Abstract
Effective therapies are urgently needed for infants with forms of pulmonary hypertension that develop or persist beyond the first week of life. The L-arginine nitric oxide (NO) precursor, L-citrulline, improves NO signalling and ameliorates pulmonary hypertension in newborn animal models. In vitro studies demonstrate that manipulating L-citrulline transport alters NO production.
Conclusion
Strategies that increase the supply and transport of L-citrulline merit pursuit as novel approaches to managing infants with chronic, progressive pulmonary hypertension.
Keywords: pulmonary hypertension, bronchopulmonary dysplasia, premature infants, nitric oxide, oxidative stress
Introduction
Infants suffering from chronic cardiopulmonary diseases, such as chronic lung disease and cyanotic congenital heart disease, can develop chronic, progressive pulmonary hypertension. Although the true prevalence of pulmonary hypertension in infants with cardiopulmonary disease is not known, echocardiographic evidence of pulmonary hypertension is found in 25–37% of infants with bronchopulmonary dysplasia, the most common form of chronic lung disease in newborns [1,2]. Moreover, persistent pulmonary hypertension in infants with bronchopulmonary dysplasia is associated with up to 40% mortality [3]. Alarmingly, this high rate of death has not improved in the past three decades [4]. The need for novel, effective therapies for infants with chronic progressive pulmonary hypertension is well acknowledged [5].
The pathobiology of chronic pulmonary vascular disease in infants must be better understood in order to devise therapies to improve outcomes for these patients. It is widely appreciated that persistent or episodic hypoxia contributes to the progressive changes in pulmonary vascular structure and function that characterise the worsening pulmonary vascular disease in infants [6]. Accumulating evidence implicates impairments in nitric oxide (NO) signalling in the pathogenesis of chronic pulmonary vascular disease [7], particularly in the case of chronic hypoxia-induced pulmonary hypertension [8].
Piglet model of chronic, progressive neonatal pulmonary hypertension
Piglets provide a useful model for studying progressive chronic hypoxia-induced pulmonary hypertension in newborns. We have established that pulmonary arterial hypertension develops in newborn piglets within three days exposure to hypoxia and progresses when hypoxia is extended to ten days [9]. The progressive elevations in pulmonary vascular resistance are accompanied by more pronounced degrees of pulmonary vascular remodelling [10]. Notably, pulmonary resistance level arteries (pulmonary arteries less than 300 microns in diameter) from piglets with chronic hypoxia-induced pulmonary hypertension exhibit impairments in NO-dependent pulmonary vascular responses that worsen with more prolonged exposure to hypoxia [11]. Furthermore, reductions in lung NO production directly correlate with duration of hypoxic exposure [12,13]. These findings in newborn piglets are consistent with the accumulating body of evidence in both humans [14] and other animal models that impaired NO signalling plays a pivotal role in chronic pulmonary hypertension [7,15].
Impaired NO signalling in chronic pulmonary hypertension: bioavailability of L-arginine
Because NO is synthesised from the amino acid, L-arginine, by NO synthase (NOS), L-arginine supplementation has been studied as a therapy to improve NO signalling and ameliorate chronic pulmonary hypertension. We and other investigators have shown that limited bioavailability of L-arginine contributes to vascular dysfunction in chronic pulmonary hypertension [11,16,17]. Short-term administration of L-arginine reduces pulmonary arterial pressure in adult human patients with chronic pulmonary hypertension [17]. In our newborn piglet model of chronic hypoxia-induced pulmonary hypertension, acute administration of L-arginine improves endothelium-dependent vascular relaxation [11] and increases lung NO production [16]. In adult rats, prolonged administration of L-arginine inhibits the development of chronic pulmonary hypertension [18].
The mechanisms underlying impaired L-arginine bioavailability and the beneficial therapeutic responses to L-arginine are not yet clear. Data suggest that intracellular L-arginine levels exceed the Km for eNOS [19]. The phenomenon of increased NO production with L-arginine supplementation despite eNOS-saturating intracellular levels of L-arginine has been termed the arginine paradox. One possible explanation is that the bulk of intracellular endothelial L-arginine is not available to eNOS for NO production [19] and that the extracellular arginine transported into the cells is preferentially delivered to the site of the eNOS synthetic machinery [20]. This explanation is supported by the finding that the major transporter for arginine in endothelial cells, cationic amino acid transporter 1, CAT-1, co-localises with eNOS in caveolae [20, 21].
