Abstract
Background and Purpose
The psychoactive cannabinoid Δ9-tetrahydrocannabinol (THC) and the non-psychoactive cannabinoid cannabidiol (CBD) can both reduce cancer progression, each through distinct anti-tumour pathways. Our goal was to discover a compound that could efficiently target both cannabinoid anti-tumour pathways.
Experimental Approach
To measure breast cancer cell proliferation/viability and invasion, MTT and Boyden chamber assays were used. Modulation of reactive oxygen species (ROS) and apoptosis was measured using dichlorodihydrofluorescein and annexin/propidium iodide, respectively, in combination with cell flow cytometry. Changes in protein levels were evaluated using Western analysis. Orthotopic and i.v. mouse models of breast cancer metastasis were used to test the activity of cannabinoids in vivo.
Key Results
CBD reduced breast cancer metastasis in advanced stages of the disease as the direct result of down-regulating the transcriptional regulator Id1. However, this was associated with moderate increases in survival. We therefore screened for analogues that could co-target cannabinoid anti-tumour pathways (CBD- and THC-associated) and discovered the compound O-1663. This analogue inhibited Id1, produced a marked stimulation of ROS, up-regulated autophagy and induced apoptosis. Of all the compounds tested, it was the most potent at inhibiting breast cancer cell proliferation and invasion in culture and metastasis in vivo.
Conclusions and Implications
O-1663 prolonged survival in advanced stages of breast cancer metastasis. Developing compounds that can simultaneously target multiple cannabinoid anti-tumour pathways efficiently may provide a novel approach for the treatment of patients with metastatic breast cancer.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| CB1 receptor | Δ9-tetrahydrocannabinol (THC) |
| CB2 receptor | Cannabidiol (CBD) |
| SR141716A (rimonabant) | |
| SR144528 |
This Table lists key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Tumour metastasis accounts for 90% of cancer-related deaths, but most existing cancer therapies were not developed to specifically target metastatic progression (Editorial, 2011). The processes leading to metastatic progression are still not well understood (Kraljevic Pavelic et al., 2011), and despite all currently available treatments, breast cancer is usually incurable once clinically apparent metastases develop. Therefore, there is an urgent need to develop non-toxic therapeutic interventions that specifically target metastatic progression.
The two most abundant cannabinoids in Cannabis sativa (CS) are tetrahydrocannabinol (THC) and cannabidiol (CBD). THC activates CB1 and CB2 receptors and is the primary psychoactive cannabinoid in CS as a result of its interactions with CB1 receptors in the CNS (Pertwee, 2006). CBD is the second most abundant cannabinoid in CS, but does not interact efficiently with CB1 and CB2 receptors and is not psychoactive (Showalter et al., 1996).
A recent, exciting area of study for the therapeutic applications of THC and CBD has been their anti-tumour activity against a variety of aggressive cancers (Velasco et al., 2012). Each compound, however, has its own unique mechanism of action. In culture and in vivo, the primary mechanisms leading to the inhibition of tumour progression by THC include de novo synthesis of ceramide, leading to endoplasmic reticulum stress and autophagy-mediated cell death (Carracedo et al., 2006a,b; Salazar et al., 2009). As opposed to THC, the pathways responsible for anti-tumour activity of CBD have not been well defined, particularly in vivo. In culture, the most unifying theme is the production of reactive oxygen species (ROS) (Ligresti et al., 2006; Massi et al., 2006; McKallip et al., 2006).
It has been proposed by our group and others that targeting both cannabinoid anti-tumour pathways simultaneously may provide enhanced anti-tumour activity (Marcu et al., 2010; Torres et al., 2011). The clinical application of this approach is limited when combining THC and CBD because of the psychoactivity of THC mediated through the activation of CB1 receptors. However, it has been shown that similar anti-tumour activity of mixed CB receptor agonists (those targeting CB1 and CB2) could be reproduced using non-psychoactive CB2 selective agonists (Blazquez et al., 2008).
We have previously reported that CBD inhibits Id1 gene expression and corresponding breast cancer aggressiveness in culture (McAllister et al., 2007). Id1 is a transcriptional regulator that has been shown to play a critical role in mediating breast cancer tumourigenicity and metastasis to the lung (Fong et al., 2003; Minn et al., 2005; Gupta et al., 2007; Swarbrick et al., 2008). Based on our discovery that CBD targets Id1 and our past experience with structure-activity relationship of cannabinoids (McAllister et al., 2002; 2003), we hypothesized that if it were possible to retain the anti-tumour activity of CBD in a single cannabinoid analogue that could also target CB2 receptors, this would lead to further enhancement of anti-tumour activity. Since THC and CBD individually have also been shown to have therapeutic efficacy against other disease states, there is also a potential broad-based use for this approach.
While some potential targets, such as Id1, explaining the anti-metastatic activity of CBD have been identified in culture, limited investigations have determined whether these targets are modulated in secondary tumours derived from disseminated cancer cells. The lack of a viable biomarker predicting successful targeting of metastasis by CBD limits the development of future clinical trials and the potential synthesis of more potent and efficacious analogues. Additionally, the pharmacological assessment of cannabinoids, particularly CBD, has been limited, with most studies incorporating the use of only a single dose and no cannabinoid has been previously shown to extend survival in a model of metastasis.
In this investigation, we determined that the anti-metastatic activity of CBD was directly related to the down-regulation of Id1 in vivo and, in addition, discovered that CBD was also effective at inhibiting advanced stages of metastasis. Based on these results, we then screened compounds and discovered a cannabinoid analogue that was more active than CBD at down-regulating Id1 and was also a CB2 selective agonist that could target CB2 receptor anti-tumour pathways. We present mechanistic data unique to this analogue that demonstrates inhibition of advanced stages of metastasis in preclinical models leading to prolonged survival.
Methods
Cell culture and drugs
All cell lines were cultured as we previously described (McAllister et al., 2007). Human MDA-MB231-luc-D3H2LN cells (PerkinElmer, Waltham, MA, USA) as well as MDA-MB231 + Id1 [infected with a pLXSN-Id1 retroviral vector as we previously published (McAllister et al., 2007)] were grown under the same conditions as wild-type cells. The compounds cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), SR141716A and SR144528 were obtained from National Institutes of Health (NIH; Bethesda, MD, USA) through the National Institute of Drug Abuse, and O-1663 was obtained from Organix, Inc. For the in vivo experiments, ethanol stocks of CBD and O-1663 were dissolved in a solution containing 2% ethanol, 2% Tween 80 and 96% saline.
Mouse models of breast cancer
For the in vivo studies, 6–8 week old female mice were used. Ten mice per group were used for the orthotopic studies and 6–8 mice per group were used for the i.v. model of metastasis. Mice were cared for as we previously described (McAllister et al., 2011). Survival studies were carried out in accordance with the National Institutes of Health’s guidelines involving experimental neoplasia and our approved IACUC protocol. Animals in all the different groups (control and treated) were removed from the study when they demonstrated any single sign indicative of significant tumour burden development in the lung including labored breathing, hunched back, closed eyes or decreased general activity. These signs generally preceded any significant decrease in weight. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Orthotopic model of breast cancer
Breast primary tumours and metastases were generated in 6–8 week old female BALB/c mice by injection of 4T1 cells into the mammary fat pad (MFP) as previously described (McAllister et al., 2011). CBD treatment was initiated upon first detection of the primary tumours (1 week) until the completion of the study (approximately 1 month). Treatment and analysis were performed as previously described (McAllister et al., 2011).
An i.v. model of breast cancer
The mouse 4T1 i.v. model was carried out as previously described (McAllister et al., 2011). For i.v. model experiments assessing the activity of CBD in human MDA-MB231 breast cancer cells, female athymic nu/nu mice were i.v. injected with 200 μL of RPMI containing 5 × 105 MDA-MB231 cells. One or 7 days after the injection, the tumour-bearing mice were i.p. injected once a day with vehicle (control) or varying doses of CBD or O-1663 until the completion of the study (approximately 8 weeks).
For experiments assessing the activity of cannabinoids in human MDA-MB231-luc-D3H2LN cells, female athymic nu/nu mice were i.v. injected with 200 μL of RPMI containing 0.25 × 106 cells. Two or 18 days after the injection, the tumour-bearing mice were i.p. injected once a day, for 5 days, with vehicle (control) or varying doses of cannabinoid until the completion of the study (approximately 8 weeks). Starting on week two, bioluminescence imaging (BLI) on mice was performed using an IVIS Lumina II (PerkinElmer). Fifteen minutes before imaging, mice were i.p. injected with 150 mg·kg−1 of luciferin (Gold Biotechnology, Olivette, MO, USA). Comparison of tumour progression over time was analysed between groups by comparing the absolute unit of radiance in photons s−1·cm−2 steradian−1 (p·s−1·cm−2·sr−1).
