Abstract
This study described the cerebrospinal fluid (CSF) exposure of vancomycin in 8 children prescribed intravenous vancomycin therapy for cerebral ventricular shunt infection. Vancomycin CSF concentrations ranged from 0.06 to 9.13 mg/L and the CSF: plasma ratio ranged from 0 to 0.66. Two children out of three with a staphylococcal CSF infection had CSF concentrations > minimal inhibitory concentration at the end of the dosing interval.
Keywords: vancomycin, pharmacokinetics, cerebrospinal fluid, children, cerebral shunt
Cerebral ventricular shunt infections are a common cause of shunt failure and occur in 11% of children with shunts.1 In this setting, vancomycin is the most commonly used antimicrobial drug because staphylococcal species account for nearly 75% of shunt infections.2 Successful cerebrospinal fluid (CSF) sterilization depends on optimal drug exposure and, despite its widespread use, limited vancomycin CSF pharmacokinetic (PK) data are available in children. This study describes the CSF exposures of vancomycin in children with a shunt.
Methods
This was a prospective, single-center, open label PK study of intravenous (IV) vancomycin in children with suspected or documented ventricular shunt infection. Children <18 years of age were included if they received IV vancomycin and had CSF collected per standard care. Vancomycin dosing, infusion rate and duration of treatment were prescribed at the discretion of the treating physician. This trial was approved by the institutional review board of the Duke University Medical Center.
CSF was collected as part of standard of care through ventricular or CSF reservoir tap, if the subject had a shunt, or by lumbar puncture if no shunt was present. Vancomycin serum concentrations and dosing were retrospectively recorded from routine therapeutic drug monitoring.
Serum PK samples were analyzed by immunoassay in the Duke Hospital Clinical Laboratory using a validated assay under Good Laboratory Practice (GLP) standards. CSF PK samples were analyzed using a validated liquid chromatography–tandem mass spectrometry assay (HPLC/MS/MS).
The serum PK of vancomycin was characterized using nonlinear regression in WinNonlin v.6.3 (Pharsight Co., St. Louis, MO). A one-compartment model with zero-order infusion was used to fit the serum PK data based on previous vancomycin PK models in children.3 The model fit was evaluated using standard model goodness of fit criteria. Vancomycin CSF penetration was measured using the ratio of CSF to serum concentrations (CSF: serum) at the same time point. Because serum and CSF concentrations (Cserum and CCSF) were not sampled simultaneously, Cserum was predicted at each CSF sample time point using the following equation:
where Cmax is the predicted maximal serum concentration; ke the elimination constant in serum and t the time of CSF sampling. Using the same equation, serum trough concentration (Cmin) was also predicted during the dosing interval when CSF was sampled.
As a pharmacodynamics (PD) endpoint, CCSF was compared with minimum inhibitory concentration (MIC) of vancomycin for children with bacteriologically proven infection. MIC was determined by the clinical microbiology laboratory using validated broth microdilution method or Etest.
Demographics and clinical characteristics were summarized using descriptive statistics using STATA 12 (College Station, TX).
Results
Serum and CSF samples were obtained from 8 children (4 males) with a median (range) age of 4.3 years (0.2, 17), a weight of 14 kg (1, 116), and a serum creatinine of 0.42 mg/dL (0.13, 0.82) (Table 1). All children had a cerebral ventricular shunt except for one subject who had a history of external ventricular drain that was removed 7 days prior to the lumbar puncture. Among the 5 children (63%) who had a positive CSF culture, 2 had a methicillin-resistant Staphylococus aureus (MRSA) (vancomycin MIC of 0.75 and 1 mg/L) and 1 had a Staphylococcus epidermidis (vancomycin MIC of 2 mg/L). All children with confirmed infection underwent externalization of their ventriculo-peritoneal shunt and placement of an external ventricular drain. Shunt was internalized when CSF cultures were negative as decided by the treating physician. All children with confirmed infection were cured and did not have recurrent infection over a 6 month period. Of the 3 children with confirmed Staphylococus sp infection, subject #2 and #8 cleared the infection after 3 days of vancomycin therapy, whereas subject #5 had persistent MRSA in CSF on day 3 and had his first negative CSF culture on day 5. The median vancomycin dose was 19 mg/kg/dose (11, 30) every 8 hours (7–13) for a mean duration of 17 days (4, 27). The median number of doses received prior to PK sampling was 47 (7–74).