Other explanations for limited L-arginine bioavailability include increased intracellular concentrations of arginase or the methylated analogs of L-arginine. Arginase converts arginine to ornithine and urea, limiting the availability of NO substrate. Increased arginase expression and activity were found in pulmonary endothelial cells of adult patients with pulmonary hypertension [22], in human lung endothelial cells exposed to hypoxia [23], in lungs of newborn rats exposed to hyperoxia who develop a BPD phenotype and pulmonary hypertension [24] and in adult rats with monocrotaline-induced pulmonary hypertension [25]. The methylated analogs of L-arginine act as false substrates, competing with L-arginine, thereby inhibiting NOS activity. Asymmetric NGNG-dimethylarginine (ADMA) is considered to be the major endogenous NOS inhibitor. Elevated levels of ADMA have been found in some adult patients with pulmonary hypertension [26] and in both newborn [27] and adult [25] animal models of chronic pulmonary hypertension. Supplemental L-arginine could counteract elevations in ADMA or arginase and explain, at least in part, some of the improvements found with L-arginine supplementation.
Results with L-arginine supplementation have not been consistent, with some studies showing no benefit from either acute [28] or prolonged [29] L-arginine supplementation in animals or humans with chronic pulmonary hypertension. In addition, there is evidence that chronic supplementation with L-arginine may be harmful [30,31]. The feasibility and logic of chronic oral L-arginine supplementation are questionable because the presence of arginase in gut bacteria, intestinal epithelial cells and hepatocytes dictates that orally administered L-arginine will largely be catabolised to ornithine and urea. This catabolic loss of L-arginine necessitates the administration of massive L-arginine doses to achieve increases in circulating levels that are therapeutically effective [31]. These large doses are often poorly tolerated and patient compliance can be difficult to maintain [32]. Some limitations of oral L-arginine therapy can be avoided by intravenous administration of L-arginine [32]. However, intravenous therapies are challenging to sustain long-term and have potential for adverse consequences, including infection and thrombosis. Thus, alternative means of restoring impaired NO production are worthy areas for investigation.
Citrulline supplementation: an alternative approach to delivering bioavailable L-arginine and increasing NO production
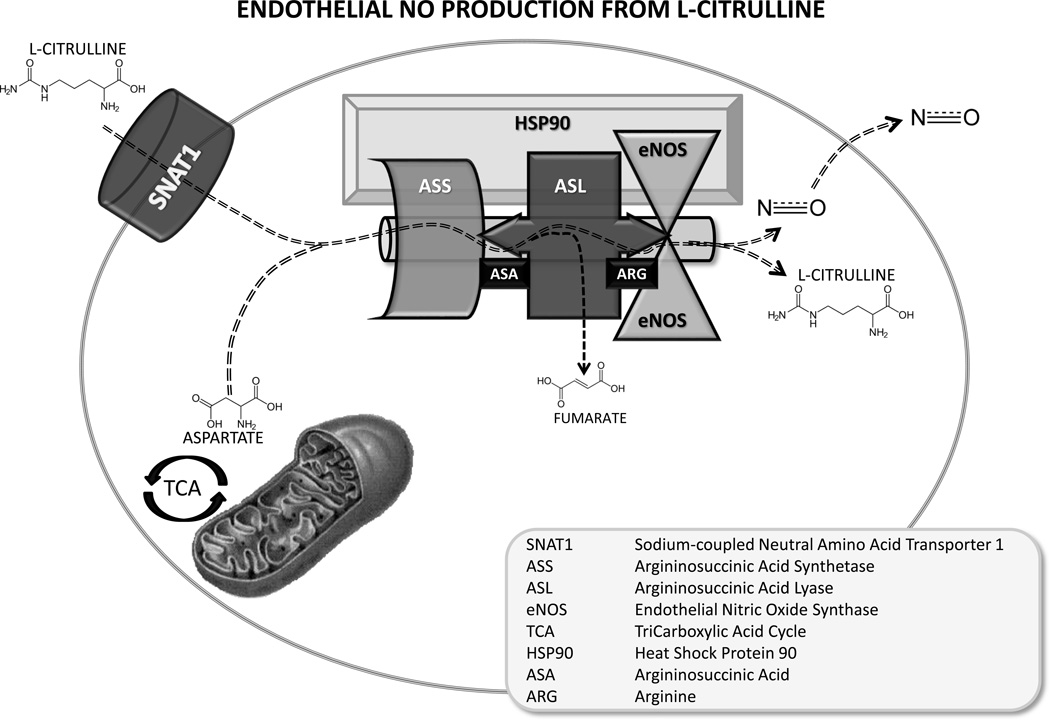
L-citrulline provides an intracellular source for L-arginine via a two-step biosynthetic pathway involving the co-substrate, aspartate, and the enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) [33] (Figure 1). Through this recycling pathway, L-citrulline both serves as substrate for arginine and as end product when arginine is converted to NO by NOS. Thus, L-citrulline, potentially provides an alternate approach to deliver bioavailable L-arginine to increase synthesis of pulmonary vascular NO.