Western blotting
Western blotting was performed as previously described (McAllister et al., 2007). The blots were probed with anti-Id1 or anti-Id2 (McAllister et al., 2007), anti-αLC3 (Cell Signaling, Danvers, MA, USA), anti-CB2 (Abcam, Cambridge, MA, USA) and anti-actin that was used as a loading control. The relative amounts of protein were quantified using densitometry and the software programme ImageJ (NIH). All values in separate experiments were normalized to the corresponding vehicle (control) values. Resulting values were then converted to % change relative to control.
Proliferation and invasion assays
MTT assays (measuring cell proliferation/viability) and Boyden chamber invasion assays were performed as previously described (McAllister et al., 2007).
Apoptosis analysis
Cells were grown in 6-well culture dishes and treated with the appropriate compounds every 24 h for 2 days. Cells attached to the plate as well as cells in the media were collected, pelleted and processed for labelling with FITC-tagged annexin and propidium iodide (PI) using the Muse cell death and apoptosis kit (Millipore, Billerica, MA, USA). Labelled cells were analysed by cell flow cytometry using the Muse cell analyzer.
cAMP assay
The cAMP assay was carried out by Cerep (l’Evêque, France) as previously described (Felder et al., 1995).
Data analysis and statistical procedures
The IC50 values with corresponding 95% confidence limits were compared by analysis of logged data. When the confidence limits of the IC50 values overlapped, significant differences were determined using Student’s unpaired t-test. Significant differences were also determined using a one-way anova or the Student’s unpaired t-test, where suitable. Dunnett’s post hoc analyses were conducted when appropriate. Survival between groups was compared using a log-rank Mantel–Cox test. P-values < 0.05 defined statistical significance. All analyses were performed using GraphPad Prism software (La Jolla, CA, USA). Additional methods are described in the Supporting Information.
Results
CBD increases survival in a syngeneic mouse model of breast cancer
While CBD has been shown to inhibit breast cancer metastasis in vivo, a detailed pharmacological analysis to determine potency and efficacy has not been performed. Utilizing the 4T1 i.v. mouse model of breast cancer metastasis, we determined that CBD reduced the total breast cancer metastasis up to 75%, with an EC50 value of 0.3 mg·kg−1 (CI = 0.2–0.5), and when administered at 1 mg·kg−1, CBD increased survival (P < 0.006) (Supporting Information Fig. S1A–C).
The potency of CBD at targeting metastasis in the i.v. model was similar to that previously reported by our group using the 4T1 orthotopic model of metastatic progression (McAllister et al., 2011). CBD was also effective at reducing metastatic progression in an orthotopic model utilizing 4T1 cells (Supporting Information Fig. S2A–C) even when the drug was administered three times a week as opposed to daily, but the cannabinoid did not inhibit primary tumour growth. In both the i.v. and the orthotopic models, we observed that CBD was highly effective at targeting metastatic foci ≥2 mm. Since orthotopic models suffer from significant variability and CBD did not inhibit primary tumour growth, we continued our investigations into the anti-metastatic activity of CBD using the i.v. model of breast cancer metastasis.
The anti-metastatic activity of CBD is directly related to the down-regulation of Id1 expression in vivo
In the culture, the ability of CBD to inhibit breast cancer aggressiveness is the direct result of down-regulation of Id1 expression (McAllister et al., 2007). To determine whether the anti-metastatic activity of CBD was directly related to the down-regulation of Id1 in vivo, we first determined whether CBD down-regulated Id1 gene expression in the tumour tissue. We found that treatment with CBD produced a significant down-regulation of Id1 expression in metastatic foci in the lung (Figure 1A and B, and Supporting Information Table S1). CBD also produced a significant down-regulation of Ki67, demonstrating its ability to reduce tumour cell proliferation in metastatic foci (Figure 1C). To determine whether Id1 represented a key mediator of the effects of CBD in vivo, ectopic Id1 was constitutively expressed into MDA-MB231 cells (MDA-MB231+Id1) using the pLXSN retroviral vector. We have previously shown that CBD does not inhibit Id1 expression and invasion in these cells in the culture (McAllister et al., 2007). In cells expressing the control vector, treatment with CBD reduced lung metastasis, whereas CBD did not inhibit lung metastasis in tumours derived from MDA-MB231 + Id1 cells (Figure 1D and E).
Figure 1.
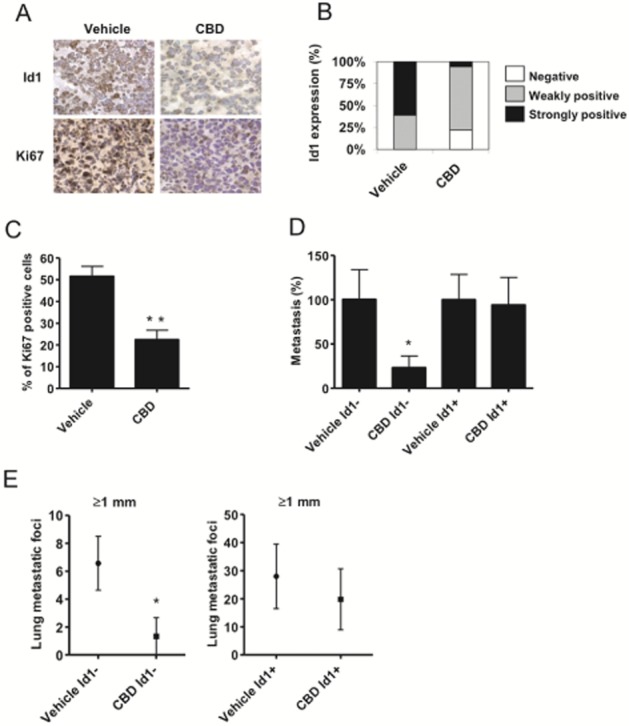
Inhibition of Id1 expression in vivo is necessary for the anti-metastatic activity of CBD. (A) Immunohistochemical detection of Id1 and Ki67 was performed in lung tissues of vehicle (left) and CBD (right) treated 4T1-derived tumours. Nuclei are visible in blue (haematoxylin staining). Pictures are ×400 magnification. (B) The intensity of Id1 expression is shown. (C) The percentage of Ki67 positive cells per lung metastatic foci was evaluated. (D) Lung metastases were generated in athymic nu/nu mice after i.v. injection of 5 × 105 human MDA-MB231 expressing control MDA-MB231 cells (Id1−) or MDA-MB231 cells that ectopically expressed Id1 (Id1+). Two days after the injection, the tumour bearing mice were injected i.p. once a day with vehicle or 1 mg·kg−1 CBD for 6 weeks. % metastatic foci = total metastatic foci in treated/vehicle × 100. (E) The number of lung metastatic foci ≥1 mm was compared between vehicle and CBD-treated groups. Data are presented as metastatic foci ≥1 mm in control MDA-MB231 cells (Id1−) compared with MDA-MB231 cells that ectopically expressed Id1 (Id1+). *P < 0.04 and **P < 0.002 indicate statistically significant differences from control.
To further confirm the correlation between the effects of CBD and inhibition of Id1 expression in culture and in vivo in human breast cancer cells, we established stable pooled populations of MDA-MB231 cells expressing Id1shRNA (Supporting Information Fig. S3A). We observed similar reductions in cell proliferation and invasion rate in the culture, and metastasis in vivo in MDA-MB231 cells expressing Id1shRNA or in parental MDA-MB231 cells treated with CBD (Supporting Information Fig. S3B–D).
CBD produces a dose-dependent inhibition of metastasis in advanced stages of breast cancer progression
CBD was most effective in targeting metastatic foci ≥2 mm, suggesting that the compound could be effective at inhibiting the growth of secondary tumours even after their initial establishment in lungs. We therefore treated mice at a time point where visual lung metastatic foci were already formed (day 7, Figure 2A). We found that CBD dose-dependently reduced the growth of established lung metastatic foci, reduced the formation of new metastatic foci and increased survival (Figure 2B–D). While the median increase in survival was only a day, a subset of animals did live 3–5 days longer (P < 0.02). Based upon these findings, we expected that synthesis of more active analogues based upon CBD would result in the development of a compound that could produce more robust inhibition of advanced stages of metastasis.
Figure 2.