Table 1.
Clinical characteristics and vancomycin concentrations in cerebrospinal fluid
| Subject number |
Age (years) |
Sex | Weight (kg) |
Dose* (mg/kg/dose) |
Dosing interval* (h) |
Days of vancomycin therapy |
Serum creatinine* (mg/dL) |
Ventricular CSF culture |
Vancomycin MIC (mg/L) |
CCSF (mg/L) |
Time after last dose (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.2 | M | 1 | 30 (30, 30) | 11 (8, 12) | 4 | 0.12 (0.10, 0.20) | Negative | NA | 0.98 | 5.0 |
| 0.71 | 10.0 | ||||||||||
| 2 | 0.3 | F | 6 | 18 (15, 20) | 7 (4, 18) | 18 | 0.27 (0.20, 0.40) | E. coli | NA | 0.67 | 7.5 |
| 3 | 1.7 | M | 10 | 21 (19, 21) | 8 (7, 24) | 27 | 0.35 (0.20, 0.50) | MRSA E. coli |
0.75 NA |
1.02 | 4.5 |
| 1.12 | 4.6 | ||||||||||
| 1.15 | 14.5 | ||||||||||
| 4 | 4.3 | F | 12 | 17 (15, 20) | 8 (6, 11) | 8 | 0.22 (0.20, 0.30) | Negative | NA | 0.31 | 9.5 |
| 5 | 4.4 | M | 17 | 18 (15, 21) | 13 (6, 24) | 23 | 0.69 (0.40, 1.00) | MRSA | 1 | 4.79 | 7.0 |
| 9.13 | 8.0 | ||||||||||
| 6 | 10.4 | F | 30 | 20 (17, 22) | 9 (6, 59) | 23 | 0.51 (0.40, 0.70) | Gordonia sp/Rhodococcus sp group | Unknown§ | 3.01 | 7.0 |
| 7 | 14.8 | M | 53 | 19 (19, 19) | 8 (5, 24) | 9 | 0.80 (0.70, 0.90) | Negative¥ | NA | 6.60 | 4.0 |
| 8 | 17.3 | F | 116 | 11 (9,13) | 7 (5, 16) | 16 | 0.60 (0.50, 0.70) | CONS | 2 | 0.06 | 5.3 |
| Median (range) | 4.3 (0.2, 17.3) | 4 M (50%)** | 14 (1, 116) | 19 (11, 30) | 8 (7, 13) | 17 (4, 27) | 0.43 (0.13, 0.82) | 1.07 (0.06, 9.13) | 7.0 (4.0, 14.5) |
mean values and range over the vancomycin therapy,
n(%),
the identified bacteria was susceptible to vancomycin but the minimal inhibitory concentration was not available;
CSF sampled through lumbar puncture
F indicates female; M, male; CCSF, vancomycin concentration in cerebrospinal fluid; CONS, coagulase negative staphylococcus sp; E. coli, Escherichia coli; MIC, minimal inhibitory concentration; MRSA, methicillin resistant Staphylococcus aureus; NA: not applicable
Of the 8 children enrolled, one was excluded from the serum PK analysis because only a single serum sample was collected. The median number of serum and CSF samples per child was 5 (1, 14) and 2 (1, 3), respectively, resulting in a total of 43 and 12 serum and CSF samples, respectively. In serum, the median CL was 0.08 L/h/kg (0.05, 0.15), V was 0.70 L/kg (0.22, 4.46) and ke was 0.12 /h (0.02, 0.26). The median predicted serum Cmin was 11.5 mg/L (3.9, 32.1).