Figure 1.
Model for L-citrulline transport, arginine channelling and nitric oxide (NO) metabolism in pulmonary endothelial cells. Circulating L-citrulline is taken up by the sodium-dependent neutral amino acid transporter, SNAT1, and is delivered to a multi-protein complex containing the urea cycle enzymes, argininosuccinate synthetase, ASS, and argininosuccinate lyase, ASL, endothelial nitric oxide synthase, eNOS, and the chaperone protein, heat shock protein 90, Hsp90, for efficient metabolism of L-citrulline to arginine and arginine to NO and L-citrulline. This model would explain the so called arginine paradox and would predict that citrulline is an effective precursor for NO production.
Citrulline is a neutral amino acid that was first identified in and named for watermelon, citrullus vulguaris [34]. Although watermelon is unusually rich in citrulline, very little citrulline is contained in a normal diet. Human milk, infant formulas and parenteral nutrition solutions contain minimal or no citrulline. In mammals, L-citrulline is formed from ornithine and carbamoyl phosphate by the mitochondrial enzymes of the urea cycle in the liver and proximal intestines [35]. Enterocytes are the major source of endogenously produced, blood-borne L-citrulline because the L-citrulline produced in the liver is compartmentalised as an intermediate of the urea cycle [34]. After it has been produced and released into the circulation by enterocytes, L-citrulline passes through the liver without major metabolism. Some of the circulating L-citrulline is taken up and metabolised into L-arginine by cells in the proximal tubules of the kidney, via a two-step enzymatic process involving ASS and ASL. Therefore, a significant portion of the L-citrulline produced by the enterocytes reaches the systemic circulation as L-arginine. Oral L-citrulline has been shown to have high bioavailability. Humans administered oral L-citrulline achieve dramatic elevations in circulating L-citrulline with minimal urinary loss [36].
Pulmonary arterial endothelial cells (PAECs) express ASS and ASL and therefore can use L-citrulline to produce NO [37]. Indeed, there is evidence that ASS and ASL are co-localised with eNOS in plasmalemmal caveolae of endothelial cells [19] in a multi-protein complex with the chaperone protein, heat shock protein 90 [38–40]. The co-localisation of these enzymes makes it feasible that, instead of equilibrating with bulk intracellular levels, L-citrulline-induced increases in L-arginine could be directly channelled to eNOS [41] (Figure 1).
L-citrulline availability and cardiovascular disease: genetic considerations
It is conceivable that a reduction in the capacity to endogenously produce L-citrulline or to metabolise L-citrulline to L-arginine may affect in vivo NO synthesis and contribute to vascular disease. Thus, it is possible that genetic variations in enzymes involved in citrulline synthesis may contribute to the susceptibility to develop pulmonary hypertension. For example, the enzyme carbamoyl-phosphate synthetase 1 is one of the urea cycle enzymes involved in the mitochondrial synthesis of citrulline. A single nucleotide polymorphism in the carbamoyl-phosphate synthetase 1 gene designated T1405N is located at an important cofactor binding site and could affect enzyme activity, thereby impacting NO production from L-citrulline [42]. Compared with the general population, newborns with respiratory distress were found to have a significantly skewed distribution of carbamoyl-phosphate synthetase 1 genotypes for this polymorphism [43]. In addition, the carbamoyl-phosphate synthetase 1 T1405 genotype distribution differed between those newborns with respiratory distress who developed pulmonary hypertension compared to those who did not [43]. The distribution of the carbamoyl-phosphate synthetase 1 T1405 genotype has also been shown to differ between patients with congenital heart defects who developed postoperative pulmonary hypertension compared to those who did not [44]. Carbamoyl-phosphate synthetase 1 polymorphisms were also shown to influence measurements of venous NO and forearm blood flow responses to NOS-dependent and -independent vasodilation in a heterogeneous population of adults [45]. More studies are needed to determine if the carbamoyl-phosphate synthetase 1 genotype predicts mortality and morbidity from cardiovascular diseases.