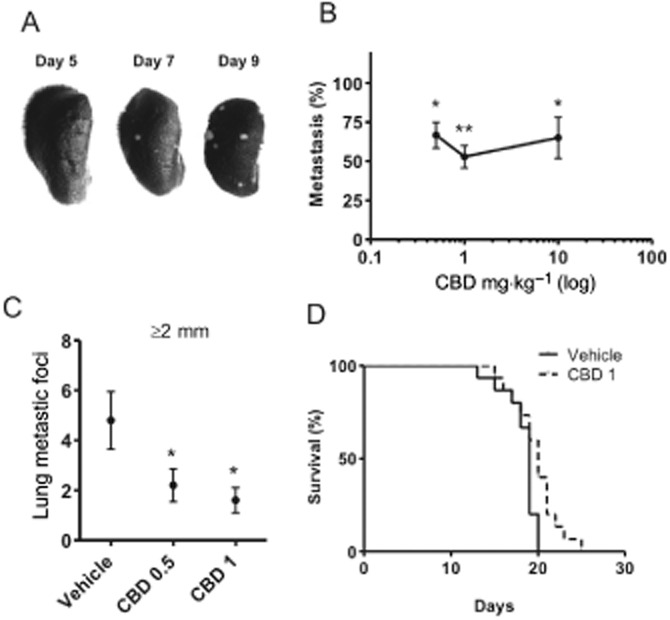
CBD reduces the formation of metastatic foci and increases survival in advanced stages of metastatic progression. Lung metastases were generated in BALB/c mice after i.v. injection of 2 × 104 mouse 4T1 cells. (A) The pictures are representative of tumour formation observed at days 5, 7 and 9. (B) Seven days after the injection of tumour cells, mice were injected i.p. once a day with vehicle, 0.5, 1 or 10 mg·kg−1 CBD for 7 days. % metastasis = total metastatic foci in treated/vehicle × 100. (C) The number of lung metastatic foci ≥2 mm was compared between vehicle and CBD-treated groups. (D) Mice treated with vehicle or 1 mg·kg−1 CBD, starting 7 days after i.v. injection of 2 × 104 4T1 cells, were observed until they demonstrated signs of disease progression that necessitated killing. Survival between groups was compared using a log-rank Mantel–Cox test. *P < 0.05 and **P < 0.01 indicate statistically significant differences from control.
O-1663 is more active than CBD at inhibiting cell proliferation, invasion and Id1 expression
Our past studies (McAllister et al., 2007) and a limited structural activity comparison based upon the targeting of Id1 (Supporting Information Fig. S4A and B) suggested the unique activity (inhibition of Id1 gene expression) of CBD was related to the opened pyran ring, the possession of an extended alkyl side chain and was not due to interactions with the abnormal CBD receptor. CBD has been reported to be an antagonist at the abnormal CBD receptor (Jarai et al., 1999); however, the abnormal CBD receptor antagonist O-1918 (Mo et al., 2004) did not inhibit Id1 expression. While selective activation of CB2 receptors leads to anti-tumour activity (Blazquez et al., 2008), we have shown that classical CB1 and CB2 receptor agonists are not efficient inhibitors of Id1, particularly THC (McAllister et al., 2007). We reasoned however that a CB2 selective cannabinoid agonist, having limited activity at CB1 receptors (psychoactivity), could be developed to target Id1, resulting in a single compound that could efficiently target multiple pathways associated with cannabinoid activity leading to enhanced anti-tumour activity.
We screened over 40 resorcinol derivatives because of their structural similarity with CBD and discovered that the analogue O-1663 was significantly more potent at inhibiting human (MDA-MB231) and mouse (4T1) breast cancer cell proliferation (Table 1). The rank order of potency for the compounds tested in MDA-MB231 cells was O-1663 > CBD > THC. The rank order of potency for the compounds tested in 4T1 cells was O-1663 > CBD = THC.
Table 1.
Inhibition of breast cancer cell proliferation/viability by cannabinoids
| Cell line | Compound | IC50 ( μM) | Confidence limits |
|---|---|---|---|
| MDA-MB231 | CBD | 1.9 | (1.5–2.5) |
| O-1663 | 0.85 | (0.79–0.91) | |
| THC | 3.0 | (2.3–3.9) | |
| 4T1 | CBD | 1.8 | (1.2–2.7) |
| O-1663 | 0.83 | (0.79–0.87) | |
| THC | 2.3 | (1.9–2.7) |
Experiments were carried out as previously described (McAllister et al., 2007). Data represent the mean with corresponding confidence limits for three to six independent determinations.
O-1663 was previously synthesized in a series of bicyclic resorcinol derivatives that resembled CBD (Supporting Information Fig. S4A) (Wiley et al., 2002). It has been previously shown to have lower affinity for CB1 receptors compared with THC and produces little activity in the tetrad assay (measure of psychoactivity in vivo) (Wiley et al., 2002). In a standard cannabinoid functional assay (cAMP inhibition), we also determined that O-1663 acted as a full agonist in the activation of CB2 receptors when compared with the potent and efficacious CB1 and CB2 receptor agonist, WIN 55,212-2 (Supporting Information Table S2). However, O-1663 was less potent than WIN 55,212-2 at producing cAMP inhibition.
O-1663 but not CBD reduces breast cancer cell aggressiveness through the activation of CB2 receptors
To directly test whether our analogue could co-target two distinct cannabinoid anti-tumour pathways, we investigated the effects of CBD and O-1663 on breast cancer cell viability/proliferation using multiple antagonists (Figure 3A and B). In all studies, the drugs were co-administered. The ROS scavenger, α-TOC, was able to reverse the inhibitory effects of CBD on cell viability/proliferation, while CB1 (SR141716A – SR1) and CB2 (SR144528 – SR2) selective antagonists produced no significant reversal. The ROS scavenger also reversed the activity of O-1663, but in contrast to CBD, SR14458 was able to partially reverse the inhibitory effects of O-1663 on breast cancer cell viability/proliferation. Mouse 4T1 and human MDA-MB231 breast cancer cells were both shown to express CB2 receptors (Supporting Information Fig. S5A). The antago nist alone had no significant effects on cell viability (Supporting Information Fig. S5B).
Figure 3.
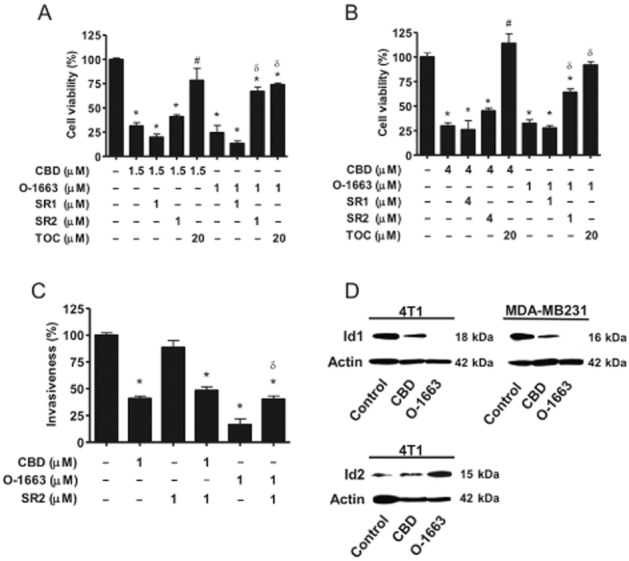
O-1663 and CBD are compared for their ability to inhibit breast cancer cell proliferation/viability, invasion and Id1 expression. (A) Mouse 4T1 and (B) human MDA-MB231, breast cancer cells were treated with vehicle, CBD or O-1663 for 2 days in the absence or in the presence of α-tocopherol (TOC), the CB1 receptor antagonist (SR141716A – SR1) or the CB2 receptor antagonist (SR144528 – SR2). Cell proliferation/viability was then evaluated using the MTT assay. (C) MDA-MB231 breast cancer cells were treated with CBD or O-1663 for 3 days in the absence or in the presence of the CB2 receptor antagonist (SR144528 – SR2). The ability of the cells to migrate and invade in modified Boyden chambers was then determined. The percentage relative proliferation/viability and invasion were calculated as the effect on treated cells/vehicle cells × 100. Vehicle-treated cells were set as 100%. (D) Proteins from 4T1 and MDA-MB231 cells treated with vehicle (control), 1.0 μM CBD or 1 μM O-1663 for 3 days were extracted and analysed for Id1 (4T1 and MDA-MB231 cells) using Western blot analysis. Id2 expression was also determined in 4T1 cells treated with vehicle (control), 1.5 μM CBD or 1.5 μM O-1663. *, # and δ indicate statistically significant differences from control, CBD and O-1663 respectively (P < 0.01).
