Abstract
Rationale
Inflammation drives atherogenesis. Animal and human studies have implicated interleukin (IL)-1β in this disease. Moderate hypoxia, a condition that prevails in the atherosclerotic plaque, may conspire with inflammation and contribute to the evolution and complications of atherosclerosis through mechanisms that remain incompletely understood.
Objective
This study investigated the links between hypoxia and inflammation by testing the hypothesis that moderate hypoxia modulates IL-1β production in activated human macrophages.
Methods and Results
Our results demonstrated that hypoxia enhances pro-IL-1β protein — but not mRNA — expression in LPS-stimulated human macrophages. We show that hypoxia limits the selective targeting of pro-IL-1β to autophagic degradation, thus prolonging its half-life and promoting its intracellular accumulation. Furthermore, hypoxia increased the expression of NLRP3, a limiting factor in NLRP3 inflammasome function, and augmented caspase-1 activation in LPS-primed macrophages. Consequently, hypoxic human macrophages secreted higher amounts of mature IL-1β than did normoxic macrophages after treatment with crystalline cholesterol, an endogenous danger signal that contributes to atherogenesis. In human atherosclerotic plaques, IL-1β localizes predominantly to macrophage-rich regions that express activated caspase-1 and the hypoxia markers hypoxia-inducible factor 1α (HIF-1α) and hexokinase-2 (HK-2), as assessed by immunohistochemical staining of carotid endarterectomy specimens.
Conclusions
These results indicate that hypoxia potentiates IL-1β expression in cultured human macrophages and in the context of atheromata, therefore unveiling a novel pro-inflammatory mechanism that may participate in atherogenesis.
Keywords: Interleukin-1β, hypoxia, human macrophages, autophagy, inflammasome, interleukin, inflammation
INTRODUCTION
Inflammation plays a primordial role in all phases of atherogenesis, from the initial recruitment of innate and acquired immune cells to the arterial wall to later atherothrombotic events. Pro-inflammatory cytokines and chemokines secreted from these immune cells critically mediate the inflammatory response that operates in atherosclerosis. Among these cytokines, interleukin (IL)-1α and IL-1β have received particular attention.1 Both cytokines signal through the IL-1 type I receptor. The balance of these two IL-1 isoforms and IL-1 receptor antagonist (IL-1Ra), a member of the IL-1 family that acts as an endogenous competitive inhibitor, modulates cytokine–receptor interactions and downstream signaling. IL-1α generally remains associated with the plasma membrane of the cell that produces it and therefore acts locally, whereas IL-1β can act at a distance after being proteolytically processed and secreted primarily by monocytes and macrophages.1, 2 A diverse array of extracellular danger signals triggers the assembly of inflammasomes — large multiprotein platforms containing caspase-1 that process and activate the IL-1β precursor.1, 3 In vitro and in vivo experiments have also implicated several non-inflammasome proteases in the processing of pro-IL-1β, including the matrix metalloproteinases MMP-2, MMP-3, and MMP-9;4 the sulfhydryl proteases cathepsin (Cat) B and CatL5; and several neutrophil proteases.1
Human gene polymorphisms that cause variations in circulating levels of IL-1Ra, along with animal studies that used overexpression of IL-1Ra or genetic ablation of IL-1R or IL-1Ra in atherosclerosis-prone mice, have implicated IL-1 signaling — without resolving the involvement of individual members of the IL-1 family — in atherogenesis.6–10 Experimental studies have demonstrated a causal relationship between IL-1 isoforms and arterial disease by showing that IL-1α or IL-1β deficiency alleviate atherosclerosis in mice,7, 11–13 and that chronic treatment with IL-1β induces coronary artery intimal lesions in pigs.14 Clinical studies showing increased levels of IL-1β in atherosclerotic coronary arteries compared to normal arteries15, and the association of circulating levels of IL-1β with risk factors of coronary artery disease,16 have indicated the participation of IL-1β in the development of atherosclerosis in human subjects. The ongoing Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), a large phase 3 clinical trial, tests directly the hypothesis that neutralization of IL-1β reduces the incidence of thrombotic events in patients following myocardial infarction that remain at high risk due to persistent inflammation despite standard of care treatment including intensive statin therapy.17
