antagonists have not been studied in vivo. In a set of experiments with anaesthetized rats where spontaneous motility was monitored with a strain gauge, NOS inhibition caused a dramatic and long-lasting increase in spontaneous motility, whereas MRS2500 induced a single but prominent contraction without a major effect on subsequent contractions (Gil et al., 2010). These results further confirm the hypothesis that both inhibitory neurotransmitters have complementary physiological functions (Gil et al., 2012). Studies performed to investigate colonic motility in vitro using transit of pellets showed that both incubation with MRS2500 (in WT animals) and depletion of P2Y1 receptors (in KO mice) induced delayed colonic transit. These findings indicate that both nitrergic and purinergic inhibitory pathways are necessary to accomplish a proper motor function (Hwang et al., 2012). Although complementary roles are suggested by these contractile and electrophysiological experiments, it is possible that one pathway might partially compensate for the other one when it is blocked pharmacologically or genetically removed in KO mice.
The ‘intercalation’ theory
Two theories are currently supported by different groups regarding the cell types involved in neuromuscular transmission:
The ‘intercalation’ theory suggests that a non-muscular cell type [interstitial cells of Cajal (ICC) or platelet-derived growth factor receptor α (PDGFRα+) cells] mediates neuromuscular transmission.
The direct theory suggests a direct contact between motor neurons and smooth muscle cells without any kind of intermediate cell.
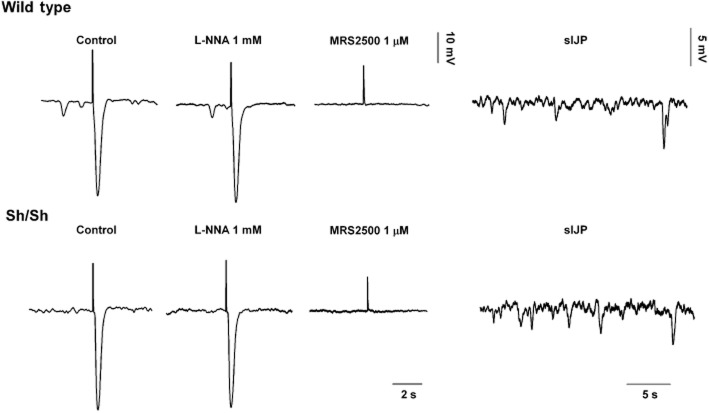
This is a controversial issue, and contradictory experimental data supporting both theories have been published by outstanding groups in the field of neurogastroenterology. Conditionally, KO mice lacking GC in smooth muscle have functional nitrergic neurotransmission (Groneberg et al., 2011). These experiments support the intercalation theory, suggesting that GC in ICC might transduce nitrergic inputs from inhibitory motor neurons to muscle (Burns et al., 1996; Ward and Sanders, 2001; Suzuki et al., 2003). However, nitrergic neuromuscular transmission is present in genetically modified animals where GC is removed from ICC, suggesting that both direct and indirect communications are possible (Groneberg et al., 2013). Purinergic neurotransmission is largely independent of the ICC. Mutant animals with impaired development of ICC including Ws/Ws rats (Alberti et al., 2007) and Wsh/Wsh mice (Figure 8) have intact purinergic neurotransmission. Recordings from colonic tissue display MRS2500-sensitive ‘spontaneous’ IJP and IJPf. Consequently, purinergic neurotransmission is independent of ICC. PDGFRα+ cells (fibroblast-like cells) can transduce purinergic signals and have the apparatus to do so (Cobine et al., 2011; Kurahashi et al., 2011; 2012) as shown by (i) the presence of P2Y1 receptors in these cells (Kurahashi et al., 2011); (ii) the abundance of KCa2.3 (previously known as SK3) channels (Vanderwinden et al., 