We next compared the ability of CBD and O-1663 to inhibit cancer cell invasion (Figure 3C). O-1663 was 1.7-fold more potent than CBD at inhibiting the invasion of MDA-MB231 cells. The IC50 value and the corresponding confidence limits for O-1663 and CBD were 0.6 μM (0.5–0.7) and 1 μM (0.8–1.2) respectively. While no reversal of CBD activity was observed using the CB2 receptor antagonist SR144528, it was able to partially reverse the inhibitory effects of O-1663.
O-1663 is more potent than CBD at inhibiting Id1 expression and up-regulating ROS
Whereas CBD produced a partial reduction of Id1 expression, treatment with the same concentration of O-1663 produced almost a complete down-regulation of Id1 expression in mouse 4T1 and human MDA-MB231 breast cancer cells (Figure 3D). Id2 is a marker of good prognosis in breast cancer patients and is specifically up-regulated following inhibition of Id1 expression (Itahana et al., 2003). As demonstrated in Figure 3D, O-1663 was more potent than CBD at up-regulating Id2 expression in 4T1 breast cancer cells.
In the culture, CBD-induced generation of ROS (Supporting Information Fig. S5C) is a primary mechanism that leads to the inhibition of Id1 expression, cell growth, invasion and survival across multiple cancers (Ligresti et al., 2006; Massi et al., 2006; McKallip et al., 2006; McAllister et al., 2011). Using the approximate IC50 for CBD, O-1663 and THC for inhibition of cell proliferation/viability, we found that CBD and O-1663 produced a robust up-regulation of ROS, whereas THC produced no significant increase in ROS (Figure 4A). In the presence of SR144528, there was no significant reversal of ROS produced by CBD, whereas there was more than a 50% reduction in ROS produced by O-1663 (Figure 4B).
Figure 4.
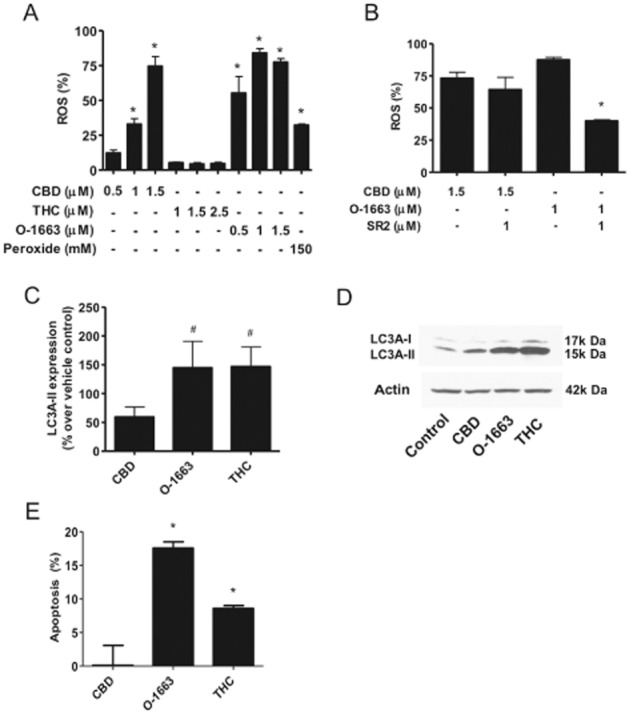
O-1663 targets anti-tumour pathways unique to both CBD and THC and is more potent than CBD at generating ROS. (A) Human MDA-MB231 cells were treated with vehicle (control), CBD, THC or O-1663 (μM) for 2 days and the production of ROS was then measured using 2′-7′dichloro-dihydrofluorescein and cell flow cytometry. The % increase in ROS was calculated as the FL2 emission shift in treated cells/vehicle cells × 100. (B) MDA-MB231 cells were treated with CBD or O-1663 (μM) for 2 days in the presence or absence of SR2. (C) Western blot analysis was performed using protein lysates from MDA-MB231 cells treated with vehicle, 1.5 μM CBD, 1.5 μM O-1663 or 3.0 μM THC for 2 days, and densitometric quantification of the LC3 II band was carried out using actin for normalization. The values were then converted to % change relative to vehicle (control). (D) A representative example of the Western blot analysis for LC3 is shown. (E) The number of cells positive for annexin staining after 2 days treatment with 1.5 μM CBD, 1.5 μM O-1663 or 3.0 μM THC was measured using cell flow cytometry analysis. Apoptosis (%) was calculated as positive annexin staining of the treated cells minus control cells. Data are the mean of at least three independent experiments; bars, ± SEM. * and # indicate statistically significant differences from control and CBD respectively (P < 0.01).
O-1663, but not CBD, stimulates autophagy and induces apoptosis
A primary mechanism for the anti-tumour activity of the mixed CB1 and CB2 receptor agonist (THC) and CB2 selective agonists is the up-regulation of the autophagy pathway (Velasco et al., 2012). A hallmark of autophagy is the conversion of the soluble form of LC3 (LC3-I) to the lipidated and autophagosome-associated form (LC3-II). In line with our hypothesis that O-1663 was efficiently targeting pathways that have been associated with the anti-tumour activity of CBD and THC, the analogue was as effective as THC at up-regulating LC3-II, and in addition was more effective at inducing apoptosis (Figure 4C–E). In comparison, CBD produced only a minor up-regulation of LC3-II and did not induce apoptosis at the concentration (1.5 μM) shown to produce marked up-regulation of ROS and down-regulation of Id1 gene expression.
In comparison to CBD, O-1663 is more active at reducing breast cancer metastasis
We next compared the activity of CBD to O-1663 in the 4T1 i.v. model of breast cancer metastasis (Figure 5A and B). We found that O-1663 was 2.3-fold more potent at inhibiting total metastasis [O-1663: EC50 = 0.13 (0.10–0.20); CBD: EC50 = 0.29 (0.18–0.49)] and 7.0-fold more potent than CBD at inhibiting lung metastatic foci ≥2 mm [O-1663: EC50 = 0.02 (0.01–0.05); CBD: EC50 = 0.15 (0.10–0.24)]. Further, 60% of the mice treated with O-1663 did not show any lung metastatic foci ≥2 mm. O-1663 was also more potent than CBD at inhibiting metastasis in the MDA-MB231 i.v. model of breast cancer metastasis (Figure 5C). Moreover, no overt toxicity was noted with O-1663 in the mouse models of metastasis as assessed by weight, appearance and general activity (data not shown).
Figure 5.
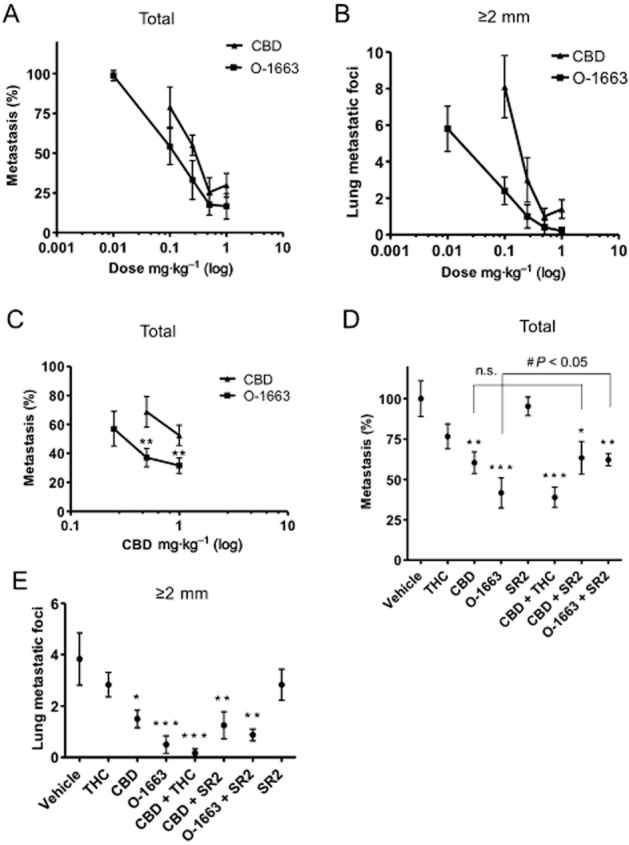
O-1663 is more potent than CBD at inhibiting breast cancer metastasis. (A, B) Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 mouse 4T1 cells. One day after the injection, the tumour-bearing mice were i.p. injected once a day with vehicle, CBD or O-1663 for 14 days. In (A), the % metastasis (total metastatic foci in treated/vehicle × 100), and in (B) the number of lung metastatic foci ≥2 mm, were evaluated. (C) Lung metastases were generated in athymic nu/nu mice after i.v. injection of 5 × 105 human MDA-MB231 cells. One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle, CBD or O-1663 for 6 weeks and % metastasis was compared. (D, E) Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 mouse 4T1 cells. One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle or 1 mg·kg−1 of cannabinoids for 14 days. In (D) the % metastasis, and in (E) the number of lung metastatic foci ≥2 mm, were compared. * P < 0.05, ** P < 0.01 and *** P < 0.001 indicate statistically significant differences from control.
