Abstract
The delayed immune response to stroke is responsible for the increased neural injury that continues to occur after the initial ischemic event. This delayed immune response has been linked to the spleen, as splenectomy prior to middle cerebral artery occlusion (MCAO) is neuroprotective. Interferon gamma (IFNγ) is linked to the splenic response, which enhances neural injury following MCAO. IFNγ activates the expression of the inflammatory chemokine interferon-inducible protein 10 (IP-10). This study was designed to determine the role of IFNγ signaling in the inflammatory response following MCAO. Expression of IP-10 increased in the brain and the spleen following MCAO. Splenectomy inhibited the increase of IP-10 in the brain post-MCAO, while recombinant IFNγ administration to splenectomized rats returned IP-10 levels in the brain to levels found in rats after MCAO only. Systemic administration of an IFNγ neutralizing antibody to MCAO-treated rats reduced infarct volume and IP-10 levels in the brain. T cell infiltration was reduced in the MCAO-damaged brains of IFNγ antibody-treated animals relative to those that received isotype control antibodies. Additionally, inhibiting IFNγ signaling with splenectomy or an IFNγ neutralizing antibody blocked the induction of IP-10 expression and decreased neurodegeneration following MCAO. Targeting this pro-inflammatory pathway following stroke could be a promising stroke therapeutic.
Keywords: Spleen, IP-10, Monocytes, Chemokines, Lymphocytes
Introduction
The inflammatory response after stroke has been well documented in enhancing delayed neurodegeneration after ischemic injury (Leonardo and Pennypacker 2011). Downstream signaling of the pro-inflammatory cytokine interferon gamma (IFNγ) induces the expression of the chemokine interferon-inducible protein 10 (IP-10), also known as CXCL10. IP-10 is a pro-inflammatory chemokine that selectively drives the propagation of the Th1 response by interacting with the CXCR3 receptor (Loetscher et al. 2001). Microglia/macrophages produce IP-10 in response to IFNγ stimulation (Luster 2002), and IP-10 induces chemotaxis of Th1 cells to the site of injury. In addition, IP-10 can prevent the activation of Th2 cells by competitive antagonism of the CCR3 receptor (Loetscher et al. 2001). These interactions of IP-10 with CXCR3 and CCR3 create a pro-inflammatory feed forward mechanism that effectively recruits more IFNγ producing cells to the site of injury, leading to more IP-10 production through increased IFNγ signaling.
Investigations of IP-10 in experimental mouse models of stroke have shown that IP-10 mRNA is upregulated early in the brain at 6 and 22 h following MCAO. In the spleen, IP-10 mRNA levels are increased at 22 h post-MCAO (Offner et al. 2006; Hurn et al. 2007). Although IP-10 transcript has been measured at selected time points following stroke, IP-10 protein levels have not been quantified in the brain or spleen following MCAO or at longer time points after MCAO. Further investigation into the role of IP-10 following brain ischemia is necessary to elucidate the IFNγ/T cell response in stroke.
The contribution of the peripheral immune response to ischemic stroke injury has been directly linked to the spleen, and IFNγ signaling appears to play a key role in stroke-induced neurodegeneration. The spleen is a large reservoir for immune cells and splenectomy prior to middle cerebral artery occlusion (MCAO) is neuroprotective in rats (Ajmo et al. 2008) and mice (Jin et al. 2013). IFNγ is a pro-inflammatory cytokine that relays the splenic response to MCAO. IFNγ levels increase in the spleen 24 h post-MCAO (Seifert et al. 2012b), and are upregulated in the injured brain at 72 h post-MCAO in rats (Seifert et al. 2012b) and mice (Jin et al. 2013). Interestingly, splenectomy prior to MCAO blocks post-stroke IFNγ elevations in the brain (Seifert et al. 2012b; Jin et al. 2013), and administration of recombinant IFNγ to splenectomized rats negates the protective effects of splenectomy. These data demonstrate that spleen-derived IFNγ plays a detrimental role following stroke.
Since splenocytes have also been tracked in vivo and were found to migrate to the injured brain after MCAO (Seifert et al. 2012a), we have been investigating the downstream effects of IFNγ signaling that may be responsible for promoting degenerative injury following stroke. Previous studies have demonstrated increased levels of IFNγ, the main inducer of IP-10 synthesis, in the brain (Seifert et al. 2012b; Jin et al. 2013) and the spleen (Seifert et al. 2012b) post-MCAO. In this study, we assessed the time course of IP-10 expression in the spleen and brain after MCAO to determine whether IP-10 is a critical mediator of the peripheral immune response following stroke. Splenectomy and/or administration of an IFNγ neutralizing antibody were utilized to inhibit IFNγ signaling. Results showed that blocking IFNγ signaling effectively reduced IP-10 expression and decreased neural injury following stroke. T cell recruitment to the brain was also investigated, as IP-10 is a chemoattractant for Th1 cells, and decreased IP-10 protein expression was associated with diminished T cell infiltration into the infarct. These data indicate that IFNγ/IP-10 is a major inflammatory signal pathway in the immune response to stroke.
Materials and Methods
Animal Care
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Male Sprague-Dawley rats (300–350g) were used for the following experiments. All rats were purchased from Harlan Labs (Indianapolis, IN), maintained on a 12 h light/dark cycle (6 am – 6 pm) and given access to food and water ad libitum.