2002; Fujita et al., 2003; Iino and Nojyo, 2009) that might contribute to the hyperpolarization; and (iii) the fact that potential agonists of P2Y1 receptors activate large-amplitude apamin-sensitive currents that were blocked by MRS2500 (Fujita et al., 2003; Kurahashi et al., 2011). Accordingly, PDGFRα+ cells, as described previously for smooth muscle cells, have the potential/capacity to transduce purinergic inputs (Figure 9). Animals with a decreased number of PDGFRα+ cells will be important to investigate the relative contribution of each cell type to the intercalation hypothesis. One important issue that needs to be solved is how two different cell populations (ICC and PDGFRα+ cells) can transduce in parallel two neurotransmitters apparently co-transmitted from the same neuron. Another unknown mechanism is how signals are transduced from intercalated cells to smooth muscle (Figure 9). Finally, if the intercalation hypothesis is confirmed, then, as it was suggested long time ago by Ed Daniel for NO, exogenous addition of purines might not always exactly mimic (see below) the effect of endogenous release of neurotransmitters. ‘If receptor to the same mediator on interstitial cells and on smooth muscle differ in the response they initiate, the actions of mediator added to the bath may not duplicate those of receptor mediate from nerves’. (Daniel and Posey-Daniel, 1984). With this sentence, Daniel already postulated that according to the intercalation hypothesis, exogenous addition of neurotransmitters might not exactly mimic the endogenous release if different receptors are located in smooth muscle and ICC. This might also be applicable for purinergic neurotransmission if the intercalation theory is validated (Figure 9).
Figure 8.
Purinergic fast and spontaneous IJP are recorded in deficient mouse (Wsh/sh) with impaired ICC development (unpublished data).
Figure 9.
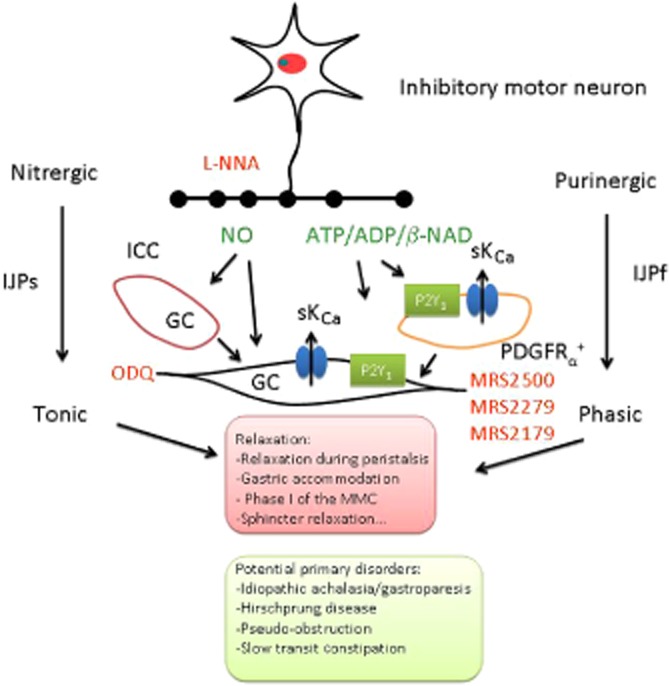
Smooth muscle relaxation is accomplished by enteric inhibitory motor neurons in the gastrointestinal tract. NO and a purine (ATP/ADP/β-NAD) are possibly co-released by inhibitory motor neurons. GC (ODQ sensitive) mediates the nitrergic slow component of the IJP. P2Y1 receptors (MRS2179-, MRS2279- and MRS2500-sensitive) mediate the purinergic fast component of the IJP. Smooth muscle can transduce both nitrergic and purinergic signals through a direct communication. ICC and PDGFRα+ cells are potential intercalated cells that might transduce nitrergic and purinergic inputs to smooth muscle cells respectively. Due to the electrophysiological profile of the response, nitrergic IJP is tonic since it can be time-sustained, whereas purinergic IJP is phasic because the response runs down. The combination of both mechanisms is responsible for relaxation in different regions of the gastrointestinal tract that might be potentially impaired in primary and secondary disorders affecting the neuromuscular junction. [agonists (neurotransmitters) are depicted in green and antagonists (blocking these pathways at different levels) are depicted in red].