The anti-metastatic activity of O-1663, but not CBD, is partially reversed by a CB2 receptor antagonist
To determine whether a portion of the anti-tumour activity of O-1663 was linked to CB2 receptor activation, we treated our mice-bearing 4T1 tumours with CBD or O-1663 in the presence of the CB2 receptor antagonist (Figure 5D and E). In agreement with our findings in the culture, the anti-metastatic activity of CBD was not affected by co-administration with SR144528 (SR2), whereas the anti-metastatic activity of O-1663 was partially reversed by the antagonist. In addition, we included a combination treatment with 1 mg·kg−1 CBD and 1 mg·kg−1 THC, the latter cannabinoid being able to activate CB2 receptors and up-regulate autophagy. We found that this combination CBD + THC produced the same level of anti-metastatic activity as 1 mg·kg−1 O-1663 (Figure 5E). Again, CBD and O-1663 were most active at targeting lung metastatic foci ≥2 mm. Interestingly, co-administration of O-1663 with SR144528 (SR2) only produced a minor non-significant reversal of the inhibitory effects of the analogue on lung metastatic foci ≥2 mm, suggesting that the CB2 receptor component of the anti-metastatic activity of O-1663 is a result of targeting the entire population of lung metastatic foci.
O-1663 inhibits advanced stages of metastasis and increases survival
To further compare the activity of CBD to O-1663, we carried out survival studies (Figure 6A–C). Seven days after i.v. injection of 4T1 cells, mice were treated daily with vehicle, 1 mg·kg−1 CBD (a dose producing maximum anti-metastatic activity) or 1 mg·kg−1 O-1663. CBD produced a medium increase in survival of 4 days that was not significant (P < 0.1), whereas O-1663 produced a medium increase in survival of 30 days (P < 0.006). In the group treated with O-1663, 50% of the mice were still alive and demonstrated no signs of disease progression at time of killing (2 months). Importantly, few visible lung metastatic foci were present in 20% of these mice (Supporting Information Fig. S6A).
Figure 6.
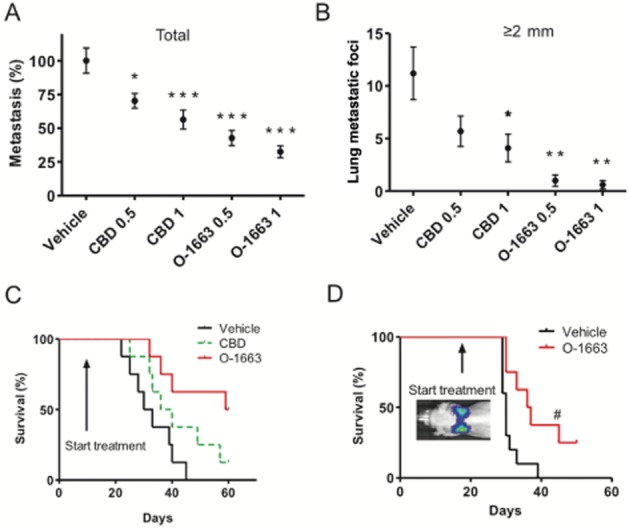
O-1663 produces a significant inhibition of advanced stage breast metastasis. Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 mouse 4T1 or 0.25 × 106 human MDA-MB231-luc-D3H2LN cells. (A, B) One week after the injection of 4T1 cells, the tumour-bearing mice were injected i.p. once a day with vehicle, CBD or O-1663 for 14 days. In (A) the % metastasis (total metastatic foci in treated/vehicle × 100) and (B) the lung metastatic foci ≥2 mm were evaluated. (C) Mice treated with vehicle, CBD or O-1663 (1 mg·kg−1) starting 1 week after i.v. injection of 4T1 cells were observed until they demonstrated signs of disease progression that necessitated killing. (D) Mice were treated with vehicle or O-1663 (1 mg·kg−1) starting 18 days after i.v. injection of MDA-MB231-luc-D3H2LN cells and were observed until they demonstrated signs of disease progression that necessitated killing. The mice were periodically imaged using an IVIS Lumina II instrument. Fifteen minutes before imaging, mice were injected i.p. with 150 mg·kg−1 of luciferin. Comparison of tumour progression over time was analysed between studies by comparing the absolute unit of radiance in photons· s−1·cm−2·sr−1. *P < 0.05, ** P < 0.01 and *** P < 0.001 indicate statistically significant differences from control. Survival between groups was compared using a log-rank Mantel–Cox test. #Indicates where one animal responding well to treatment with O-1663 based on BLI was removed in order to stain and visualize lung metastatic foci.
We next utilized MDA-MB231-luc-D3H2LN cells in order to determine whether O-1663 would produce robust anti-metastatic activity against a human breast cancer cell line in advanced stages of disease progression. MDA-MB231-luc-D3H2LN cells are a luciferase-expressing cell line that was derived from a spontaneous lymph node metastasis from a D3H1 MFP tumour. This variant is significantly more aggressive than the parental line with a rapid disease progression with a time course more closely resembling the 4T1 model. Additionally, the expression of luciferase allowed us to assess the activity of the drug treatments longitudinally in real time using BLI and to demonstrate that metastases were present in the lung when the treatment was initiated. The IC50 for inhibition of MDA-MB231-luc-D3H2LN cell proliferation/viability in the culture for CBD and O-1663 were 2.8 and 1.0 μM respectively. This cell line was therefore significantly less sensitive to the effects of CBD but not O-1663.
Since CBD was not effective at inhibiting advanced stages of metastatic progression and was less active at inhibiting the proliferation/viability of MDA-MB231-luc-D3H2LN, we focused our studies on O-1663. Mice were i.v. injected with MDA-MB231-luc-D3H2LN cells. Eighteen days later, after the presence of lung metastasis was confirmed using the in vivo imager, mice were treated daily, 5 days per week, with vehicle or 1 mg·kg−1 O-1663 (Figure 6D and Supporting Information Fig. S6B and C). The cannabinoid analogue produced a significant increase in survival (P < 0.01) where the median survival for vehicle and O-1663 treated mice were 30 and 37 days respectively. Additionally, 38% of the animals were still alive at day 50 when the study was terminated. Using the in vivo imager, we were able to observe tumour regression, beyond the initial size of the tumour when the treatment started, in 25% of the animals (data not shown). While regression did occur beyond the initial size of the tumour in some mice, the tumour did adapt over time and began to progress again. One mouse, where the tumour was regressing in the lung, was removed from the study to confirm the imaging results by visualizing the lung using an India ink stain and a dissecting microscope (Supporting Information Fig. S6D). While the MDA-MB231-luc-D3H2LN cells were significantly less sensitive to the effects of CBD, we did test whether treatment at an earlier stage of metastatic progression would yield beneficial effects on survival. We therefore initiated treatment with CBD 2 days after mice were i.v. injected with MDA-MB231-luc-D3H2LN cells. While CBD inhibited disease progression in a subset of the mice, overall survival was not significantly improved (Supporting Information Fig. S6E).
Discussion
CBD has been reported to have a wide variety of therapeutic indications and has been shown to be non-toxic, safe and well-tolerated in clinical trials (Zuardi, 2008). One of the most exciting recent areas of study for the therapeutic application of CBD resides in its ability to decrease cancer cell invasion and metastasis (Ligresti et al., 2006; Ramer et al., 2010; McAllister et al., 2011). In this investigation, we determined that CBD was effective at inhibiting metastatic progression, leading to prolonged survival in multiple preclinical models of breast cancer.