Laser Doppler Blood Flow Measurement
Laser Doppler was used to monitor blood perfusion (Moor Instruments Ltd, Devon, England). A hole was drilled 1 mm posterior and 4 mm lateral from Bregma, and a guide screw was placed. The laser doppler probe was inserted into the guide screw, and the tip of the probe was placed against the surface of the brain. Rats that did not show ≥ 60% reduction in perfusion during MCAO were excluded from this study (Ajmo et al. 2006; Ajmo et al. 2008; Hall et al. 2009). Sham operated rats had the guide screw and laser doppler probe placed and blood flow was monitored to ensure that there was not a drop in cerebral blood flow during the sham procedure.
Splenectomy
Splenectomy was performed two weeks prior to MCAO as previously described (Seifert et al. 2012b). Briefly, rats were anesthetized with 5% isoflurane and maintained on 2–3%. The abdominal cavity was opened on the anatomical left. The splenic blood vessels were ligated and the spleen was removed. The incision was then closed with sutures, first closing the abdominal wall and then the skin incision. Sham operations were also performed where the spleen was exteriorized and then reinserted into the cavity. Animals were allowed to wake in fresh cages.
Permanent Middle Cerebral Artery Occlusion
MCAO surgery was performed using the intraluminal method originally described by Longa et al. (Longa et al. 1989) and previously reported (Ajmo et al. 2006; Ajmo et al. 2008; Hall et al. 2009). Briefly, rats were anesthetized with 5% isoflurane and maintained on 2–3%. Blunt dissection was performed to isolate the common carotid artery, the internal carotid artery (ICA), and the external carotid artery (ECA). The ECA was ligated and cut. Then a 40 mm monofilament was introduced into the ECA, fed distally into the ICA, and advanced to the origin of the MCA. The filament was tied off on the ECA to produce a permanent occlusion. The incision was then sutured closed and the rat was allowed to wake in a fresh cage.
Antibody Treatment Injections
A goat anti-rat polyclonal IFNγ neutralizing antibody (R&D Systems, Minneapolis, MN) and a goat IgG isotype antibody (R&D Systems) were reconstituted with phosphate buffered saline (PBS) to a concentration of 100 μg/ml. Animals in the antibody treatment study were randomly assigned to one of three treatment groups: IFNγ neutralizing antibody, IgG isotype control or the PBS control. Beginning at 24 h post-MCAO animals were administered either 5 μg (0.05 ml) of a goat anti-rat IFNγ neutralizing antibody, a goat IgG isotype control, or an equivalent amount of PBS via an intraperitoneal (i.p.) injection. Treatment was administered at 24, 48, and 72 h post-MCAO.
Recombinant IFNγ Administration
Rat recombinant IFNγ (rIFNγ) was administered as previously described (Seifert et al. 2012b). The lowest dosage of rIFNγ (ProSpec, Rehovot, Israel) that elicited a physiologic response in naïve rats was previously determined. The dosage of 20 μg (in 0.21 ml sterile ddH2O) was injected intravenously (i.v.), via the tail vein, at 48 and 72 h post-MCAO to determine the effects of IFNγ on neural injury in splenectomized and sham-splenectomized rats.
Tissue Extraction and Sectioning
The animals were euthanatized with a ketamine/xylazine mix, 75 mg/kg and 7.5 mg/kg respectively, i.p. at 24, 48, 72 or 96 h post-MCAO for the time course experiment and at 96 h post-MCAO for the antibody treatment and rIFNγ experiments. Anesthetized animals were then perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in phosphate buffer (PB). The spleen and thymus were removed prior to perfusion. Spleens were weighed immediately following removal and were subsequently snap frozen and stored in the −80°C with the thymi. The brains were harvested, post fixed in 4% paraformaldehyde, and immersed in 20% followed by 30% sucrose in PBS. Brains were frozen and sliced into 30 μm sections using a cryostat. Coronal sections were taken at six points from 1.7 to −3.3 mm from Bregma. Sections were either thaw mounted on glass slides or placed in Walter’s Anti-freeze cryopreservative and stored at −20°C.
Fluoro-Jade Staining
Brain sections mounted on glass slides were stained with Fluoro-Jade, which labels degenerating neurons. This method was adapted from that originally developed by Schmued et al. (Schmued et al. 1997) and has been described by Duckworth et al. (Duckworth et al. 2005). Slides were dried at room temperature overnight, placed in 100% ethanol for 3 min, 70% ethanol for 1 min, and then ddH2O for 1 min. Slides were oxidized using a 0.06% KMnO4 solution for 15 min followed by three 1 min rinses with ddH2O. Slides were stained in a 0.001% solution of Fluoro-Jade (Histochem, Jefferson, AR) in 0.1% acetic acid in the dark for 30 min. Slides then were rinsed 4 times with ddH2O for 3 min each time, allowed to dry at 45°C for 20 min, cleared twice with xylene and then cover slipped with DPX mounting medium (Electron Microscopy Sciences, Ft. Washington, PA).
Infarct Quantification
Fluoro-Jade stained tissue was digitally photographed with Zeiss Axioskop2 (Carl Zeiss Inc, Thornwood, NY) microscope controlled by Openlab software (Improvision, Waltham, MA) at a magnification of 1x. The area of neurodegeneration was measured using the NIH ImageJ software. The area of the contralateral hemisphere was also measured and used to compensate for possible edema in the ipsilateral hemisphere. Infarct volumes were then calculated by the total area of ipsilateral staining divided by the total contralateral area for a given animal. Infarct quantification was done for all animals.