Human and experimental atheromata have hypoxic regions that result from high oxygen consumption and diffusion limitations in the plaque. Although severe hypoxia prevails in deep regions of atherosclerotic plaques, most cells within atheromata — including macrophages in the rupture-prone shoulder regions — experience chronic moderate hypoxia (2% to 5% O2).18–20 Chronic or intermittent hypoxia augment atherogenesis in mice.21, 22 Several mechanisms may contribute to atheroma evolution and complications elicited by hypoxia.23, 24 Low oxygen tension stimulates plaque angiogenesis25, which may promote lesion growth, intraplaque hemorrhage, heme/iron-catalyzed oxidative stress, and recruitment of inflammatory cells. Hypoxia favors foam cell formation by promoting fatty acid synthesis and inhibiting fatty acid oxidation and cholesterol efflux in mononuclear phagocytes.26, 27 Hypoxic cells shift their metabolism from mitochondrial respiration to anaerobic glycolysis, resulting in production of reactive oxygen species and reduced adenosine triphosphate (ATP) availability — conditions that predispose to cell death and contribute to the formation of the plaque’s necrotic core.28, 29 Hypoxia may contribute to the catabolism of the extracellular matrix, and therefore to lesion evolution, by inducing the expression of MMP-1, MMP-7, and MMP-930–32 and by increasing the activity of the lysosomal proteinases CatS, CatK, and CatL due to the pH drop that follows lactate production.33
The studies described above have indicated that hypoxia may conspire with inflammation to promote the evolution and clinical complications of atherosclerosis, by mechanisms as yet incompletely understood. This study explored a possible intersection between hypoxia and inflammation by testing the hypothesis that moderate hypoxia potentiates IL-1β production in activated human macrophages. Our results demonstrate that cell exposure to hypoxia evokes distinct mechanisms that result in augmented release of secreted IL-1β. Hypoxia retards the autophagic degradation and therefore induces intracellular accumulation of pro-IL-1β, and also augments NLRP3 expression and caspase-1 activity in human macrophages. Furthermore, we demonstrate that IL-1β colocalizes with markers of hypoxia and with activated caspase-1 in human carotid atherosclerotic lesions, uncovering two novel mechanisms by which hypoxia can aggravate this arterial disease by promoting inflammation.
METHODS
Reagents
Ultrapure E. coli lipopolysaccharide (LPS) and bafilomycin A1 were purchased from Invivogen (San Diego, CA), cholesterol and nigericin from Sigma (Saint Louis, MO), Z-YVAD-FMK from R&D Systems (Minneapolis, MN), and GM-6001 from Enzo Life Sciences (Farmingdale, NY). Cholesterol crystals were prepared as described.34
Cell culture
Human peripheral blood monocytes were isolated from freshly prepared leukocyte concentrates from healthy donors, and differentiated to macrophages as described.28 Macrophages were pre-incubated for 24 hours in RPMI 1640 containing 5% human serum, under normoxic or hypoxic conditions, followed by the addition of the indicated reagents for various periods of time as described in each experiment. For culture in a hypoxic environment, experiments were conducted in Modular Incubation Chambers MIC-101 (Billups-Rothenberg, Del Mar, CA) filled with a gas mixture containing 2% O2, 5% CO2, and 93% N2.
Immunoblot
Whole-cell lysates from 25,000 cells were fractionated on 4%–12% gradient SDS-PAGE gels (Life Technologies, Grand Island, NY) and transferred to polyvinilidene difluoride membranes. After blocking with 5% defatted milk and incubating with the appropriate antibodies, membranes were developed using a chemiluminescence reagent (Thermo Scientific, Waltham, MA). Anti-IL-1β was from Santa Cruz (Santa Cruz, CA), and anti-β-actin, anti-sequestosome 1 (SQSTM1)/p62, anti cleaved caspase-1, anti-NLRP3, and anti-LC3B were from Cell Signaling (Danvers, MA). Densitometric analyses were performed using the National Institutes of Health Image J processing system.
Cytokine assays
The concentrations of IL-1β, TNF-α, and IL-6 in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions. The IL-1β ELISA kit was purchased from Biolegend (San Diego, CA), and the TNF-α and IL-6 kits were purchased from R&D Systems.