Id1 has been shown to play a key role in mediating breast cancer tumourigenicity and metastasis to the lung (Fong et al., 2003; Minn et al., 2005; Gupta et al., 2007; Swarbrick et al., 2008). We previously reported that CBD could down-regulate Id1 gene expression in breast cancer cell lines in culture (McAllister et al., 2007). Here, we demonstrated that treatment with CBD can also lead to the inhibition of Id1 gene expression and tumour cell proliferation in lung metastatic foci in a mouse model of breast cancer. In addition, ectopic expression of Id1 in breast cancer cells reversed the anti-metastatic activity of CBD. Overall, these data suggest that the anti-metastatic activity of CBD is directly related to the down-regulation of Id1 gene expression; Id1 therefore represents a potential biomarker for predicting whether CBD will be effective at inhibiting tumour progression. Additional mechanisms in vivo that have been implicated in the anti-metastatic activities of CBD include the up-regulation of intercellular adhesion molecule-1 and tissue inhibitor of matrix metalloproteinases-1 in lung cancer (Ramer et al., 2012).
In contrast to the moderate doses of CBD needed to inhibit metastatic progression, higher doses of CBD have been required to inhibit tumour growth after s.c. injection into the flank of athymic mice (Massi et al., 2004; Torres et al., 2011). In our previous work, daily administration of CBD only produced a minor delay in primary tumour growth in a model where 4T1 cells where s.c. implanted (McAllister et al., 2011). In the present manuscript, we observed no inhibition of primary tumour growth in the orthotopic model where the cells where injected into the MFP and when the mice were treated three times a week with CBD. Taken together, the ability of CBD to inhibit primary tumour growth at the doses evaluated in our current investigation is limited. The differences in the potency of CBD for targeting processes involved in cancer cell growth and survival versus invasion and metastasis may explain why the cannabinoid is less efficient at inhibiting primary tumour growth. In multiple studies, CBD has been shown to be at least four times more potent at inhibiting cancer cell invasion in comparison to proliferation/viability (Massi et al., 2004; Vaccani et al., 2005; McAllister et al., 2007; 2011).
We noted that, in both the orthotopic and the i.v. models of breast cancer, CBD was highly effective at targeting metastatic foci ≥2 mm. This led us to hypothesize that CBD could be effective at inhibiting the growth of secondary tumours even after their initial establishment in the lung. While CBD was effective at inhibiting metastatic progression even in more advanced stages of the disease, it was not efficacious enough to produce substantial increases in survival at this stage. We therefore underwent a screening strategy of cannabinoid analogues focused on inhibition of Id1, cell proliferation and invasion. While CBD does not interact efficiently with the CB1 and CB2 receptors, modification of this cannabinoid has been shown to lead to a return of CB1 and CB2 activity (Wiley et al., 2002). We therefore further envisioned a cannabinoid analogue that could target Id1 and activate cannabinoid receptors. THC has been shown to inhibit the progression of multiple aggressive cancer through the activation of CB1 and CB2 (Velasco et al., 2012). This effect can be reproduced using CB2 selective agonists, which is an advantage since activation of CB1 receptors leads to psychotropic effects (Salazar et al., 2009; Caffarel et al., 2010). We hypothesized that targeting of Id1 expression and cannabinoids receptors with a single compound would result in an even more robust inhibition of advanced stages of metastasis.
Screening a library of resorcinol derivatives, we discovered a cannabinoid analogue (O-1663) that could activate CB2 receptors and was more potent at inducing the formation of ROS and inhibiting Id1 expression in comparison to CBD. We have previously determined that a primary mechanism leading to down-regulation of Id1 gene expression by CBD includes induction of ROS (McAllister et al., 2011). The induction of ROS has also been implicated in the anti-tumour activity of CBD by multiple groups (Massi et al., 2006; 2008; McKallip et al., 2006). In non-transformed cell, the initial release of intracellular calcium has been linked to CBD-dependent production of ROS (Ryan et al., 2009; Mato et al., 2010). This activity was not related to the activation of CB1, CB2 or vanilloid receptor 1 similar to the results reported previously in human breast cancer cells (Ligresti et al., 2006; Shrivastava et al., 2011). Taken together, these studies suggest the existence of a unique intracellular interaction site for CBD that regulates calcium homeostasis, leading to generation of ROS. This activity may be one of the initial events leading to the downstream anti-tumour activity of CBD.
In addition to inhibiting Id1 expression, the resorcinol derivate O-1663 was also found to be an efficacious CB2 selective agonist. The ability of O-1663 to inhibit breast cancer aggressiveness in the culture and in vivo was partially reversed by a CB2 receptor antagonist. O-1663 was also more potent than the mixed CB1/CB2 agonist THC at activating the autophagy pathway. CBD did not efficiently activate autophagy and did not induce apoptosis in cell culture at concentrations that produce ROS and target Id1 gene expression. A recent study showed CBD increased autophagy-mediated cell death in breast cancer cells in the culture (Shrivastava et al., 2011). However, the concentration of CBD used in the culture to produce this effect was significantly higher (approximately three times) than that needed to target Id1 gene expression in our investigations. CBD was also shown to be ineffective at inducing autophagy in vivo when targeting glioblastoma (Torres et al., 2011).
O-1663 was significantly more potent and efficacious than CBD in multiple preclinical models of breast cancer. O-1663 also produced a significant increase in survival in advanced stages of mouse and human breast cancer metastasis, and in certain instances, O-1663 produced regression of established metastatic foci. We propose that this is the result of efficiently targeting cannabinoid anti-tumour pathways that have been associated with the activity of both CBD and THC. In agreement with this hypothesis, the combined administration of CBD and THC produced a similar magnitude of anti-metastatic activity when compared with O-1663 alone. O-1663, however, is significantly less potent than THC at targeting CB1 receptors and demonstrated limited activity in in vivo assays that predict the potential psychotropic activity of cannabinoids (Wiley et al., 2002). In addition to the direct anti-tumour activity of cannabinoids, the targeting of Id1 expression and autophagy has been shown to result in the enhanced the activity of first-line agents (Hu et al., 2009; Ponz-Sarvise et al., 2011; Torres et al., 2011) representing the potential for additional indirect anti-tumour activity by O-1663.
THC and CBD have been shown to have anti-inflammatory, analgesic and neuroprotective effects (Hampson et al., 1998; Malfait et al., 2000; Mishima et al., 2005; Pertwee, 2006; Kaplan et al., 2008; Russo, 2008). Similar to what was accomplished in this investigation using the Id1 gene as a target for CBD, if primary mechanisms for the beneficial effect of CBD in non-cancer-related diseases could be identified, then there is the potential to develop more potent analogues that retain the activity of THC and CBD.
Overall, this study suggests that the use of cannabinoid compounds may represent a potential approach for the treatment of patients with metastatic breast cancer and provides a framework for the synthesis of additional novel cannabinoid analogues based on our lead compound.
Acknowledgments
This work was supported by the National Institutes of Health (CA082548, CA135281, DA009789 and DA005488), the California Breast Cancer Research Program (12IB-0116) and the Komen Breast Cancer Research Foundation (KG090385). All authors have made substantial contributions to the conception and design of the study, or acquisition of data, or analysis and interpretation of data, or drafting of the manuscript.
Glossary
- BLI
bioluminescence imaging
- CB receptor
cannabinoid receptor
- CBD
cannabidiol
- EC
effective concentration
- IC
inhibitory concentration
- Id
inhibitor of DNA binding
- MFP
mammary fat pad
- ROS
reactive oxygen species
- THC
tetrahydrocannabinol
Conflict of interest
The authors disclose no potential conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site: http://dx.doi.org/10.1111/bph.12803
Figure S1 CBD produces a dose-dependent reduction of metastatic spread to the lung and increases survival. Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 mouse 4T1 cells. (A) One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle or CBD (0.1–5 mg·kg−1) for 14 days and metastasis was evaluated. % metastasis = total tumour number of lung metastatic foci in drug-treated group/total number of lung metastatic foci in vehicle-treated group where the respective controls (vehicle-treated mice) were set as 100%. (B) Lung metastases measured in mice treated with vehicle or CBD 1 mg·kg−1 included those with metastatic foci <2 and ≥2 mm. (C) Mice treated with vehicle or 1 mg·kg−1 CBD, starting 1 day after i.v. injection of 2 × 104 4T1 cells, were observed until they demonstrated signs of disease progression that necessitated killing. Survival between groups was compared using a log-rank Mantel–Cox test. *P < 0.05, **P < 0.01 and #P < 0.001 indicate statistically significant differences from control.