The enzyme, ornithine transcarbamylase, catalyses the final step in mitochondrial synthesis of citrulline, the conversion of ornithine to citrulline. Low serum and urine levels of NO metabolites have been reported in children with the late-onset type of ornithine transcarbamylase deficiency [46]. Citrulline production, citrulline plasma levels and NO production have been shown to be diminished in ornithine transcarbamylase deficient mice [47]. The possible contribution to cardiovascular disease from this impairment in the citrulline-arginine-NO pathway is not yet known.
The requirement of ASL for adequate NO production in vivo has recently been demonstrated. Argininosuccinic aciduria is a well-recognised inborn error of metabolism caused by a mutation in the ASL gene resulting in the inability to metabolise citrulline to arginine and characterised by life-threatening hyperammonemia. Humans with ASL deficiency are at risk of developing systemic hypertension [48]. Plasma concentrations of NO metabolites were reduced and NOS-dependent relaxation of the brachial artery was found to be impaired in human patients with ASL deficiency [41]. Moreover, tissue production of NO was reduced and systolic and diastolic blood pressures were elevated in a hypomorphic mouse model of ASL deficiency [41].
The combined findings from patients and animals, with deficiencies in enzymes involved in the ability to synthesise citrulline de novo or to metabolise citrulline to arginine, suggest that the integrity of the citrulline-arginine-NO pathway is important for vascular health. In addition, these findings lend support to the notion that providing L-citrulline or enhancing its cellular uptake may be effective ways to ameliorate vascular diseases in conditions associated with impaired NO production, such as chronic hypoxia-induced pulmonary hypertension in newborns.
Transport of L-citrulline into PAECs: the role of Na+-dependent neutral amino acid transporters (SNATs) and the impact of hypoxia
PAECs do not express the enzymes needed for de novo synthesis of L-citrulline [37]. Thus, intracellular L-citrulline concentrations depend, at least in part, on uptake of circulating L-citrulline. Surprisingly, there is scarce information about the amino acid transporters responsible for L-citrulline transport into PAECs. This knowledge is important as it could provide a means to manipulate NO production.
SNATs are one of the major systems responsible for neutral amino acid transport [49]. And are found in most mammalian cells [49]. Moreover, it is well known that a number of different transport systems, including multiple different members of the SNAT family, may occur in the same cell membrane.
Because of their potential role in L-citrulline transport, studies were performed to evaluate the expression of SNATs in lungs, pulmonary arteries, and PAECs of newborn piglets [50] and the impact of hypoxic exposure on expression of SNATs. We speculated that expression of SNATs would be reduced with hypoxic exposure and contribute to the impaired NO signalling in piglets with chronic hypoxia-induced pulmonary hypertension [11,12]. SNATs 1, 2, 3, and 5 were expressed in lungs, pulmonary arteries, and PAECs of newborn piglets; SNAT4 was not evaluated as it is considered to be liver specific [49]. Unexpectedly, we found no impact of hypoxia on expression of SNAT 2, 3, or 5, but an increase in expression of SNAT1 in lungs, pulmonary arteries, and PAECs of newborn piglets with both in vitro and in vivo hypoxia [50].
To determine whether the alteration in SNAT1 expression correlated with the functional ability to transport citrulline, 14C-L-citrulline uptake was measured in PAECs cultured under normoxic and hypoxic conditions. We found that in vitro hypoxia increased L-citrulline uptake in PAECs from newborn piglets. Using a silencing ribonucleic acid technique, we identified SNAT1 as the transporter responsible for the enhanced ability to transport L-citrulline in hypoxic PAECs [51]. Furthermore, SNAT1 knockdown abolished the ability of L-citrulline to increase NO production in both normoxic and hypoxic PAECs. Taken altogether, these findings identify a role for SNAT1 in transporting L-citrulline and modulating NO production in PAECs. In addition, these findings raise the possibility that an increase in SNAT1 expression and concomitant enhanced ability to transport citrulline could be important mechanisms to counteract, rather than contribute to, impairments in NO signalling that occur during chronic hypoxia-induced pulmonary hypertension.