Identification of the purinergic neurotransmitter
ATP was identified by Burnstock as the main purinergic inhibitory neurotransmitter in the GI tract (Burnstock et al., 1970). ATP is rapidly hydrolysed by the activity of ectonucleotidases into ADP and adenosine that might be biologically active and contribute to smooth muscle hyperpolarization and relaxation. The work to demonstrate the relevance of purinergic neurotransmission has been long and difficult (Burnstock, 2008). ATP has been considered the main purinergic neurotransmitter in the human small and large intestine (Xue et al., 1999; Gallego et al., 2006). Recently, β-nicotinamide dinucleotide (β-NAD) and ADP-ribose (Mutafova-Yambolieva et al., 2007; Durnin et al., 2012) have been proposed to be the purinergic NANC inhibitory mediators in the GI tract. These two mediators bind to P2Y1 receptors and cause apamin-sensitive and MRS2500-sensitive hyperpolarizations (Hwang et al., 2011). However, in human tissues, high concentrations of β-NAD are needed to inhibit spontaneous contractility and the effect is not blocked by P2Y1 receptor antagonists (Gallego et al., 2011). Exogenously added β-NAD induces a small hyperpolarization in human tissue that does not mimic the IJPf (Gallego et al., 2011). In the mice colon, β-NAD-induced hyperpolarization is partially blocked by MRS2500 and strongly reduced in P2Y1 KO mice (Gallego et al., 2012). However, in the caecum, β-NAD-induced hyperpolarization is insensitive to MRS2500 and still recorded in P2Y1 KO mice (Gil et al., 2013). β-NAD could bind to extrajunctional receptors, and consequently, it might not really mimic the endogenous release of the inhibitory neurotransmitter. However, this has still not been validated. Similarly, exogenously added ATP/ADP does not exactly mimic the endogenous neurotransmitter, for example, ATP-induced smooth muscle hyperpolarization in the human colon is insensitive to MRS2500 (Hwang et al., 2011). ATP overflow measured after EFS is not blocked by TTX or ω-conotoxin (GVIA) (Durnin et al., 2013). More than 40 years after the initial finding (Burnstock et al., 1970; Burnstock, 2008), the nature of the purinergic neurotransmitter in the GI tract is still not known and is debatable (Goyal, 2011).
P2Y1 receptors in other cell types
P2Y1 receptors are located in different subclasses of enteric neurons including submucosal and myenteric neurons. It has been demonstrated that slow excitatory synaptic transmission is mediated by P2Y1 receptors in guinea pigs (Hu et al., 2003; Gao et al., 2006; Gwynne and Bornstein, 2009). In this species, P2Y1 receptors might also participate in neurogenic secretion (Fang et al., 2006). P2Y1 receptors also participate in the enterochromaffin neural secretomotor arch in the human small intestine. Using calcium imaging in human submucous neurons, stimulation of intermodal strands cause the release of purines that act on post-synaptic neurons causing P2Y1/Gαq/PLC/IP3/Ca2+ signals. This effect is effectively blocked by the P2Y1 antagonist MRS2179 (Wunderlich et al., 2008). Recently, it has been demonstrated that neural purinergic release causes activation of glial cells and the response might be mediated by P2Y4 and P2Y1 receptors (Gomes et al., 2009; Gulbransen and Sharkey, 2009). Altogether, these results demonstrate purinergic neural communication between enteric neurons and glial cells.