Immunohistochemistry in the Brain
The slides were dried at 37°C for 1 h then rinsed with PBS pH 7.4. Slides were placed in permeabilization buffer containing 10% serum, 3% 1M lysine, and 0.3% Triton X-100 in PBS for 1 h at room temperature. Next, sections were incubated overnight at 4°C in a primary antibody solution (PBS with 2% serum and 0.3% Triton X-100) in a humidified chamber. Slides were subsequently rinsed with PBS and incubated with a secondary antibody solution (PBS, 2% serum, 0.3% Triton X-100) for 1 h. Slides were washed thoroughly with PBS and dried for 1 h at 45°C. Once dry, the slides were rinsed twice with x ylene and cover slipped using DPX (Electron Microscopy Sciences). Slides were protected from light during these steps. As a negative control, sections were processed as above without the primary antibody.
Double label immunohistochemistry for IP-10 and immune cell surface markers was achieved by incubating the slides with primary antibodies, followed by incubation with secondary antibodies conjugated to 594nm or 488nm fluorophores.
The following primary antibodies were used: rabbit anti-rat IP-10 (1:5,000; abcam; Cambridge, MA), mouse anti-rat CD3 for T cells (1:2,000; BD Biosciences, San Jose, CA), and mouse anti-rat CD11b for microglia/macrophages (1:3,000; Serotec, Raleigh, NC). Alexa-Fluor® 488 goat anti-rabbit (1:300; Life Technologies, Grand Island, NY) secondary was used for all IP-10 staining. Alexa-Fluor® 594 goat anti-mouse (1:300; Life Technologies) secondary was used in conjunction the immune cell surface markers for double staining with IP-10. Alexa-Fluor® 488 rabbit anti-mouse (1:300; Life Technologies) secondary was used when only staining for CD3 T cell.
IP-10 Immunohistochemistry in the Spleen
Spleens were fixed in 4% paraformaldehyde overnight. The spleens were then placed in a solution of 20% glycerol and 2% dimethyl sulfoxide (DMSO) and embedded in a gelatin matrix using MultiBrain Technology© (NeuroScience Associates, Knoxville, TN). The block of spleens was rapidly frozen in isopentane with crushed dry ice (-70°C). Using a microtome the block was sliced into 25 μm sections. Six consecutive sections were taken and collected in Antigen Preservation solution (50% ethylene glycol, 49% PBS pH 7.0, 1% polyvinyl pyrrolidone). The spleen sections were stained free floating in Tris-buffered saline (TBS) solutions. Endogenous peroxide activity was extinguished by treatment with 3% hydrogen peroxide for 15 min. After washing with TBS sections were incubated for 30 min in permeabilization buffer (TBS with 0.3% TritonX-100 and 10% serum). Following permeabilization, slides were incubated overnight at room temperature with primary antibody in TBS with 2% serum. The sections were rinsed with TBS and incubated in secondary biotinylated antibody in TBS with 2% serum for 1 h. After being rinsed with TBS, sections were incubated with an avidin/biotin/horseradish peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) for 1 h. Staining was visualized with 3,3′-Diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St. Louis, MO). The sections were then mounted on gelatinized slides, dried, dehydrated, cleared with xylene, and cover slipped with Permount (Fischer Scientific, Pittsburg, PA). The primary antibody used was rabbit anti-rat IP-10 (1:500; abcam) and the secondary antibody was biotinylated goat anti-rabbit (Vector Laboratories). The IP-10 antibody’s specificity was determined by Western blotting using recombinant IP-10.
Immunohistochemistry Quantification
IP-10 stained brain tissue sections were digitally photographed with Zeiss Axioskop2 microscope controlled by Openlab software at a 10x magnification. Three images from each of 4 Bregma points (1.7 mm to −1.3 mm) were taken for a total of twelve images per brain. The area selected for quantification was the striatum of the ipsilateral hemisphere for all animals. The images were analyzed for percent of immunostaining per area with ImageJ software. These twelve values were then averaged for each brain. Percent immunostaining was used for the brain sections since the IP-10 staining was diffuse which didn’t lend itself to an “intensity” measure as noted below for the more prevalent staining of the spleen.
CD3 stained brain tissue sections were digitally photographed with Zeiss Axioskop2 microscope controlled by Openlab software at a 10x magnification. Images from Bregma point +0.7 mm were taken of the peri-infract region of the striatum near the lateral ventricle of the ipsilateral hemisphere. The images were analyzed for percent of immunostaining per area with ImageJ software.
Splenic IP-10 images were taken with a Nikon 90i microscope using a 20x objective and NIS Elements BR 4.00.07 software at a high resolution. The images were processed and analyzed with Photoshop CS5 (Adobe Systems Inc., San Jose, CA). The intensity of the staining was measured in the histogram for the entire image and the amount of staining per image was analyzed. Six sections per spleen were analyzed for each rat.
Confocal Imaging
Tissue sections that were double labeled were viewed on the Leica SP2 confocal microscope (Leica Microsystems, Buffalo Grove, IL). Images were taken at a magnification of 63x. Each fluorophore was scanned sequentially and then the two images were merged.
Statistical Analysis
All data are expressed as group mean ± SEM. Significance of the data was determined by ANOVA with Fischer’s Least Significant Difference post hoc test for all analysis. A value of p<0.05 was considered significant. All sections were blinded prior being analyzed by an investigator.