L-citrulline improves NO signalling by modulating eNOS coupling
Although the accumulating data implicating NO signalling impairments in chronic pulmonary vascular disease are compelling, better understanding the mechanistic underpinnings of this association are essential for the development of effective therapies. There is evidence that uncoupled eNOS is at least one of the signalling abnormalities underlying the diminished NO production found in chronic pulmonary vascular disease, including chronic hypoxia-induced pulmonary hypertension in newborn piglets [18,52,53]. Both L-arginine and tetrahydrobiopterin promote eNOS coupling [54]. As supplementation with L-citrulline is a potential means to increase L-arginine, we pursued the possibility that L-citrulline improves NO signalling in hypoxic PAECs by modulating eNOS coupling.
eNOS has a dimeric structure with oxygenase, N-terminal, and reductase, C-terminal, domains. In the homo-dimeric or coupled state, electrons are transferred from the reductase domain to the oxygenase domain and NO is produced. When eNOS becomes uncoupled, electrons are diverted to molecular oxygen producing superoxide instead of NO. Uncoupled eNOS can be demonstrated as a loss of eNOS dimer formation and increase of eNOS monomers. Consistent with eNOS uncoupling, we found that superoxide generation was increased and both NO production and eNOS dimer-to-monomer ratios were reduced in PAECs cultured under hypoxic conditions [51]. Treatment with L-citrulline reduced superoxide generation and increased NO production as well as eNOS dimer-to-monomer ratios in hypoxic PAECs [51]. Thus, at least one mechanism by which L-citrulline improves NO signalling in hypoxic PAECs is via re-coupling eNOS.
Having identified a role for SNAT1 in both L-citrulline transport and NO production, we determined whether SNAT1 modulates eNOS coupling in PAECs using a silencing ribonucleic acid technique. SNAT1 knockdown reduced eNOS dimer-to-monomer ratios and increased superoxide generation in both normoxic and hypoxic PAECs [51]. Superoxide generation was reduced with L-citrulline treatment of hypoxic PAECs, regardless of SNAT1 knockdown. However, SNAT1 knockdown prevented the ability of L-citrulline to increase eNOS dimer-to-monomer ratios in hypoxic PAEC [51]. Thus, SNAT1 modulates basal superoxide generation and eNOS coupling in both normoxic and hypoxic PAEC and is integral to the ability of L-citrulline to re-couple eNOS in hypoxic PAECs.
L-citrulline and the management of chronic, progressive pulmonary hypertension in newborns
The impact of strategies that increase the supply or transport of L-citrulline to modulate NO production in infants with chronic, progressive pulmonary hypertension has not yet been evaluated. There are some data in newborn animal models to suggest that supplying exogenous L-citrulline may be of benefit in the context of neonatal chronic pulmonary hypertension. In the newborn piglet model of chronic hypoxia-induced pulmonary hypertension, we have shown that oral treatment with L-citrulline increases pulmonary vascular NO production and attenuates the development of elevated pulmonary vascular resistance [55]. In a newborn rat model of hyperoxia-induced BPD, subcutaneous injection of L-citrulline ameliorated pulmonary vascular remodelling and reduced right ventricular hypertrophy, two cardiovascular abnormalities that reflect the presence of pulmonary hypertension [24]. Moreover, in this newborn rat model of BPD, L-citrulline treatment preserved alveolar septation [24]. The ability of L-citrulline to improve alveolar and vascular morphology in hyperoxic newborn rats has been corroborated by findings in a separate study [56]. These findings from newborn animal models suggest that carefully designed clinical studies of L-citrulline supplementation are worthy of pursuit in human infants at risk of developing pulmonary hypertension.
The therapeutic use of L-citrulline in human cardiovascular diseases
There is some evidence that L-citrulline may be beneficial for patients with some types of cardiovascular disease, including pulmonary hypertension, although large clinical trials are needed for verification. One group of investigators recently reported that adults with heart failure had improvements in left ventricular ejection fraction, functional class, and endothelial function assessed by photoplethysmography after treatment with oral L-citrulline for four months [57]. Evidence of improved endothelial function was also found in a small number of adult patients with stable diastolic heart failure after treatment with oral L-citrulline for 60 days [58]. Obese adults with prehypertension or stage one hypertension showed evidence of improved arterial function after six weeks treatment with watermelon powder as a source of L-citrulline [59]. Also of interest, oral supplementation with L-citrulline reduced brachial-ankle pulse wave velocity, a non-invasive assessment of arterial stiffness, in healthy middle-aged men [60].
