Abstract
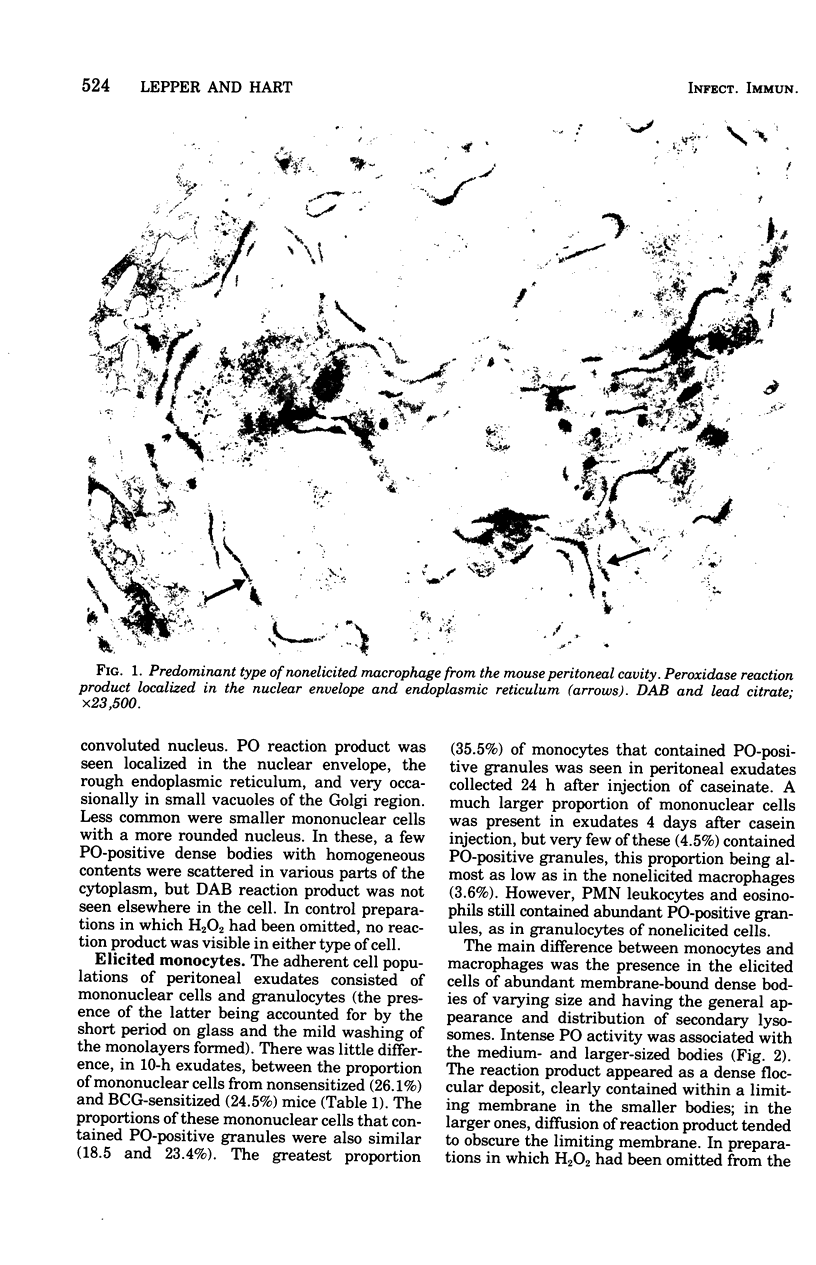
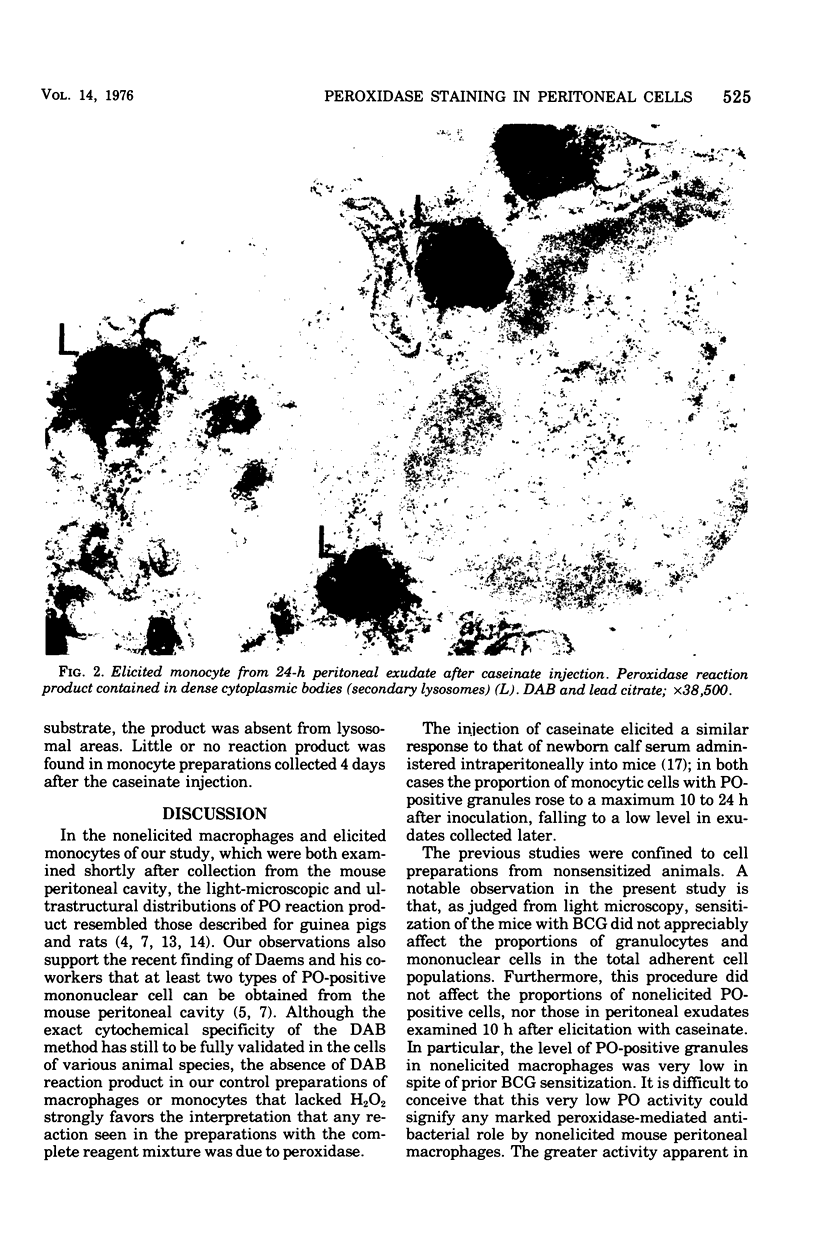
The peroxidase (PO) activity in nonelicited macrophages and in casein-elicited monocytes, obtained from peritoneal cavities of nonsensitized and BCG-sensitized mice and cultivated on glass for 1 or 2 h, was studied by light and electron microscopy, using the 3,3'-diaminobenzidine technique. These two types of glass-adherent peritoneal cells differed in PO activity. In macrophages, PO activity was predominantly in the nuclear envelope, rough endoplasmic reticulum, and occasionally in vesicles of the Golgi apparatus. In monocytes, PO activity was confined to cytoplasmic dense bodies resembling lysosomes, and was greater at 10 and 24 h after elicitation than at 96 h. The BCG sensitization did not significantly alter the proportion of cells with PO-positive granules in macrophages or monocytes from that observed in nonsensitized mice. From its lysosomal site, the PO in monocytes could come into contact with those microorganisms whose ingestion by these cells was followed by phagolysosome formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Litt M. Ultrastructural localization of horseradish peroxidase and endogenous peroxidase activity in guinea pig peritoneal macrophages. J Immunol. 1970 Dec;105(6):1536–1546. [PubMed] [Google Scholar]
- Daems W. T., Brederoo P. Electron microscopical studies on the structure, phagocytic properties, and peroxidatic activity of resident and exudate peritoneal macrophages in the guinea pig. Z Zellforsch Mikrosk Anat. 1973 Nov 5;144(2):247–297. doi: 10.1007/BF00307305. [DOI] [PubMed] [Google Scholar]
- Daems W. T., Poelman R. E., Brederoo P., van Lohuzen E. J. Peroxidatic activity in resident peritoneal macrophages and exudate monocytes of the guinea pig after ingestion of latex particles. J Histochem Cytochem. 1973 Jan;21(1):93–95. doi: 10.1177/21.1.93. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D., Fahimi H. D., Cotran R. S. Fine structural cytochemical localization of peroxidase activity in rat peritoneal cells: mononuclear cells, eosinophils and mast cells. J Histochem Cytochem. 1971 Sep;19(9):571–575. doi: 10.1177/19.9.571. [DOI] [PubMed] [Google Scholar]
- Simmons S. R., Karnovsky M. L. Iodinating ability of various leukocytes and their bactericidal activity. J Exp Med. 1973 Jul 1;138(1):44–63. doi: 10.1084/jem.138.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]