Results
IP-10 levels are elevated in the brain following MCAO
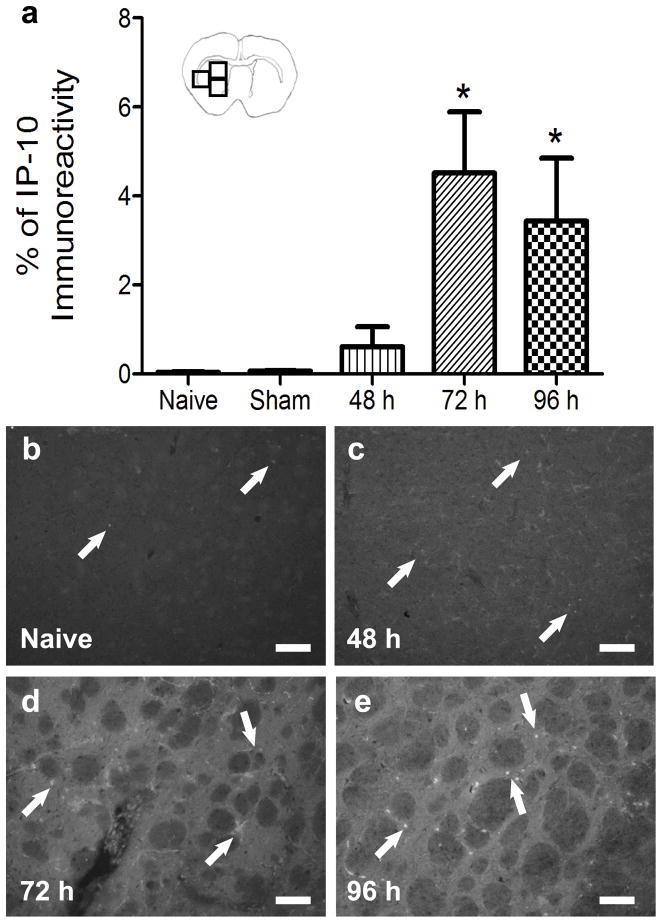
To determine if IP-10 protein is expressed in the brain following MCAO, its protein expression in the striatum of the ipsilateral hemisphere was characterized over time. To quantify IP-10 levels, immunohistochemistry for IP-10 was performed on brain sections from animals euthanized at 48, 72, and 96 h following MCAO and naïve (Figs 1b–e) or sham operated rats. IP-10 protein levels were significantly increased at 72 h and remained elevated at 96 h (p<0.01) compared to sham operated rats 96 h after surgery (Fig 1a).
Fig 1.
Quantification of IP-10 levels in the brain post-MCAO. Immunohistochemical quantification of striatal IP-10 protein levels in the brains of naïve, sham, 48, 72, and 96 h post-MCAO (n= 4, 3, 3, 4, and 4 respectively) demonstrate IP-10 levels are significantly elevated at 72 and 96 h post-MCAO compared to naïve brains (*p<0.01; a). Sham animals had a survival time of 96 h. Representative micrographs of IP-10 stained brains from naïve (b), 48 h (c), 72 h (d), and 96 h (e) post-MCAO. Scale bar = 100μm. Arrows indicate areas of staining. Boxes in the brain graphic indicated the three regions used for quantification.
Splenic IP-10 levels increase after MCAO and remain elevated
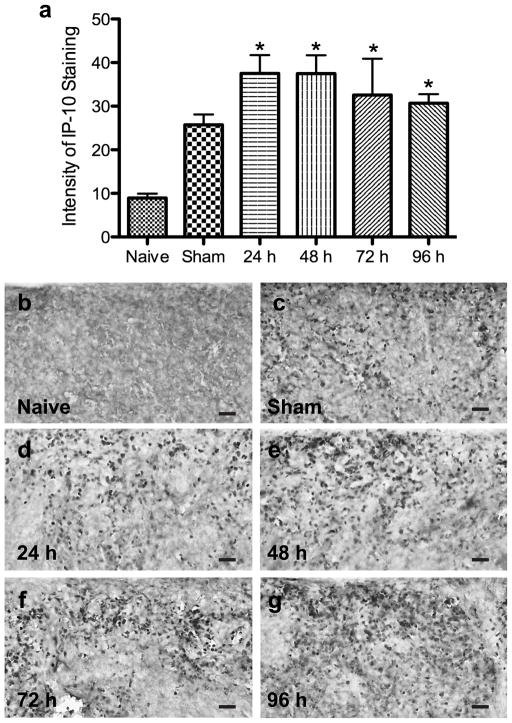
In the spleen, IP-10 protein levels were quantified using immunohistochemistry. Spleens from naïve or sham rats euthanized at 96 h and animals euthanized 24, 48, 72, and 96 h post-MCAO (Figs 2b–g) were used to perform immunohistochemistry to determine IP-10 protein expression. IP-10 levels were significantly elevated at 24 h and remained elevated out to 96 h following MCAO compared to naïve spleens (p<0.0007). The sham operated animals had increased levels of IP-10 but this did not reach statistical significance (Fig 2a).
Fig 2.
Quantification of IP-10 levels in the spleen post-MCAO. Immunohistochemical quantification of IP-10 protein levels in the spleens of naïve, sham, 24, 48, 72, and 96 h post-MCAO (n= 4, 3, 7, 3, 3, and 7 respectively) demonstrate IP-10 levels are significantly elevated at 24 h and remain elevated out to 96 h post-MCAO compared to naïve spleens (*p<0.0007; a). Representative micrographs of IP-10 stained spleens from naïve (b), 96 h sham (c), 24 h (d), 48 h (e), 72 h (f), and 96 h (g) post-MCAO. Scale bar = 120μm.