In the paediatric age group, a pilot Phase two clinical trial of L-citrulline was shown to decrease vascular complications and increase the overall wellbeing of children with sickle cell disease [61]. Postoperative pulmonary hypertension did not develop in children undergoing cardiopulmonary bypass who had naturally elevated levels of citrulline or who achieved plasma levels greater than 37 micromolar with oral citrulline supplementation [62]. Although evaluation for efficacy is ongoing, intravenous L-citrulline administered to a similar patient population was found to be safe and well-tolerated [63]. L-citrulline is used as replacement therapy for children with certain types of urea cycle defects. These children receive L-citrulline for decades and show no evidence of toxicity from its use. Therefore, evaluation of the safety and efficacy of L-citrulline therapy for cardiovascular diseases, particularly those involving L-arginine/NO deficiencies, and requiring prolonged treatment for effective inhibition or resolution of the disease process is warranted. However, it is important to caution against clinical use of L-citrulline in neonatal or paediatric populations outside of well-controlled randomized trials. L-citrulline has been shown to cause a marked increase in the expression of arginase two in lungs of newborn rats exposed to hyperoxia [24] and in human lung endothelial cells exposed to hypoxia [23], which could have an adverse impact on lung development and structural remodeling of the pulmonary architecture.
Conclusions
There is a growing body of evidence that impairments in the L-citrulline-L-arginine-NO pathway are involved in the pathogenesis of chronic cardiovascular diseases, including chronic hypoxia-induced pulmonary hypertension in newborns. While L-arginine supplementation has been shown to be effective in some experimental models and in some studies with adult humans, detrimental effects from L-arginine supplementation have also been reported and results from L-arginine treatment have been variable. L-citrulline provides an alternate means for improving NO signalling. Studies with PAECs show that eNOS re-coupling is one of the mechanisms by which L-citrulline can restore impaired NO signalling. In addition, studies with PAECs indicate that manipulating L-citrulline transport can modulate NO production. Additional research is needed to determine effective ways to enhance L-citrulline transport in vivo and to evaluate the physiological impact of such manipulations of amino acid transport. Studies in newborn animal models support further basic and translation investigations of L-citrulline supplementation as a means to improve NO production, reduce free radical generation, and ameliorate pulmonary hypertension. Clinical trials of the safety and efficacy of L-citrulline therapy in human infants with and at risk of developing chronic, progressive forms of pulmonary hypertension deserve consideration as a novel and potentially cost-effective approach to treat this life-threatening condition in term and preterm infants.
Key Notes.
L-citrulline, a precursor of L-arginine, increases production of nitric oxide (NO) in vitro and ameliorates pulmonary hypertension in experimental models in vivo.
One mechanism by which L-citrulline improves NO signalling is via re-coupling of endothelial NO synthase, reducing superoxide production and enhancing NO synthesis.
Therapeutic strategies that increase the transport or abundance of L-citrulline may represent a novel approach to the management of pulmonary hypertension in infants.
Acknowledgements
This work was supported by R01HL097566 (CDF) and U01HL101456 (JLA)
Abbreviations
- ADMA
asymmetric NGNG-dimethylarginine
- ASL
argininosuccinate lyase
- ASS
argininosuccinate synthetase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAEC
pulmonary arterial endothelial cell
- SNAT
sodium-dependent neutral amino acid transporter
REFERENCES
- 1.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ. 2010;J 40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 3.Fouron JC, Guennec JCL, Villemant D. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatr. 1980;65:529. [PubMed] [Google Scholar]
- 4.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatr. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 5.Fike CD, Aschner JL. Looking behond PPHN: The unmet challenge of chronic progressive pulmonary hypertension in the newborn. Pulm Circ. 2013 doi: 10.1086/674438. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25:329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ. 2013;3:20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiological Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 9.Fike CD, Kaplowitz MR. Chronic hypoxia alters nitric oxide-dependent pulmonary vascular responses in lungs of newborn pigs. J Appl Physiol. 1996;81:2078–2087. doi: 10.1152/jappl.1996.81.5.2078. [DOI] [PubMed] [Google Scholar]
- 10.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol. 1994;77:2853–2862. doi: 10.1152/jappl.1994.77.6.2853. [DOI] [PubMed] [Google Scholar]