Translational studies
A problematic issue regarding purinergic neuromuscular transmission has been the difficulty in convincing clinicians about the relevance of the mechanism in the human GI motility (Sanger et al., 2013). Probably, the identification of NO early in the 1990s (Bult et al., 1990; Christinck et al., 1991a; Stark et al., 1991; Boeckxstaens et al., 1993; Keef et al., 1993; Goyal and He, 1998) and the association of enteric pathologies with the lack of nitrergic neurons (Mearin et al., 1993; Boeckxstaens et al., 1994) was a strong argument to postulate that NO is the ‘main’ inhibitory mediator in the GI tract. Nowadays, gastroenterologists with great expertise in motility are usually not aware of purinergic nerve-mediated relaxation, probably due to the apparent lack of diseases associated with an impairment of purinergic neurotransmission. The general approach for clinicians to study neuromuscular diseases is pathological studies with tissue samples (Knowles et al., 2010; 2011). Purinergic neurons are not routinely labelled with histopathological techniques. Only the quinacrine technique has been proposed as a potential marker of purinergic neurons (Olson et al., 1976; Burnstock et al., 1978). Recently, staining of the vesicular nucleotide transporter (V-NUT) SLC17A9 has been proposed as a marker for purinergic neurons (Chaudhury et al., 2012) but the exclusiveness of the transporter in purinergic vesicles and not in other non-purinergic vesicles needs further evidence. Therefore, only experimental functional studies demonstrating purinergic neurotransmission in apparently healthy tissue have been demonstrated. Very few studies have been performed to investigate a possible impairment of purinergic neurotransmission in pathological conditions and most of them have been performed in animal models (Roberts et al., 2012). In inflamed guinea pig distal colon, a marked decrease in the fast component of the IJP has been reported (Strong et al., 2010). The reduction was attributed to an altered release or degradation of ATP acting on P2Y1 receptors. Interestingly, the nitrergic component was not affected, suggesting a selective damage of the purinergic neurotransmission causing peristalsis impairment. A very interesting study has been recently published demonstrating selective impairment of purinergic release due to oxidative stress in two models of colonic inflammation (Roberts et al., 2013). It is not known if purinergic neuromuscular transmission is impaired in inflamed samples from human tissue. Neuropharmacological studies on prokinetic drugs such as 5-HT4 receptor agonists are usually focused on promoting excitatory neurotransmission and the general belief is that an increase in excitatory neurotransmission will promote transit. Unfortunately, it is usually not known if these drugs also promote inhibitory neurotransmission and no data are available about the effect of these drugs on purinergic neurotransmission, which, in turn, might also facilitate transit.
Conclusions
The research in purinergic neurotransmission in the GI tract started more than 40 years ago (Burnstock et al., 1970). Recently, new important data have been generated using the newly developed selective P2Y1 receptor antagonists and genetically modified animals that lack P2Y1 receptors. According to these recent data, we have now strong reasons to believe that the receptor that contributes to purinergic smooth muscle relaxation has been identified. Inhibitory neuromuscular transmission in the GI tract therefore involves at least two inhibitory co-transmitters: a purine and NO (Figure 9). During the last 10 years, efforts have been made to demonstrate that this co-transmission is the general mechanism of neural-mediated relaxation in the human small and large intestine. It is feasible that different types of mechanical relaxation and consequently physiological roles can be ascribed to each inhibitory neurotransmitter (Figure 9). An effort should be made to further investigate possible purinergic neurotransmission involvement in neuromuscular diseases as P2Y1 receptors are possible pharmacological/genetic targets to consider. The effect of drugs that modulate purinergic neuromuscular transmission should be also studied to find better treatments for GI motility disorders. Without this effort, purinergic neurotransmission will remain a crucial physiological finding without ‘apparent’ clinical or pharmacological relevance.
Acknowledgments
The work of these authors and time spent on this review was supported by the following grants of the Spanish (SAF2003-05830, BFU2006-05055/BFI and BFU2009-11118/BFI) and Catalan government (2009SGR-708 and 2011 CTP 00032). D. G. is funded by Centro de Investigación Biomédica en red (CIBERehd), Instituto de Salud Carlos III. The authors thank the work of Ricard Farré, Victor Gil, Míriam Martinez-Cutillas, Noemí Mañé, Alvaro Opazo, Begoña Lecea and Maite Martin for their contribution to their knowledge in understanding purinergic neurotransmission in the gastrointestinal tract.