IP-10 levels in the brain are reduced by splenectomy and restored by rIFNγ administration
Splenectomy prior to MCAO is neuroprotective and reduces the amount of IFNγ in the brain (Seifert et al. 2012b; Jin et al. 2013). IP-10 expression is induced by IFNγ; therefore, brains of splenectomized rats that underwent MCAO were stained for IP-10 protein expression. Splenectomy significantly reduces the amount of IP-10 in the infarct area of rats post-MCAO. Whereas, administration of rIFNγ to splenectomized rats increases IP-10 levels to those not significantly different from sham splenectomized rats post-MCAO (p≤0.05; Fig 3a). However, splenic IP-10 expression was unaffected by administration of rIFNγ (Fig 3b).
Fig 3.
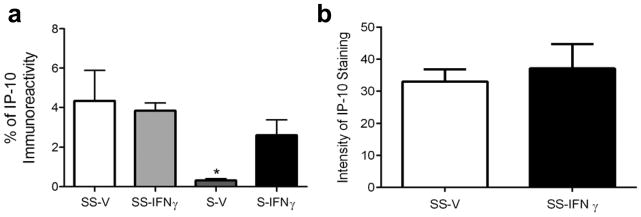
Splenectomy and rIFNγ administration affects IP-10 in the brain and not the spleen. Rats that underwent splenectomy two weeks prior to MCAO had significantly reduced levels of IP-10 in the brain at 96 h post-MCAO (*p≤0.05) and IP-10 levels were not significantly different from sham splenectomized rats when splenectomized rats were administration rIFNγ following MCAO (a). Administration of rIFNγ to sham splenectomized rats did not cause a significant increase in IP-10 levels in the spleen post-MCAO (b). Treatment groups: sham-splenectomy-vehicle (SS-V, n=3 brains and 5 spleens), sham-splenectomy-rIFNγ (SS-IFNγ, n=3 brains and 6 spleens), splenectomy-vehicle (S-V, n=3), and splenectomy-rIFNγ (S-IFNγ, n=4).
IP-10 is produced in monocytic cells in the brain following MCAO
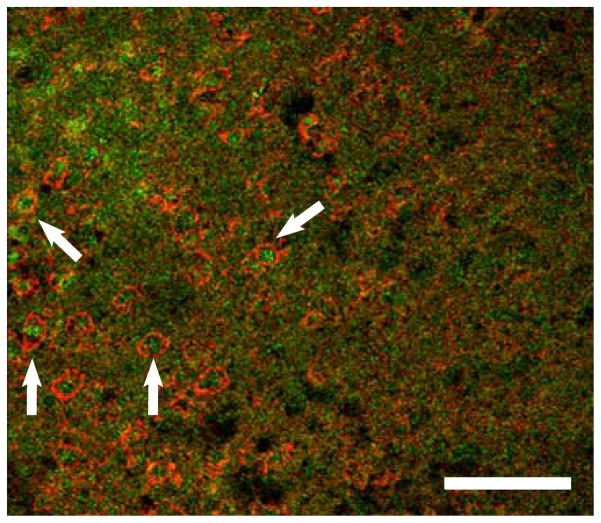
IP-10 expression is induced by IFNγ in cells of monocytic origin. Double staining with IP-10 and CD11b, a marker for monocytes, was performed on the brains from animals 96 h post-MCAO. Expression of IP-10 and CD11b were co-localized in the infarcted area of the ipsilateral hemisphere (Fig 4).
Fig 4.
IP-10 is produced in monocytic cells in the brain following MCAO. Confocal micrograph shows amoeboid CD11b positive cells (red) with intracellular IP-10 (green) in the striatum of the ipsilateral hemisphere 96 h following MCAO. Scale bar = 75μm. Arrows indicate areas of co-localization.
IFNγ neutralizing antibody administration decreases infarct following MCAO
To inhibit the pro-inflammatory IFNγ signaling pathway that increases IP-10 transcription following MCAO, an IFNγ neutralizing antibody was administered 24, 48, and 72 h post-MCAO. Infarct volumes, as measured by Fluoro-Jade staining, at 96 h post-MCAO were significantly decreased in the IFNγ neutralizing antibody group compared to the vehicle and isotype control groups (p<0.007; Fig 5d). Representative micrographs from Fluoro-Jade stained brains show decreased staining in the IFNγ neutralizing antibody treated group (Fig 5c) compared to the staining in the PBS (Fig 5a) and IgG isotype control (Fig 5b) groups.
Fig 5.
IFNγ neutralizing antibody administration following MCAO decreases infarct volume. Administration of an IFNγ at 24, 48, and 72 h post-MCAO (n=3) significantly decreased infarct volume, as measured by Fluoro-Jade staining, at 96 h when compared to the vehicle (n=4) and isotype (n=5) control groups (*p<0.007; d). Representative micrographs of Fluoro-Jade stained brains from PBS vehicle (a), IgG isotype (b), and IFNγ antibody (c) treatment groups 96 h post-MCAO. Scale bars = 2 mm.