- 11.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn pigs. Am J Physiolo Lung Cell Mol Physiol. 2004;286:1244–1254. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol Lung Cell Mol Physiol. 1998;274:L517–L526. doi: 10.1152/ajplung.1998.274.4.L517. [DOI] [PubMed] [Google Scholar]
- 13.Turley JE, Nelin LE, Kaplowitz MR, Zhang Y, Fike CD. Exhaled nitric oxide is decreased at an early stage of hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiolo Lung Cell Mol Physiol. 2003;284:489–500. doi: 10.1152/ajplung.00246.2002. [DOI] [PubMed] [Google Scholar]
- 14.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, et al. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med. 2005;172:352–357. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 15.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. doi: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 16.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. L-arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol. 2000;88:1797–1803. doi: 10.1152/jappl.2000.88.5.1797. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Uematshu M, Oya H, Sato N, Sakamaki F, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163:887–891. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 18.Ou Z-J, Wei W, Huang D, Luo D, Wang Z, et al. L-arginine restores endothelial nitric oxide synthase coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab. 2010;298:E1131–E1139. doi: 10.1152/ajpendo.00107.2010. [DOI] [PubMed] [Google Scholar]
- 19.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccnate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 20.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric oxide synthase may explain the "arginine paradox". J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Huang W, Harris MB, Goolsby JM, Venema RC. Interaction of the endothelial nitric oxide sythase with CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem J. 2005;386:567–574. doi: 10.1042/BJ20041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Kaneko FT, Zheng X, Comhair SAA, Janocha AJ, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 23.Krotova K, Patel JM, Block ER, Zharikov S. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol. 2010;299:C1541–C1548. doi: 10.1152/ajpcell.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, et al. L-Citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res. 2010;68:519–525. doi: 10.1203/PDR.0b013e3181f90278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki A, Doi S, Mizutani S, Azuma H. Roles of accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, and attenuated nitric oxide synthase activity in endothelial cells from pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1480–L1487. doi: 10.1152/ajplung.00360.2006. [DOI] [PubMed] [Google Scholar]
- 26.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, et al. Increased levels and reduced catabolism of asymmetric and symmetriic dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 27.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of asymmetric dimethylarginine is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation. 2003;107:1195–1201. doi: 10.1161/01.cir.0000051466.00227.13. [DOI] [PubMed] [Google Scholar]
- 28.Baudouin SV, Bath P, Martin JF, Bois RD, Evans TW. L-arginine infusion has no effect on systemic haemodynamics in normal volunteers, or in sytemic and pulmonary hemodynamics in patients with elevated pulmonary vascular resistance. Br J Clin Pharmacol. 1993;36:5–49. doi: 10.1111/j.1365-2125.1993.tb05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laursen BE, Dam MY, Mulvany MJ, Simonsen Hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy is unaltered by long-term oral L-arginine administration. Vasc Pharmacol. 2008;49:71–76. doi: 10.1016/j.vph.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Boger RH. L-arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr and Met Care. 2008;11:55–61. doi: 10.1097/MCO.0b013e3282f2b0c3. [DOI] [PubMed] [Google Scholar]
- 31.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, et al. L-arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Tenenbaum A, Fisman EZ, Motro M. L-arginine: rediscovery in progress. Cardiology. 1998;90:153–159. doi: 10.1159/000006837. [DOI] [PubMed] [Google Scholar]
- 33.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regneration system for NO production in endothelial cells. J Exp Biol. 2003;206:2083–2087. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 34.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 35.Morris SM. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 36.Rouge C, Robert CD, Robbins A, Bacquer OL, Volteau C, et al. Manipulation of citrulline availability in humans. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1061–G1067. doi: 10.1152/ajpgi.00289.2007. [DOI] [PubMed] [Google Scholar]
- 37.Neill MA, Aschner J, Barr F, Summar ML. Quantitative RT-PCR comparison of the urea and nitric oxide cycle gene transcripts in adult human tissues. Mol Genet Metab. 2009;97:121–127. doi: 10.1016/j.ymgme.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physio. 2007;292:L1515–L1125. doi: 10.1152/ajplung.00252.2006. [DOI] [PubMed] [Google Scholar]