Glossary
- β-NAD
β-nicotinamide dinucleotide
- EB
oesophageal body
- EFS
electrical field stimulation
- GI
gastrointestinal
- ICC
interstitial cells of Cajal
- IJP
inhibitory junction potential
- IJPf
fast inhibitory junction potential
- IJPs
slow inhibitory junction potential
- IP3
inositol 1,4,5-trisphosphate
- KO
knockout
- LES
lower oesophageal sphincter
- L-NNA
Nω-nitro-L-arginine
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- MRS2279
(1R*,2S*)-4-[2-chloro-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester
- MRS2500
(1R*,2S*)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2 (phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PDGFRα
platelet-derived growth factor receptor α
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- SKCa
small conductance calcium-activated potassium channel
- KCa2.3 (SK3)
small conductance calcium-activated potassium channel 3
- TTX
tetrodotoxin
- VIP
vasointestinal polypeptide
- WT
wild type
Author contributions
M. J. wrote the manuscript. P. C. and A. A. provided human samples during the last years and contributed to the discussion of the manuscript. D. G. performed experiments presented as figures and contributed to the discussion, revision and editing of the manuscript.
Conflict of interest
The authors disclose no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1562. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1603. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013c;170:1607–1640. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013d;170:1676–1703. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013e;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Rayment S, Eames T, Mundey M, Nisar P, Scholefield J, et al. Investigation of the role of adrenergic and non-nitrergic, non-adrenergic neurotransmission in the sheep isolated internal anal sphincter. Neurogastroenterol Motil. 2009;21:335–345. doi: 10.1111/j.1365-2982.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- Alberti E, Mikkelsen HB, Wang X, Diaz M, Larsen JO, Huizinga JD, et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- Auli M, Martinez E, Gallego D, Opazo A, Espin F, Marti-Gallostra M, et al. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol. 2008;155:1043–1055. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- Blottiere HM, Loirand G, Pacaud P. Rise in cytosolic Ca2+ concentration induced by P2-purinoceptor activation in isolated myocytes from the rat gastrointestinal tract. Br J Pharmacol. 1996;117:775–780. doi: 10.1111/j.1476-5381.1996.tb15259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens GE, Pelckmans PA, Herman AG, Van Maercke YM. Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology. 1993;104:690–697. doi: 10.1016/0016-5085(93)91003-z. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, Mebis J, Janssens J, Geboes K, De Man JG, Herman AG, et al. Clinical relevance of nitric oxide in the gut. Lancet. 1994;344:129. doi: 10.1016/s0140-6736(94)91315-3. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl. 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Cocks T, Crowe R. Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol. 1978;64:13–20. doi: 10.1111/j.1476-5381.1978.tb08635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, et al. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab FS, Daniel EE. Role of sarcoplasmic reticulum in inhibitory junction potentials and hyperpolarizations by nitric oxide donors in opossum oesophagus. Br J Pharmacol. 1996;118:2185–2191. doi: 10.1111/j.1476-5381.1996.tb15661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, He XD, Goyal RK. Role of myosin Va in purinergic vesicular neurotransmission in the gut. Am J Physiol Gastrointest Liver Physiol. 2012;302:G598–G607. doi: 10.1152/ajpgi.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinck F, Jury J, Cayabyab F, Daniel EE. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 1991a;69:1448–1458. doi: 10.1139/y91-217. [DOI] [PubMed] [Google Scholar]
- Christinck F, Jury J, Cayabyab F, Daniel EE. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 1991b;69:1448–1458. doi: 10.1139/y91-217. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Guan Z, Wood JD, Baidan LV, Stokes BT. Purinergic Ca2+ signaling in myenteric neurons via P2 purinoceptors. Am J Physiol. 1997;272:G463–G473. doi: 10.1152/ajpgi.1997.272.3.G463. [DOI] [PubMed] [Google Scholar]
- Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- De Man JG, De Winter BY, Seerden TC, De Schepper HU, Herman AG, Pelckmans PA. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol. 2003;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with beta-NAD(+) J Physiol. 2012;590:1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of beta-NAD(+) and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil. 2013;25:e194–e204. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, et al. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol. 2006;536:113–122. doi: 10.1016/j.ejphar.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Auli M, Clave P, Jimenez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008a;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Gallego D, Vanden Berghe P, Farre R, Tack J, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008b;20:159–168. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clave P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23:792–e338. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol. 2012;590:1943–1956. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Malagelada C, Accarino A, De Giorgio R, Malagelada JR, Azpiroz F, et al. Nitrergic and purinergic mechanisms evoke inhibitory neuromuscular transmission in the human small intestine. Neurogastroenterol Motil. 2014;26:419–429. doi: 10.1111/nmo.12293. [DOI] [PubMed] [Google Scholar]
- Gao N, Hu HZ, Zhu MX, Fang X, Liu S, Gao C, et al. The P2Y purinergic receptor expressed by enteric neurones in guinea-pig intestine. Neurogastroenterol Motil. 2006;18:316–323. doi: 10.1111/j.1365-2982.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Grasa L, Martin MT, Jimenez M. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G158–G169. doi: 10.1152/ajpgi.00448.2009. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Moha Ou Maati H, Peyronnet R, Martinez-Cutillas M, Heurteaux C, et al. Relative contribution of SKCa and TREK1 channels in purinergic and nitrergic neuromuscular transmission in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;303:G412–G423. doi: 10.1152/ajpgi.00040.2012. [DOI] [PubMed] [Google Scholar]
- Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M, Gallego D. P2Y(1) knockout mice lack purinergic neuromuscular transmission in the antrum and cecum. Neurogastroenterol Motil. 2013;25:e170–e182. doi: 10.1111/nmo.12060. [DOI] [PubMed] [Google Scholar]
- Gomes P, Chevalier J, Boesmans W, Roosen L, Van den Abbeel V, Neunlist M, et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil. 2009;21:870–e62. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- Goyal RK. Evidence for beta-nicotinamide adenine dinucleotide as a purinergic, inhibitory neurotransmitter in doubt. Gastroenterology. 2011;141:e27–e28. doi: 10.1053/j.gastro.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Goyal RK, He XD. Evidence for NO. redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. Am J Physiol. 1998;275:G1185–G1192. doi: 10.1152/ajpgi.1998.275.5.G1185. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Sullivan MP, Chaudhury A. Progress in understanding of inhibitory purinergic neuromuscular transmission in the gut. Neurogastroenterol Motil. 2013;25:203–207. doi: 10.1111/nmo.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D, Konig P, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology. 2011;140:1608–1617. doi: 10.1053/j.gastro.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Groneberg D, Lies B, Konig P, Jager R, Seidler B, Klein S, et al. Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology. 2013;145:188–196. doi: 10.1053/j.gastro.2013.03.042. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Gwynne RM, Bornstein JC. Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2009;297:G179–G186. doi: 10.1152/ajpgi.90700.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CH, Knight GE, Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol. 2003;550:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, et al. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–115. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- Jury J, Boev KR, Daniel EE. Nitric oxide mediates outward potassium currents in opossum esophageal circular smooth muscle. Am J Physiol. 1996;270:G932–G938. doi: 10.1152/ajpgi.1996.270.6.G932. [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Yoneda S, Takaki M. Involvement of a purinergic pathway in the sympathetic regulation of motility in rat ileum. Auton Neurosci. 2003;104:10–16. doi: 10.1016/s1566-0702(02)00257-6. [DOI] [PubMed] [Google Scholar]
- Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- Keef KD, Saxton SN, McDowall RA, Kaminski RE, Duffy AM, Cobine CA. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J Physiol. 2013;591:1489–1506. doi: 10.1113/jphysiol.2012.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem. 1996;66:604–612. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- Kimball BC, Yule DI, Mulholland MW. Extracellular ATP mediates Ca2+ signaling in cultured myenteric neurons via a PLC-dependent mechanism. Am J Physiol. 1996;270:G587–G593. doi: 10.1152/ajpgi.1996.270.4.G587. [DOI] [PubMed] [Google Scholar]
- King BF. Prejunctional autoinhibition of purinergic transmission in circular muscle of guinea-pig ileum; a mechanism distinct from P1-purinoceptor activation. J Auton Nerv Syst. 1994;48:55–63. doi: 10.1016/0165-1838(94)90159-7. [DOI] [PubMed] [Google Scholar]
- King BF. Resolution and concordance in dissecting the compound inhibitory junction potential. J Physiol. 2012;590:1777–1778. doi: 10.1113/jphysiol.2012.230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59:882–887. doi: 10.1136/gut.2009.200444. [DOI] [PubMed] [Google Scholar]
- Knowles CH, Veress B, Kapur RP, Wedel T, Farrugia G, Vanderwinden JM, et al. Quantitation of cellular components of the enteric nervous system in the normal human gastrointestinal tract – report on behalf of the Gastro 2009 International Working Group. Neurogastroenterol Motil. 2011;23:115–124. doi: 10.1111/j.1365-2982.2010.01657.x. [DOI] [PubMed] [Google Scholar]
- Koh SD, Dick GM, Sanders KM. Small-conductance Ca(2+)-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol. 1997;273:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- Kong ID, Koh SD, Sanders KM. Purinergic activation of spontaneous transient outward currents in guinea pig taenia colonic myocytes. Am J Physiol Cell Physiol. 2000;278:C352–C362. doi: 10.1152/ajpcell.2000.278.2.C352. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–1404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecea B, Gallego D, Farre R, Opazo A, Auli M, Jimenez M, et al. Regional functional specialization and inhibitory nitrergic and nonnitrergic coneurotransmission in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2011;300:G782–G794. doi: 10.1152/ajpgi.00514.2009. [DOI] [PubMed] [Google Scholar]
- Lecea B, Gallego D, Farre R, Clave P. Origin and modulation of circular smooth muscle layer contractions in the porcine esophagus. Neurogastroenterol Motil. 2012;24:779–789. doi: 10.1111/j.1365-2982.2012.01936.x. [DOI] [PubMed] [Google Scholar]
- MacMillan D, Kennedy C, McCarron JG. ATP inhibits Ins(1,4,5)P3-evoked Ca2+ release in smooth muscle via P2Y1 receptors. J Cell Sci. 2012;125:5151–5158. doi: 10.1242/jcs.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañe N, Gil V, Martinez-Cutillas M, Martin MT, Gallego D, Jimenez M. Dynamics of inhibitory co-transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1500-8. doi: 10.1007/s00424-014-1500-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Cutillas M, Gallego D, Gil V, Martin MT, Jimenez M. Pharmacological characterization of purinergic receptors in human colonic smooth muscle cells using calcium imaging. Neurogastroenterol Motil. 2011;23(1):50. [Google Scholar]
- Matsuyama H, Unno T, El-Mahmoudy AM, Komori S, Kobayashi H, Thapaliya S, et al. Peptidergic and nitrergic inhibitory neurotransmissions in the hamster jejunum: regulation of vasoactive intestinal peptide release by nitric oxide. Neuroscience. 2002;110:779–788. doi: 10.1016/s0306-4522(01)00580-2. [DOI] [PubMed] [Google Scholar]
- McDonnell B, Hamilton R, Fong M, Ward SM, Keef KD. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1041–G1051. doi: 10.1152/ajpgi.00356.2007. [DOI] [PubMed] [Google Scholar]
- Mearin F, Mourelle M, Guarner F, Salas A, Riveros-Moreno V, Moncada S, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993;23:724–728. doi: 10.1111/j.1365-2362.1993.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L, Alund M, Norberg KA. Fluorescence-microscopical demonstration of a population of gastro-intestinal nerve fibres with a selective affinity for quinacrine. Cell Tissue Res. 1976;171:407–423. doi: 10.1007/BF00220234. [DOI] [PubMed] [Google Scholar]