Metabolic labeling and pulse-chase experiments
Macrophages were primed for 3 hours with 5 ng/ml LPS in RPMI 1640 containing 5% human serum and radiolabeled with 0.4 mCi/ml of [35S] methionine and [35S] cysteine (EXPRESS, Perkin Elmer, Waltham, MA) in the same medium for 4 hours. Cells were harvested (0 min) or washed 3 times with PBS and incubated for various time intervals in complete medium supplemented with 1 mM Met and 1 mM Cys. After lysing cells with NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, and a cocktail of protease inhibitors [Boston Bioproducts, Ashland, MA]) and centrifuging cell lysates at 10,000 rpm for 15 minutes, aliquots from the supernatants containing the same amount of protein were immunoprecipitated with IL-1β antibody, washed 3 times with NP-40 lysis buffer, separated by SDS-PAGE, and analyzed by fluorography.35
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) and reverse transcribed by Superscript II (Invitrogen, Carlsbad, CA). Quantitative PCR was performed in a MyiQ Single-Color Real-Time PCR system using SYBR Green I (Bio-Rad, Hercules, CA). The mRNA levels of the genes tested were normalized to 18S as an internal control. The primer sequences were: 18S, 5′-ATGGCCGTTCTTAGTTGGTG-3′ and 5′-GAACGCCACTTGTCCCTCTA-3′; IL-β, 5′-AAAGCTTGGTGATGTCTGGTC-3′ and 5′-GGACATGGAGAACACCACTTG-3′; NLRP3, 5′-ACCTGGGGGTCATGATGTT-3′ and 5′-CGCACTTTTTGTCTCATAATTGA-3′.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 20 min at 4°C, permeabilized with 0.1% Triton X-100, blocked with 10% goat serum and 1% bovine serum albumin in PBS, and incubated with rabbit polyclonal anti-IL-1β (Santa Cruz) and mouse monoclonal anti-SQSTM1/p62 (Abcam, Cambridge, MA) antibodies, followed by incubation with goat anti-rabbit IgG-Alexa 535 and goat anti-mouse IgG-Alexa 488 (Invitrogen). After staining nuclei with DAPI (Invitrogen), slides were mounted with Dako fluorescent mounting medium (Dako, Carpintería, CA). Images were acquired in an Olympus BX63 fluorescence microscope, processed with Fluoview imaging software (Olympus), and analyzed blindly for fluorescent label colocalization using Image-Pro Plus (Media Cybernetics, Rockville, MD).
Immunohistochemical study
Surgical specimens of human carotid plaques from endarterectomies (n=8) and of non-atherosclerotic arteries from cardiac transplantation donors (n=3) were obtained through protocols approved by the Human Investigation Review Committee at Brigham and Women’s Hospital. For colocalization of hypoxia-inducible factor 1α (HIF-1α) with hexokinase-2 (HK-2), caspase-1, and IL-1β, we used serial 6-μm frozen sections. The sections were incubated with 0.3% hydrogen peroxide to block endogenous peroxidase activity, followed by overnight incubation at 4°C with mouse monoclonal antibodies to CD68 (1:900, clone KP1, Dako) or HIF-1α (1:350, BD Biosciences, San Diego, CA), or rabbit polyclonal antibodies to cleaved caspase-1 (1:10, Cell Signaling), HK-2 (1:20, Cell Signaling) or IL-1β (1:150, Santa Cruz). Subsequent processing was performed with the Universal Dako LSAB kit (peroxidase, Dako Cytomation). Nuclear HIF-1α staining was visualized with DAB (brown nuclei), and sections were counterstained with methyl green (ready-to-use, Dako). Staining performed with all other antibodies was visualized with 3-amino-9-ethyl carbazole (red cytoplasm, ready-to-use, Dako) and counterstained with Gill’s hematoxylin solution (Sigma). The specificity of the antibodies used was determined by omitting primary antibodies, which led to negligible staining (not shown). For oil red O staining, air-dried sections were rehydrated in PBS, fixed in 4% paraformaldehyde, briefly rinsed with 60% isopropanol, and stained with oil red O for 30 min. After two short rinses with 60% isopropanol, sections were washed with water and counterstained with hematoxylin. 36 Cholesterol crystals were visualized by cross-polarized light microscopy in adjacent sections fixed with 10% formalin that were not treated with any organic solvent. Micrographs of stained sections were taken with a Nikon (DXM1200F) digital camera attached to a Nikon (OPTIPHOT-2) microscope.
RESULTS
Hypoxia induces IL-1β expression by stabilizing pro-IL-1β protein in human macrophages
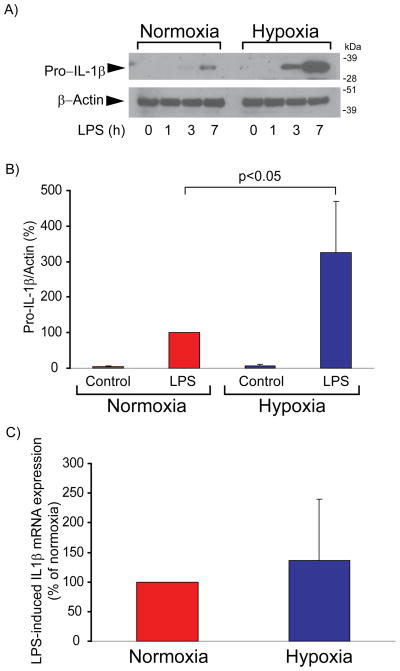
Treatment of human macrophages with LPS, a potent Toll-like receptor-4 (TLR4) agonist, induces the expression of many pro-inflammatory genes, including IL-1β. To test the effect of moderate hypoxia on LPS-induced pro-IL-1β expression, we exposed cells to 2% O2 for 24 hours, followed by treatment with LPS under the same hypoxic conditions for various periods of time. Compared to cells maintained under normal O2 tension, cells subjected to hypoxia accumulated substantial pro-IL-1β protein, as evaluated by immunoblot (Figures 1A and 1B), but contained similar levels of IL-1β mRNA after LPS stimulation (Figure 1C). Thus, hypoxia exerts strong post-transcriptional control of pro-IL-1β concentration.