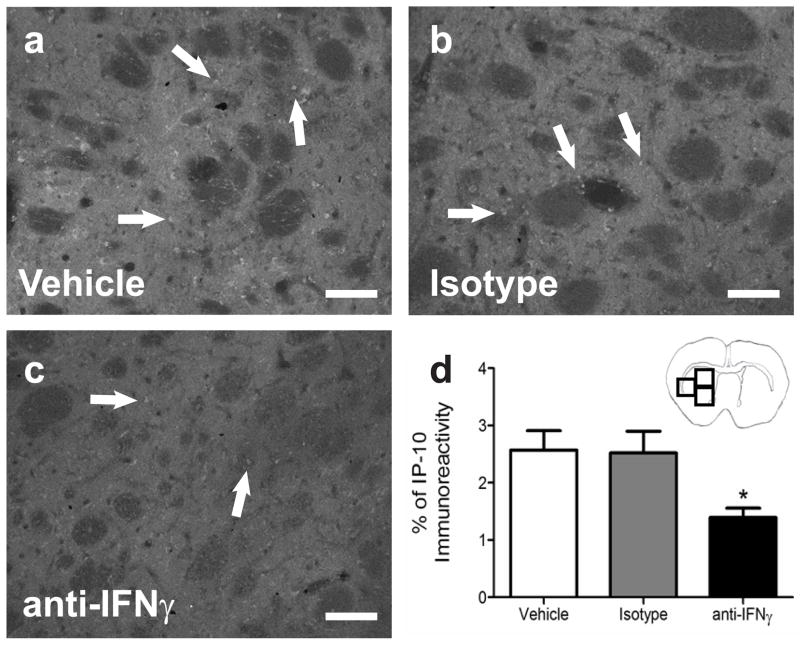
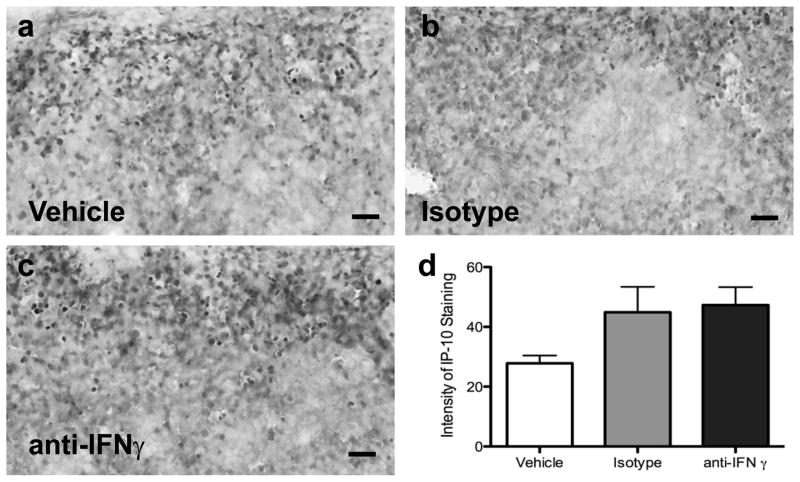
IFNγ neutralizing antibody decreased IP-10 in the brain
IP-10 protein expression was quantified in the striatum of the ipsilateral hemisphere from rats treated with PBS (Fig 6a), control IgG (Fig 6b), or IFNγ antibody (Fig 6c). IP-10 immunoreactivity was significantly decreased in the IFNγ antibody treatment group compared to the IgG isotype and PBS control groups (p<0.009; Fig 6d).
Fig 6.
Quantification of IP-10 levels in the brain post-MCAO with administration of an IFNγ neutralizing antibody. Immunohistochemical quantification of striatal IP-10 protein levels in the brains of vehicle (n=3), IgG Isotype (n=4), and IFNγ antibody (n=4) 96 h post-MCAO demonstrate IP-10 levels are significantly decreased in the IFNγ antibody treated group compared to the vehicle treated group (*p<0.009; d). Representative micrographs of IP-10 stained brains from PBS vehicle (a), IgG isotype (b), and IFNγ antibody (c) treatment groups 96 h post-MCAO. Scale bars = 100μm. Arrows indicate areas of staining. Boxes in the brain graphic indicated the three regions used for quantification.
Splenic IP-10 is not altered by antibody administration
IP-10 levels were measured in the spleen of animals treated with PBS, IgG isotype, or IFNγ neutralizing antibody. Representative micrographs show IP-10 staining in the spleens of PBS treated animals (Fig 7a) and in the groups of animals that received IgG (Fig 7b) or IFNγ neutralizing antibody (Fig 7c). Quantification showed that splenic IP-10 staining intensity was not significantly increased in either group that received antibody compared to the vehicle-treated group (Fig 7d).
Fig 7.
Quantification of IP-10 levels in the spleen post-MCAO with administration of an IFNγ neutralizing antibody. Immunohistochemical quantification of IP-10 protein levels in the spleens of vehicle (n=4), IgG Isotype (n=7), and IFNγ antibody (n=7) 96 h post-MCAO demonstrate IP-10 levels are elevated in the IFNγ antibody and the IgG groups compared to the vehicle treated group (d). Representative micrographs of IP-10 stained spleens from the PBS vehicle (a), IgG isotype antibody (b), and IFNγ antibody (c) treatment groups 96 h post-MCAO. Scale bars = 120μm.