- Opazo A, Lecea B, Gil V, Jimenez M, Clave P, Gallego D. Specific and complementary roles for nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol Motil. 2011;23:e11–e25. doi: 10.1111/j.1365-2982.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Feolde E, Frelin C, Loirand G. Characterization of the P2Y-purinoceptor involved in the ATP-induced rise in cytosolic Ca2+ concentration in rat ileal myocytes. Br J Pharmacol. 1996;118:2213–2219. doi: 10.1111/j.1476-5381.1996.tb15665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AK, Fida R, Bywater RA. Ongoing nicotinic and non-nicotinic inputs to inhibitory neurons in the mouse colon. Clin Exp Pharmacol Physiol. 2001;28:792–798. doi: 10.1046/j.1440-1681.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Lukewich MK, Sharkey KA, Furness JB, Mawe GM, Lomax AE. The roles of purinergic signaling during gastrointestinal inflammation. Curr Opin Pharmacol. 2012;12:659–666. doi: 10.1016/j.coph.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN, Mawe GM. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol. 2013;591:3725–3737. doi: 10.1113/jphysiol.2013.254136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez R, Yarova P, Winter P, Dora KA. Desensitization of endothelial P2Y1 receptors by PKC-dependent mechanisms in pressurized rat small mesenteric arteries. Br J Pharmacol. 2009;158:1609–1620. doi: 10.1111/j.1476-5381.2009.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ, Broad J, Kung V, Knowles CH. Translational neuropharmacology: the use of human isolated gastrointestinal tissues. Br J Pharmacol. 2013;168:28–43. doi: 10.1111/j.1476-5381.2012.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RA, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Syst. 1998;69:115–121. doi: 10.1016/s0165-1838(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991;444:743–761. doi: 10.1113/jphysiol.1991.sp018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol. 2010;588:847–859. doi: 10.1113/jphysiol.2009.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undi S, Benko R, Wolf M, Illenyi L, Vereczkei A, Kelemen D, et al. The NANC relaxation of the human ileal longitudinal and circular muscles is inhibited by MRS 2179, a P2 purinoceptor antagonist. Life Sci. 2009;84:871–875. doi: 10.1016/j.lfs.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Van Crombruggen K, Van Nassauw L, Timmermans JP, Lefebvre RA. Inhibitory purinergic P2 receptor characterisation in rat distal colon. Neuropharmacology. 2007;53:257–271. doi: 10.1016/j.neuropharm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, de Kerchove d’Exaerde A, Jr, Gillard K, Panthier JJ, de Laet MH, et al. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- Vigne P, Pacaud P, Loirand G, Breittmayer JP, Frelin C. PPADS inhibits P2Y1 purinoceptors in rat brain capillary endothelial cells and in rat ileal myocytes by an indirect mechanism. Biochem Biophys Res Commun. 1998;244:332–335. doi: 10.1006/bbrc.1998.8262. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Goyal RK. Activation of small conductance Ca(2+)-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J Physiol. 1997;502(Pt 3):497–508. doi: 10.1111/j.1469-7793.1997.497bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, et al. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–G1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang Y, Grants I, et al. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2008;294:G554–G566. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Sarr MG, Szurszewski JH. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Szurszewski JH. Effect of exogenous ATP on canine jejunal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2000;278:G725–G733. doi: 10.1152/ajpgi.2000.278.5.G725. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mashimo H, Paterson WG. Regional differences in nitrergic innervation of the smooth muscle of murine lower oesophageal sphincter. Br J Pharmacol. 2008;153:517–527. doi: 10.1038/sj.bjp.0707573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther. 2010;333:602–611. doi: 10.1124/jpet.109.160978. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R. Inhibitory purinergic transmission in mouse caecum: role for P2Y1 receptors as prejunctional modulators of ATP release. Neuroscience. 2007;150:658–664. doi: 10.1016/j.neuroscience.2007.09.055. [DOI] [PubMed] [Google Scholar]