Figure S2 CBD reduces metastasis but not primary tumour growth. Primary tumours and subsequent secondary tumours (metastases) were generated in BALB/c mice by injection of 5 × 104 mouse 4T1 cells into the mammary fat pad between the second and third nipple. Treatment with CBD was initiated upon detection of the first palpable tumour (day 7). The tumour-bearing mice were i.p. injected three times a week with vehicle or CBD (0.1–2.5 mg·kg−1) for 3 weeks. (A) To determine the tumour size in situ, the perpendicular largest diameters of the tumours were measured in millimetres as (L × W2)/2 based upon a modified ellipsoidal formula. (B) Visible lung metastases were counted and measured using a dissecting 4 microscope and % metastasis (total metastatic foci in treated/vehicle × 100) was calculated. (C) The number of metastatic foci ≥2 mm was also determined. *P < 0.05 and **P < 0.005 indicate statistically significant differences from control.
Figure S3 CBD or Id1 knockdown produce comparable anti-metastatic activity against human breast cancer cells. (A) To confirm the correlation between the effects of CBD and inhibition of Id1 expression in vivo, we established stable pooled populations of MDAMB231 cells expressing Id1 shRNA that showed a down-regulation of Id1 protein levels (lower panel) comparable to parental cells treated with 2 μM CBD for 2 days (upper panel). (B) In culture, cell proliferation/viability (left panels) and invasion (right panels) rates were significantly reduced in parental MDA-MB231 cells treated with CBD or in MDA-MB231 cells expressing Id1 shRNA. (C) Left panel: lung metastases were generated in athymic nu/nu mice after i.v. injection of 5 × 105 MDA-MB231. One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle or 0.5–1 mg·kg−1 CBD for 6 weeks. The percentage of metastatic foci (total metastatic foci in treated/vehicle × 100) was compared between vehicle and CBD-treated groups. Right panel: the percentage of metastatic foci was compared between athymic nu/nu mice injected i.v. with 5 × 105 MDA-MB231 cells stably expressing Ctl shRNA or Id1 shRNA. (D) Left panel: the number of lung metastatic foci ≥1 mm was compared between vehicle and CBD-treated groups. Right panel: the number of lung metastatic foci ≥1 mm was compared between groups injected with cells stably expressing Ctl shRNA or Id1 shRNA. *P < 0.05 and **P < 0.01 indicate statistically significant differences from the control.
Figure S4 The activity of various cannabinoids was compared for the down-regulation of Id1 expression. (A) Structures of CBD, CP55940, THC, abnormal (Abn)-CBD, O- 1918 and O-1663. (B) Proteins from MDA-MB231 cells treated with 1.5 μM of multiple cannabinoids for 3 days were extracted and analysed for Id1 by Western blot analysis. Normalization was carried out by stripping the blots and re-probing with a monoclonal antitubulin antibody. Densitometry readings of the blots were taken and the percentage relative expression was calculated as the expression of Id1 in the treated cells/vehicle cells × 100.
Figure S5 CB2 receptor expression and CBD-dependent generation of ROS in breast cancer cells. (A) Protein lysate from mouse spleen, mouse 4T1 cells or human MDAMB231 breast cancer cells was analysed for CB2 receptor expression. (B) Mouse 4T1 cells (upper panel) were treated with 20 μM α-tocopherol (TOC), 1 μM the CB1 receptor antagonist (SR141716A – SR1) or 1μM the CB2 receptor antagonist (SR144528 – SR2). Human MDAMB231 cells (lower panel) were treated with 20 μM α-tocopherol (TOC), 4 μM the CB1 receptor antagonist (SR141716A – SR1) or 4 μM the CB2 receptor antagonist (SR144528 – SR2). Cell proliferation/viability was then evaluated using the MTT assay. (C) Human MDA-MB231 cells were treated with vehicle or CBD (μM) for 2 days and the production of ROS was then measured using 2′-7′dichloro-dihydrofluorescein and cell flow cytometry.
Figure S6 O-1663 produces a significant inhibition of advanced stage breast metastasis. (A) Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 4T1. One week after the injection of the cells, the tumour-bearing mice were injected i.p. once a day with vehicle or O-1663 for 14 days. In the treated group, 50% of the mice were still alive and 6 demonstrated no signs of disease progression at time of killing (2 months), few metastatic foci were observed when lungs were stained with India ink and visualized using a dissecting microscope. (B) Athymic nu/nu mice were treated with vehicle or O-1663 (1 mg·kg−1) starting 18 days after i.v. injection of human MDA-MB231-luc-D3H2LN breast cancer cells, a time point where the presence of lung tumours was confirmed using BLI. Fifteen minutes before imaging, mice were injected i.p. with 150 mg·kg−1 of luciferin. Mice were evenly distributed between vehicle and O-1663-treated groups before the initiation of treatment. Comparison of tumour progression over time was analysed between studies by comparing the absolute unit of radiance in photons per second per centimetre2 per steradian (p· s−1·cm−2·sr−1). (C) All athymic nu/nu mice where then imaged 1 week (left panel) and 10 days (right panel) later after the initiation of the study until animals in the vehicle group demonstrated signs of disease and began to be killed. (D) Regression of tumour burden, beyond the initial size the tumour when the treatment started, was observed in 25% of the athymic nu/nu mice. One mouse, where the tumour was regressing in the lung, was removed from the study to confirm the imaging results by visualizing the lung using an India ink stain and a dissecting microscope. (E) Athymic nu/nu mice treated with vehicle or CBD (1 mg·kg−1) starting 2 days after i.v. injection of MDA-MB231-luc-D3H2LN cells were observed until they demonstrated signs of disease progression that necessitated killing.
Table S1 CBD inhibits Id1 expression in lung metastatic foci. The data presented correspond to the number of metastatic foci in the lungs harvested from vehicle- and CBD-treated mice, where immunohistochemical detection of Id1 was either negative, weakly positive or strongly positive. P-value < 0.0004 was calculated using chi-square test.
Table S2 Inhibition of cAMP by O-1663 and WIN55,212-2 in CB2-transfected CHO cells (CHO-CB2). Experiments were carried out as previously described (Felder et al., 1995). Data represent the mean with corresponding confidence limits (CL) for three independent determinations.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1562. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez C, Salazar M, Carracedo A, Lorente M, Egia A, Gonzalez-Feria L, et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008;68:1945–1952. doi: 10.1158/0008-5472.CAN-07-5176. [DOI] [PubMed] [Google Scholar]
- Caffarel MM, Andradas C, Mira E, Perez-Gomez E, Cerutti C, Moreno-Bueno G, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9:196. doi: 10.1186/1476-4598-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006a;66:6748–6755. doi: 10.1158/0008-5472.CAN-06-0169. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006b;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Editorial. Cancer drugs: remedy required. Nat Med. 2011;17:231. doi: 10.1038/nm0311-231. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y, et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2003;100:13543–13548. doi: 10.1073/pnas.2230238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Han HY, Wang YL, Zhang XP, Chua CW, Wong YC, et al. The role of Id-1 in chemosensitivity and epirubicin-induced apoptosis in bladder cancer cells. Oncol Rep. 2009;21:1053–1059. doi: 10.3892/or_00000323. [DOI] [PubMed] [Google Scholar]
- Itahana Y, Singh J, Sumida T, Coppe JP, Parrinello S, Bennington JL, et al. Role of Id-2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer Res. 2003;63:7098–7105. [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT) Biochem Pharmacol. 2008;76:726–737. doi: 10.1016/j.bcp.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: new perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63:2057–2066. doi: 10.1007/s00018-006-6156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–1100. doi: 10.1111/j.1471-4159.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- Mato S, Victoria Sanchez-Gomez M, Matute C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia. 2010;58:1739–1747. doi: 10.1002/glia.21044. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Tao Q, Barnett-Norris J, Buehner K, Hurst DP, Guarnieri F, et al. A critical role for a tyrosine residue in the cannabinoid receptors for ligand recognition. Biochem Pharmacol. 2002;63:2121–2136. doi: 10.1016/s0006-2952(02)01031-6. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Rizvi G, Anavi-Goffer S, Hurst DP, Barnett-Norris J, Lynch DL, et al. An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J Med Chem. 2003;46:5139–5152. doi: 10.1021/jm0302647. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–2927. doi: 10.1158/1535-7163.MCT-07-0371. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- Mo FM, Offertaler L, Kunos G. Atypical cannabinoid stimulates endothelial cell migration via a Gi/Go-coupled receptor distinct from CB1, CB2 or EDG-1. Eur J Pharmacol. 2004;489:21–27. doi: 10.1016/j.ejphar.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(Suppl. 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz-Sarvise M, Nguewa PA, Pajares MJ, Agorreta J, Lozano MD, Redrado M, et al. Inhibitor of differentiation-1 as a novel prognostic factor in NSCLC patients with adenocarcinoma histology and its potential contribution to therapy resistance. Clin Cancer Res. 2011;17:4155–4166. doi: 10.1158/1078-0432.CCR-10-3381. [DOI] [PubMed] [Google Scholar]
- Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–966. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ramer R, Bublitz K, Freimuth N, Merkord J, Rohde H, Haustein M, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012;26:1535–1548. doi: 10.1096/fj.11-198184. [DOI] [PubMed] [Google Scholar]
- Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4:245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- Swarbrick A, Roy E, Allen T, Bishop JM. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc Natl Acad Sci U S A. 2008;105:5402–5407. doi: 10.1073/pnas.0801505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S, Lorente M, Rodriguez-Fornes F, Hernandez-Tiedra S, Salazar M, Garcia-Taboada E, et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10:90–103. doi: 10.1158/1535-7163.MCT-10-0688. [DOI] [PubMed] [Google Scholar]
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–1036. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–444. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Beletskaya ID, Ng EW, Dai Z, Crocker PJ, Mahadevan A, et al. Resorcinol derivatives: a novel template for the development of cannabinoid CB(1)/CB(2) and CB(2)-selective agonists. J Pharmacol Exp Ther. 2002;301:679–689. doi: 10.1124/jpet.301.2.679. [DOI] [PubMed] [Google Scholar]
- Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras Psiquiatr. 2008;30:271–280. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 CBD produces a dose-dependent reduction of metastatic spread to the lung and increases survival. Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 mouse 4T1 cells. (A) One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle or CBD (0.1–5 mg·kg−1) for 14 days and metastasis was evaluated. % metastasis = total tumour number of lung metastatic foci in drug-treated group/total number of lung metastatic foci in vehicle-treated group where the respective controls (vehicle-treated mice) were set as 100%. (B) Lung metastases measured in mice treated with vehicle or CBD 1 mg·kg−1 included those with metastatic foci <2 and ≥2 mm. (C) Mice treated with vehicle or 1 mg·kg−1 CBD, starting 1 day after i.v. injection of 2 × 104 4T1 cells, were observed until they demonstrated signs of disease progression that necessitated killing. Survival between groups was compared using a log-rank Mantel–Cox test. *P < 0.05, **P < 0.01 and #P < 0.001 indicate statistically significant differences from control.