- 39.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90 (Hsp90)/eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol. 2009;296:L555–L564. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fike CD, Pfister SL, Slaughter JC, Kaplowitz MR, Zhang Y, Zeng H, Frye NR, Aschner JL. Protein complex formation with heat shock protein 90 (Hsp90) in chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Heart Circ Physiol. 2010;299:H1190–H1204. doi: 10.1152/ajpheart.01207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011;17:619–1626. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summar M, Hall LD, Eeds AM, Hutcheson HB, Kuo AN, et al. Characterization of genomic structure and polymorphisms in the human carbamyl phosphate synthetase I gene. Gene. 2003;311:51–57. doi: 10.1016/s0378-1119(03)00528-6. [DOI] [PubMed] [Google Scholar]
- 43.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, et al. Neonatal pulmonary hypertension: urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 44.Canter JA, Summar ML, Smith HB, Rice BD, Hall LD, et al. Genetic variation in the mitochondrial enzyme carbamyl-phosphate synthetase I predisposes children to increased pulmonary artery pressure following surgical repair of congenital heart defects: a validated genetic association study. Mitochondrion. 2007;7:204–210. doi: 10.1016/j.mito.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summar M, Gainer JV, Pretorius M, Malave H, Harris S, et al. Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension. 2004;43:186–191. doi: 10.1161/01.HYP.0000112424.06921.52. [DOI] [PubMed] [Google Scholar]
- 46.Nagasaka H, Komatsu H, Ohura T, Sogo T, Inui A, et al. Nitric oxide synthesis in ornithine transcarbamylase deficiency: possible involvement of low NO synthesis in clinical manifestations of urea cycle defect. J Pediatr. 2004;145:259–262. doi: 10.1016/j.jpeds.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Luiking YC, Hallemeesch MH, Poll MCvd, Dejong CHC, Jonge WJd, et al. Reduced citrulline availability by OTC deficiency in mice is related to reduced nitric oxide production. Am J Physiol Endocrinol Metab. 2008;295:E1315–E1322. doi: 10.1152/ajpendo.00055.2008. [DOI] [PubMed] [Google Scholar]
- 48.Brunetti-Pierri N, Erez A, Shchelochkov O, Craigen W, Lee B. Systemic hypertension in two patients with ASL deficiency: a result of nitric oxide deficiency? Mol Genet Metab. 2009;98:195–197. doi: 10.1016/j.ymgme.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch- Eur J Physiol. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 50.Fike CD, Sidoryk-Wegrzynowicz M, Aschner M, Summar M, Prince LS, et al. Prolonged hypoxia augments L-citrulline transport by System A in the newborn piglet pulmonary circulation. Cardiovasc Res. 2012;95:375–384. doi: 10.1093/cvr/cvs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dikalova A, Fagiana A, Aschner JL, Aschner M, Summar M, et al. Sodium-coupled neutral amino acid transporter 1 (SNAT 1) modulates L-citrulline transport and nitric oxide (NO) signaling in piglet pulmonary arterial endothelial cells. PLoS One. 2013 doi: 10.1371/journal.pone.0085730. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fike CD, Dikalova A, Slaughter JC, Kaplowitz MR, Zhang Y, et al. Reactive oxygen species reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid Redox Signal. 2013;18:1727–1738. doi: 10.1089/ars.2012.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- 54.Gielis JF, Lin JY, Wingler K, Schil PEYV, Schmidt HH, et al. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Rad Biol Med. 2011;50:765–776. doi: 10.1016/j.freeradbiomed.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:506–511. doi: 10.1152/ajplung.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grisafi D, Tassone E, Dedja A, Oselladore B, Masola V, et al. L-citrulline prevents alveolar and vascular derangement in a rat model of moderate hyperoxia-induced lung injury. Lung. 2012;190:419–430. doi: 10.1007/s00408-012-9382-z. [DOI] [PubMed] [Google Scholar]
- 57.Balderas-Munoz K, Castillo-Martinez L, Orea-Tejeda A, Infante-Vazquez O, Utrera-Lagunas M, et al. Improvement of ventricular function in systolic heart failure patients with oral L-citrulline supplementation. Cardiol J. 2012;19:612–617. doi: 10.5603/cj.2012.0113. [DOI] [PubMed] [Google Scholar]
- 58.Orea-Tejeda A, Orozco-Gutierrez JJ, Castillo-Martinez L, Keirns-Davies C, Montano-Hernandez P, et al. The effect of L-arginine and citrulline on endothelial function in patients in heart failure with preserved ejection fraction. Cardiol. 2010;J 17:464–470. [PubMed] [Google Scholar]
- 59.Figueroa A, Sanchez-Gonzalez MA, Wong A, Arjmandi BH. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese patients with prehypertension or hypertension. Am J Hypertension. 2012;25:640–643. doi: 10.1038/ajh.2012.20. [DOI] [PubMed] [Google Scholar]
- 60.Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, et al. Short-term effects of L-citrulline supplementation on arterial stiffness in middle aged-men. Int J Cardiol. 2012;155:257–261. doi: 10.1016/j.ijcard.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Waugh WH, Daeschner CW, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc. 2001;93:363–371. [PMC free article] [PubMed] [Google Scholar]
- 62.Smith HAB, Canter JA, Christian KG, Drinkwater DC, Scholl FG, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]