The median CCSF was 1.07 mg/L (0.06, 9.13), and CSF:serum ratio was 0.08 (0, 0.66). Visual inspection of scatter plots suggested no relationship between CSF:serum ratios and age, time after last dose, or CSF white blood cell count (data not shown). However, it appeared that CSF: serum ratio increased as CSF protein level increased (data not shown).
The 2 children with MRSA meningitis had CCSF (1.15 and 9.13 mg/L) above the vancomcyin MIC (0.75 and 1 mg/L) at the end of the dosing interval. The child with the S. epidermidis isolate had a CCSF (0.06 mg/L) below the MIC (2 mg/L) 5 h after the previous dose.
Discussion
Assessment of CSF vancomycin exposure of children with shunt infection is critical because this compartment represents the drug’s site of action. The range of CCSF (0.06, 9.13 mg/L) in our small cohort was similar to previous studies in children (0 to 6.6 mg/L).4–7 A wider range of concentrations has been reported in adults (0.1, 22.3 mg/L).8,9 This variability could be caused by a number of factors, including different vancomycin dosing and CSF sampling times, age-related changes in blood brain barrier (BBB) and blood-cerebrospinal fluid barrier permeability, and different disease states. Limited data suggest increased drug diffusion into CNS with decreasing age.10 However, no relationship between age and CSF penetration was observed in our small cohort. In addition to maturational change, variability could be explained by difference in disease state. Inflammation associated with meningitis is thought to increase drug BBB permeability, resulting in higher CSF concentrations.11 In the present study, the subject who had the highest CCSF (9.13 mg/L) had elevated inflammatory markers (leukocytosis, elevated protein) in the CSF. Moreover, this same subject had the highest serum creatinine (1 mg/dL) at the time of CSF collection which could have contributed to higher vancomycin and CSF exposure due to reduced renal elimination.
Vancomycin displays time dependent activity and area under the concentration-time curve (AUC):MIC is the parameter found to best correlate with antimicrobial efficacy.12 In adults with S. aureus pneumonia or bacteremia, serum AUC:MIC>400 has been associated with clinical success, and has therefore become the vancomycin PK/PD target.12,13 However, this serum PK/PD target has not been evaluated in patients with meningitis and its extrapolation may not be valid due to variable vancomycin penetration in CSF. Adult studies have led to recommendations of trough concentrations of at least 15 mg/L for complicated infections, if vancomycin MIC is ≤1 µg/mL.14 In our study cohort, serum trough concentration was < 15 mg/L in all children but 1. None of the 3 children with proven Staphylococcus spp infection met that target, but all cleared the infection.
The optimal method to assess vancomycin penetration into CSF is to compare AUC from both the CSF and serum compartments.4,15 Calculating AUC in the CSF is challenging because it requires multiple measurements of vancomycin over the dosing interval in the CSF. Because we only collected 1–3 CSF PK samples per subject, we were limited to calculating CSF:serum ratios at single time points. In the present study, distribution of vancomycin in CSF was markedly different from serum; elimination from the CSF compartment appeared to be slower than in serum allowing CCSF to be more stable than Cserum over the dosing interval (Figure 1). This difference in PK profiles partly explains why CSF:serum ratios (0–0.66) were variable between subjects in our cohort as well as in previous reports in children (0.14 to 0.68).5,7 However, a previous comparison of vancomycin AUC in CSF versus serum in 6 children on vancomycin therapy resulted in an AUC CSF:serum ratio range (0.01–0.18) comparable to the ratios observed in our study.4
Figure 1. Vancomycin concentrations over time.