Figure S2 CBD reduces metastasis but not primary tumour growth. Primary tumours and subsequent secondary tumours (metastases) were generated in BALB/c mice by injection of 5 × 104 mouse 4T1 cells into the mammary fat pad between the second and third nipple. Treatment with CBD was initiated upon detection of the first palpable tumour (day 7). The tumour-bearing mice were i.p. injected three times a week with vehicle or CBD (0.1–2.5 mg·kg−1) for 3 weeks. (A) To determine the tumour size in situ, the perpendicular largest diameters of the tumours were measured in millimetres as (L × W2)/2 based upon a modified ellipsoidal formula. (B) Visible lung metastases were counted and measured using a dissecting 4 microscope and % metastasis (total metastatic foci in treated/vehicle × 100) was calculated. (C) The number of metastatic foci ≥2 mm was also determined. *P < 0.05 and **P < 0.005 indicate statistically significant differences from control.
Figure S3 CBD or Id1 knockdown produce comparable anti-metastatic activity against human breast cancer cells. (A) To confirm the correlation between the effects of CBD and inhibition of Id1 expression in vivo, we established stable pooled populations of MDAMB231 cells expressing Id1 shRNA that showed a down-regulation of Id1 protein levels (lower panel) comparable to parental cells treated with 2 μM CBD for 2 days (upper panel). (B) In culture, cell proliferation/viability (left panels) and invasion (right panels) rates were significantly reduced in parental MDA-MB231 cells treated with CBD or in MDA-MB231 cells expressing Id1 shRNA. (C) Left panel: lung metastases were generated in athymic nu/nu mice after i.v. injection of 5 × 105 MDA-MB231. One day after the injection, the tumour-bearing mice were injected i.p. once a day with vehicle or 0.5–1 mg·kg−1 CBD for 6 weeks. The percentage of metastatic foci (total metastatic foci in treated/vehicle × 100) was compared between vehicle and CBD-treated groups. Right panel: the percentage of metastatic foci was compared between athymic nu/nu mice injected i.v. with 5 × 105 MDA-MB231 cells stably expressing Ctl shRNA or Id1 shRNA. (D) Left panel: the number of lung metastatic foci ≥1 mm was compared between vehicle and CBD-treated groups. Right panel: the number of lung metastatic foci ≥1 mm was compared between groups injected with cells stably expressing Ctl shRNA or Id1 shRNA. *P < 0.05 and **P < 0.01 indicate statistically significant differences from the control.
Figure S4 The activity of various cannabinoids was compared for the down-regulation of Id1 expression. (A) Structures of CBD, CP55940, THC, abnormal (Abn)-CBD, O- 1918 and O-1663. (B) Proteins from MDA-MB231 cells treated with 1.5 μM of multiple cannabinoids for 3 days were extracted and analysed for Id1 by Western blot analysis. Normalization was carried out by stripping the blots and re-probing with a monoclonal antitubulin antibody. Densitometry readings of the blots were taken and the percentage relative expression was calculated as the expression of Id1 in the treated cells/vehicle cells × 100.
Figure S5 CB2 receptor expression and CBD-dependent generation of ROS in breast cancer cells. (A) Protein lysate from mouse spleen, mouse 4T1 cells or human MDAMB231 breast cancer cells was analysed for CB2 receptor expression. (B) Mouse 4T1 cells (upper panel) were treated with 20 μM α-tocopherol (TOC), 1 μM the CB1 receptor antagonist (SR141716A – SR1) or 1μM the CB2 receptor antagonist (SR144528 – SR2). Human MDAMB231 cells (lower panel) were treated with 20 μM α-tocopherol (TOC), 4 μM the CB1 receptor antagonist (SR141716A – SR1) or 4 μM the CB2 receptor antagonist (SR144528 – SR2). Cell proliferation/viability was then evaluated using the MTT assay. (C) Human MDA-MB231 cells were treated with vehicle or CBD (μM) for 2 days and the production of ROS was then measured using 2′-7′dichloro-dihydrofluorescein and cell flow cytometry.
Figure S6 O-1663 produces a significant inhibition of advanced stage breast metastasis. (A) Lung metastases were generated in BALB/c mice by i.v. injection of 2 × 104 4T1. One week after the injection of the cells, the tumour-bearing mice were injected i.p. once a day with vehicle or O-1663 for 14 days. In the treated group, 50% of the mice were still alive and 6 demonstrated no signs of disease progression at time of killing (2 months), few metastatic foci were observed when lungs were stained with India ink and visualized using a dissecting microscope. (B) Athymic nu/nu mice were treated with vehicle or O-1663 (1 mg·kg−1) starting 18 days after i.v. injection of human MDA-MB231-luc-D3H2LN breast cancer cells, a time point where the presence of lung tumours was confirmed using BLI. Fifteen minutes before imaging, mice were injected i.p. with 150 mg·kg−1 of luciferin. Mice were evenly distributed between vehicle and O-1663-treated groups before the initiation of treatment. Comparison of tumour progression over time was analysed between studies by comparing the absolute unit of radiance in photons per second per centimetre2 per steradian (p· s−1·cm−2·sr−1). (C) All athymic nu/nu mice where then imaged 1 week (left panel) and 10 days (right panel) later after the initiation of the study until animals in the vehicle group demonstrated signs of disease and began to be killed. (D) Regression of tumour burden, beyond the initial size the tumour when the treatment started, was observed in 25% of the athymic nu/nu mice. One mouse, where the tumour was regressing in the lung, was removed from the study to confirm the imaging results by visualizing the lung using an India ink stain and a dissecting microscope. (E) Athymic nu/nu mice treated with vehicle or CBD (1 mg·kg−1) starting 2 days after i.v. injection of MDA-MB231-luc-D3H2LN cells were observed until they demonstrated signs of disease progression that necessitated killing.
Table S1 CBD inhibits Id1 expression in lung metastatic foci. The data presented correspond to the number of metastatic foci in the lungs harvested from vehicle- and CBD-treated mice, where immunohistochemical detection of Id1 was either negative, weakly positive or strongly positive. P-value < 0.0004 was calculated using chi-square test.
Table S2 Inhibition of cAMP by O-1663 and WIN55,212-2 in CB2-transfected CHO cells (CHO-CB2). Experiments were carried out as previously described (Felder et al., 1995). Data represent the mean with corresponding confidence limits (CL) for three independent determinations.