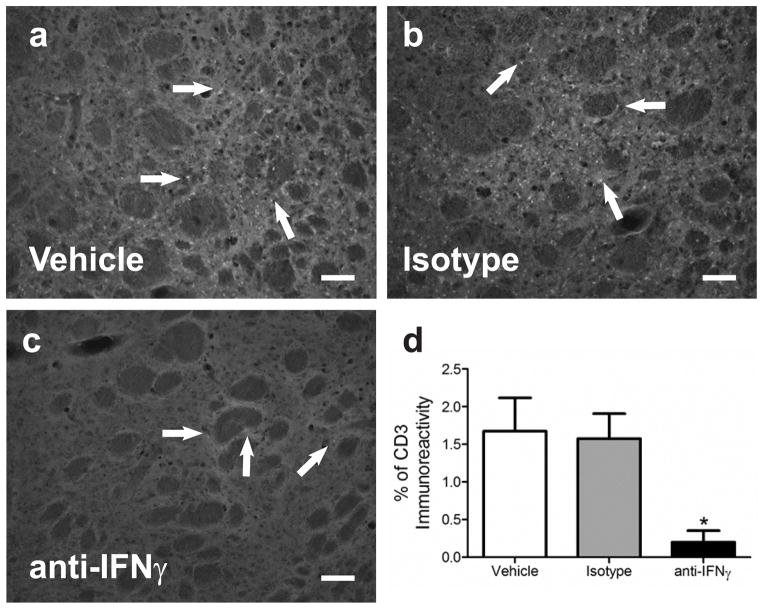
The amount of CD3 immunoreactivity decreases in the brains of IFNγ neutralizing antibody treated animals
Brain sections from animals that underwent MCAO and then administered an IFNγ neutralizing antibody, an IgG isotype antibody, or PBS were stained using an anti CD3 antibody to visualize the presence of T cells. At 96 h post-MCAO the amount of CD3 immunostaining in the ipsilateral striatum was significantly decreased in the IFNγ antibody treated group compared to the IgG and PBS control groups (p<0.04; Fig 8d). Representative micrographs of the striatum show reduced CD3 staining in the brains of IFNγ antibody (Fig 8c) treated animals compared to the CD3 staining in the IgG (Fig 8b) and vehicle (Fig 8a) groups.
Fig 8.
CD3 immunoreactivity decreased in IFNγ antibody treated brains. The amount of T cells in the brain following MCAO is decreased in the IFNγ antibody treated group (n=3) compared to controls (*p<0.04; n= 4 each;d). Images are from the striatum of the ipsilateral hemisphere. There is decreased CD3 (T cell) immunoreactivity in the brains of IFNγ antibody treated animals (c) compared to the amount of staining in the two control groups PBS vehicle (a) and isotype (b). Scale bars = 100 μm. Arrows indicate areas of staining.
Discussion
IP-10 levels are induced in the MCAO-injured hemisphere where its expression significantly increases at 72 h and remains elevated at 96 h post-MCAO. These results are consistent with a delayed response and up regulation of protein compared to an earlier increase in IP-10’s mRNA levels. Studies looking at mRNA levels of IP-10 in the brain following MCAO found increased mRNA as early as 6 h post-MCAO (Offner et al. 2006). As expected, IP-10 within the infarct is expressed in cells of the monocytic lineage (CD11b+), which are most likely microglia. IFNγ is known to activate cells of monocytic origin to produce IP-10 (Boehm et al. 1997). Splenectomy decreases IFNγ levels in the brain (Seifert et al. 2012b), subsequently prevents the increase in IP-10 in the brain following MCAO. However, we have previously shown that administration of rIFNγ after MCAO reversed the protective effects of splenectomy (Seifert et al. 2012b) and additionally this treatment increased the expression of IP-10 protein in the injured brain to levels not significantly different from those from sham splenectomized rats. Additionally, sham splenectomized rats that received rIFNγ after MCAO did not further elevate levels of IP-10 in the spleen or the brain, showing that the endogenous IFNγ response elicited by the spleen to stroke is a maximal response.
The spleen reacts to bodily injuries by eliciting an inflammatory response that further exacerbates cellular damage. Splenectomy is protective in a variety of ischemic injuries in other organs including the liver, (Okuaki et al. 1996) kidney, (Jiang et al. 2007) intestines, (Savas et al. 2003) and heart (Leuschner et al. 2010). Additionally, removal of the spleen is neuroprotective in several types of brain injuries including ischemic stroke, (Ajmo et al. 2008; Jin et al. 2013) intracerebral hemorrhage, (Lee et al. 2008) and traumatic brain injury (Li et al. 2011; Das et al. 2011; Walker et al. 2010). Radiation of the spleen following MCAO also reduces infarct volume (Ostrowski et al. 2012). The elimination of splenocytes by any means results in protection from ischemic tissue injuries. These studies reveal that splenocytes probably via the expression of inflammatory cytokines are universally detrimental to ischemic injuries in mouse and rat injury models.
In the spleen the levels of IFNγ spike at 24 h post-MCAO (Seifert et al. 2012b) while mRNA levels of IP-10 in the spleen 22 h following stroke in mice (Offner et al. 2006; Hurn et al. 2007). We show that the increase in IFNγ is followed by a prolonged increase in the expression of the pro-inflammatory chemokine IP-10 that begins at 24 h and remains elevated at least out to 96 h post-MCAO. IFNγ despite a very short half-life elicits protracted effects. An IFNγ-induced feed forward inflammatory cycle is initiated when the protracted expression of IP-10 secreted from macrophage/microglia recruits inflammatory T cells to the infarct and they express and secrete more IFNγ, leading to further activation and expression of IP-10 in macrophages/microglia ultimately resulting in delayed neural injury. Since IFNγ is not directly neurotoxic (Seifert et al. 2012b), its downstream activation of macrophages/microglia appears to be the mechanism by which this inflammatory cytokine can indirectly be responsible for delayed neurodegeneration.