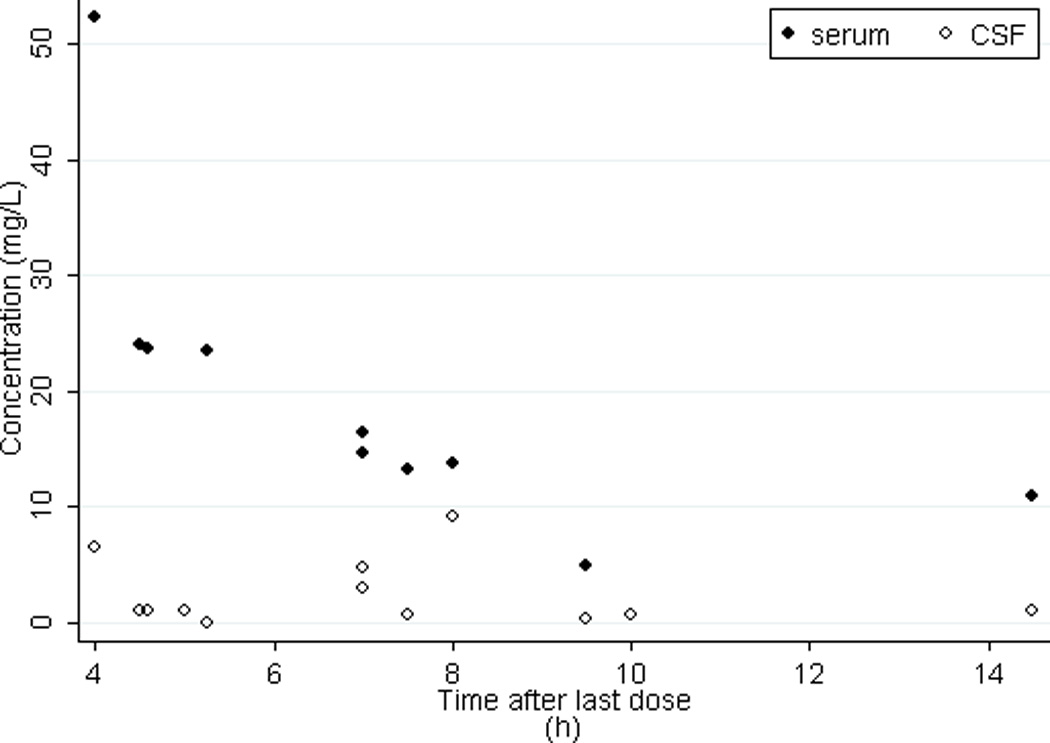
CSF, cerebrospinal fluid
Two cerebrospinal fluid concentrations at 5 and 10 h are not paired with a predicted serum concentration. These concentrations are from the child who was excluded from the pharmacokinetic analysis due to insufficient serum sampling.
Due to our inability to estimate CSF AUC:MIC as a PK/PD endpoint, we compared CCSF to the MIC for the 3 children with staphylococcal meningitis; only 2 of those achieved a CCSF above the observed MIC. Despite these findings, all three children subsequently cleared the infection. Among those 3 cases of documented staphylococcal infection, we found no correlation between CCSF and time to clear the infection. Subject #5 had the highest vancomycin CCSF (4.79 mg/L and 9.13 mg/L), but sterilization of CSF was slightly delayed compared with the 2 other children with Staphylococcus sp in their CSF (5 vs 3 days).
Results from this study highlight the need to better characterize CSF vancomycin exposure. Due to different PK profiles between serum and CSF, vancomycin AUCs need to be described in both matrices and correlated to outcomes in children with S. aureus meningitis. This will allow the determination of an AUC:MIC target in the CSF compartment and establish a valid surrogate serum PD endpoint to guide vancomycin dosing. Children with shunt are a population with easier access to CSF, would benefit from this knowledge and should be considered as a target population in future studies.
In summary, vancomycin concentrations in CSF in our cohort of children were low but persistently detectable across the dosing interval. Penetration ratios are variable probably because of a slower clearance of vancomycin in CSF compared with serum. The CSF concentration range described in this small cohort was associated with successful therapy. Future evaluations should include prospective collection of sufficient number of serum and CSF concentrations to allow estimation of AUC ratios, as well as documentation of clinical outcomes to better identify serum PD endpoints for shunt infection.