Splenectomy not only reduces infarct volume but neuroinflammation as demonstrated by decrease in levels of IFNγ, IP-10, CD3 T cell immunoreactivity, and CD11b microglial activation in the infarct (Seifert et al. 2012b; Jin et al. 2013). Removal of the spleen blunts the IFNγ mediated immune response to the brain following stroke, which includes IP-10 expression, and recruitment and activation of T cells. The importance of IFNγ in the splenic response is clearly demonstrated in that the administration of IFNγ reverses the neuroprotective effects provided by splenectomy. Using a neutralizing antibody directed against IFNγ administered after MCAO could provide further evidence that IFNγ is the neurodegenerative mediator originating from the spleen.
Systemic administration of an IFNγ neutralizing antibody significantly decreases infarct volume when compared to vehicle controls. These results are consistent with previous observations where IFNγ was blocked (Yilmaz et al. 2006; Liesz et al. 2009; Liesz et al. 2011). Studies using IFNγ−/− mice (Yilmaz et al. 2006) or inhibiting IFNγ with a neutralizing antibody injected directly into the brain three days following MCAO (Liesz et al. 2009) both decreased infarct volume. Additionally, using an antibody directed at CD49d (very late antigen 4, VLA4) prevented immune cells from entering the brain following MCAO, leading to decreased infarct volume. This treatment precluded the cells producing IFNγ from entering the injured brain (Liesz et al. 2011). These studies did not investigate the downstream effects of IFNγ inhibition on the expression of chemokines like IP-10. Our study shows that striatal IP-10 levels in the brain are significantly decreased with IFNγ neutralization. The amount of CD3 immunoreactivity is significantly reduced in the striatum of these rats as well. This is expected as IFNγ is known to induce IP-10 production (Boehm et al. 1997) and IP-10 is a strong chemoattractant for pro-inflammatory IFNγ producing T cells (Groom and Luster 2011). The infarct volumes for the IFNγ neutralizing antibody were significantly reduced compared to the IgG and vehicle controls. Moreover, a potentially more effective approach would be to use these antibodies in conjunction with pharmaceuticals that block IFNγ or its downstream intracellular signal transduction.
The increase in IP-10 expression in the spleen, and later the brain, is an important indicator of a pro-inflammatory pro-degeneration splenic response to ischemic stroke. IFNγ signaling induces the expression of IP-10. Inhibiting IFNγ signaling results in a reduction in infarct volume, IP-10 protein expression in the brain, and T cell recruitment to injured brain area leading to decreased infarct size. Selectively targeting the IFNγ signaling pathway is a potential treatment for stroke.
Acknowledgments
The authors would like to thank the Lisa Muma Weitz Laboratory for Advanced Microscopy & Cell Imaging at the University of South Florida for assistance with obtaining the confocal images. This research was funded by National Institutes of Health – Neurological Disease and Stroke 1-R01-NS052839.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3 (2):89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Das M, Leonardo CC, Rangooni S, Mohapatra SS, Mohapatra S, Pennypacker KR. Lateral fluid percussion injury of the brain induces CCL20 inflammatory chemokine expression in rats. J Neuroinflammation. 2011;8:148. doi: 10.1186/1742-2094-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR. Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042 (1):29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89 (2):207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AA, Guyer AG, Leonardo CC, Ajmo CT, Jr, Collier LA, Willing AE, Pennypacker KR. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. J Neurosci Res. 2009;87 (2):333–341. doi: 10.1002/jnr.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27 (11):1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Meng F, Li W, Tong L, Qiao H, Sun X. Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats. Surgery. 2007;141 (1):32–40. doi: 10.1016/j.surg.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Jin R, Zhu X, Liu L, Nanda A, Granger DN, Li G. Simvastatin Attenuates Stroke-induced Splenic Atrophy and Lung Susceptibility to Spontaneous Bacterial Infection in Mice. Stroke. 2013;44 (4):1135–1143. doi: 10.1161/STROKEAHA.111.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131 (Pt 3):616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Pennypacker KR. The splenic response to ischemic stroke: what have we learned from rodent models? Translational Stroke Research. 2011 doi: 10.1007/s12975-0111-0075-3. (e pub April) [DOI] [PubMed] [Google Scholar]
- Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107 (11):1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li F, Luo C, Shan Y, Zhang L, Qian Z, Zhu G, Lin J, Feng H. Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J Trauma. 2011;71 (1):141–147. doi: 10.1097/TA.0b013e3181f30fc9. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15 (2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134 (Pt 3):704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276 (5):2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- Longa E, Weinstein P, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14 (1):129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26 (5):654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Okuaki Y, Miyazaki H, Zeniya M, Ishikawa T, Ohkawa Y, Tsuno S, Sakaguchi M, Hara M, Takahashi H, Toda G. Splenectomy-reduced hepatic injury induced by ischemia/reperfusion in the rat. Liver. 1996;16 (3):188–194. doi: 10.1111/j.1600-0676.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Ostrowski R, Schulte R, Nie Y, Ling T, Lee T, Manaenko A, Gridley D, Zhang J. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res. 2012;3:473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas MC, Ozguner M, Ozguner IF, Delibas N. Splenectomy attenuates intestinal ischemia-reperfusion-induced acute lung injury. J Pediatr Surg. 2003;38 (10):1465–1470. doi: 10.1016/s0022-3468(03)00497-4. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751 (1):37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacology. 2012a;7 (4):1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012b;27 (2):131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PA, Shah SK, Jimenez F, Gerber MH, Xue H, Cutrone R, Hamilton JA, Mays RW, Deans R, Pati S, Dash PK, Cox CS., Jr Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225 (2):341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113 (17):2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]