Acknowledgments
JA receives support from Training Award, Fonds Irma-Levasseur, Pediatric Department, Sainte-Justine University Hospital Center. DG is funded by the National Institute of General Medical Sciences through a T32 grant (GM086330). EVC receives support from the United States government for his work in pediatric and developmental clnical pharmacology (U54-HD071600-2,UM1-AI068632, HHSN261200800001E, HHSN275201000003, R01 NS074409-01A1). PBS receives support for research from the National Institutes of Health and the U.S. Department of Health and Human Services (NICHD DG is funded by the National Institute of General Medical Sciences through a T32 grant (GM086330). 1K23HD060040-01 and DHHS-1R18AE000028-01). GAG receives support for research from the National Institutes of Health (K08NS075144-03). DKB receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1R01FD003519-04, 1K24HD058735-05, and NICHD contract HHSN2752010000031) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). MCW receives support for research from the National Institutes of Health (1K23HD064814); the Food and Drug Administration (1U01FD004858-01); the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org); and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). KMW receives support from NIGMS (1T32GM086330-01A1) and the Thrasher Research Fund (www.thrasherresearch.org) for his work in pediatric clinical pharmacology.
References
- 1.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858–862. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 2.Mancao M, Miller C, Cochrane B, Hoff C, Sauter K, Weber E. Cerebrospinal fluid shunt infections in infants and children in Mobile, Alabama. Acta Paediatr. 1998;87:667–670. doi: 10.1080/080352598750014085. [DOI] [PubMed] [Google Scholar]
- 3.Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Jorgenson L, Reiter PD, Freeman JE, et al. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr Neurosurg. 2007;43:449–455. doi: 10.1159/000108786. [DOI] [PubMed] [Google Scholar]
- 5.Klugman KP, Friedland IR, Bradley JS. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother. 1995;39:1988–1992. doi: 10.1128/aac.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan-Havard P, Nahata MC, Bartkowski MH, Barson WJ, Kosnik EJ. Pharmacokinetics and cerebrospinal fluid (CSF) concentrations of vancomycin in pediatric patients undergoing CSF shunt placement. Chemotherapy. 1990;36:103–108. doi: 10.1159/000238755. [DOI] [PubMed] [Google Scholar]
- 7.Reiter PD, Doron MW. Vancomycin cerebrospinal fluid concentrations after intravenous administration in premature infants. J Perinatol. 1996;16:331–335. [PubMed] [Google Scholar]
- 8.LeRoux P, Howard MA, 3rd, Winn HR. Vancomycin pharmacokinetics in hydrocephalic shunt prophylaxis and relationship to ventricular volume. Surg Neurol. 1990;34:366–372. doi: 10.1016/0090-3019(90)90238-k. [DOI] [PubMed] [Google Scholar]
- 9.Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007;44:250–255. doi: 10.1086/510390. [DOI] [PubMed] [Google Scholar]
- 10.Painter MJ, Pippenger C, Wasterlain C, et al. Phenobarbital and phenytoin in neonatal seizures: metabolism and tissue distribution. Neurology. 1981;31:1107–1112. doi: 10.1212/wnl.31.9.1107. [DOI] [PubMed] [Google Scholar]
- 11.Hieber JP, Nelson JD. A pharmacologic evaluation of penicillin in children with purulent meningitis. N Engl J Med. 1977;297:410–413. doi: 10.1056/NEJM197708252970802. [DOI] [PubMed] [Google Scholar]
- 12.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 13.Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 15.Nau R, Zysk G, Thiel A, Prange HW. Pharmacokinetic quantification of the exchange of drugs between blood and cerebrospinal fluid in man. Eur J Clin Pharmacol. 1993;45:469–475. doi: 10.1007/BF00315520. [DOI] [PubMed] [Google Scholar]