Figure 1. Hypoxia augments LPS-induced pro-IL-1β protein expression, but not mRNA expression, in human macrophages.
A) Cells were incubated with 5 ng/ml LPS for the indicated periods of time. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to IL-1β (top) and β-actin (bottom). (B) Densitometric analyses of the bands corresponding to pro-IL-1β in immunoblots of lysates from macrophages stimulated with LPS for 7 hs. β-actin served as an internal control for adjustment between samples. Data are expressed in means ± SD (N = 4 donors) relative to the values in normoxic cells stimulated with LPS, defined as 100%. C) Cells were stimulated with 5 ng/ml LPS for 4 hours, followed by RNA extraction and determination of IL-1β mRNA levels by RT-qPCR. mRNA levels of 18S served as an internal control for adjustment between samples. For each donor, the change of IL-1β mRNA expression induced by LPS in hypoxic cells was normalized to the corresponding change in normoxic cells, defined as 100%. Data are expressed in means ± SD (N = 5 donors).
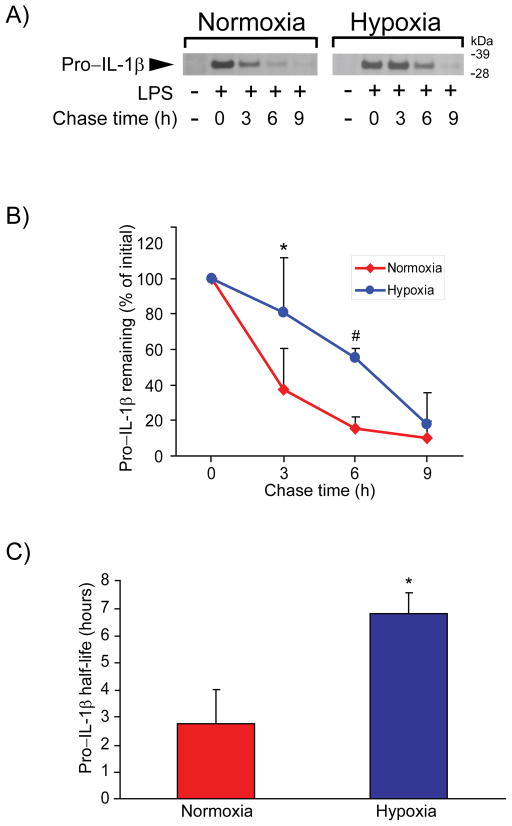
The rates of translation and of proteolytic degradation determine the steady-state levels of intracellular proteins. Radwan et al. have shown that tyrosine kinase 2 (Tyk2) exerts translational control of IL-1β production in mouse bone marrow–derived macrophages (BMDMs).37 We did not find evidence supporting a role of hypoxia on Tyk2 activation in human macrophages (not shown). We therefore hypothesized that hypoxia would reduce the degradative rate of pro-IL-1β, and tested this conjecture in pulse-chase experiments. Radiolabeled pro-IL-1β decayed rapidly in normoxic macrophages (T1/2 of 2.74 ± 1.25 hours), whereas exposure of cells to hypoxia notably stabilized pro-IL-1β (T1/2 = 6.79 ± 0.75 hours, p< 0.05 vs. normoxia) (Figure 2).
Figure 2. Hypoxia prolongs the half-life of pro-IL-1β in LPS-stimulated human macrophages.
Cells were primed with 5 ng/ml LPS for 3 hours, radiolabeled with [35S] methionine and [35S] cysteine for 4 hours, and chased with complete medium containing excess unlabeled methionine and cysteine for the indicated periods of time. A) Cell lysates were immunoprecipitated with anti-IL-1β antibody, and the precipitates were analyzed by SDS/PAGE and fluorography. B) Densitometric analysis of the bands corresponding to pro-IL-1β in A. Data are expressed in means ± SD (N = 4 donors) relative to the respective values at the beginning of the chase. * denotes p < 0.05; # denotes p < 0.005. C) Half-life of pro-IL-1β, calculated from the graphs in B. Data are expressed in means ± SD (N = 4 donors). * denotes p < 0.005.
Paradoxically reduced autophagic catabolism contributes to pro-IL-1β protein accumulation in hypoxic human macrophages
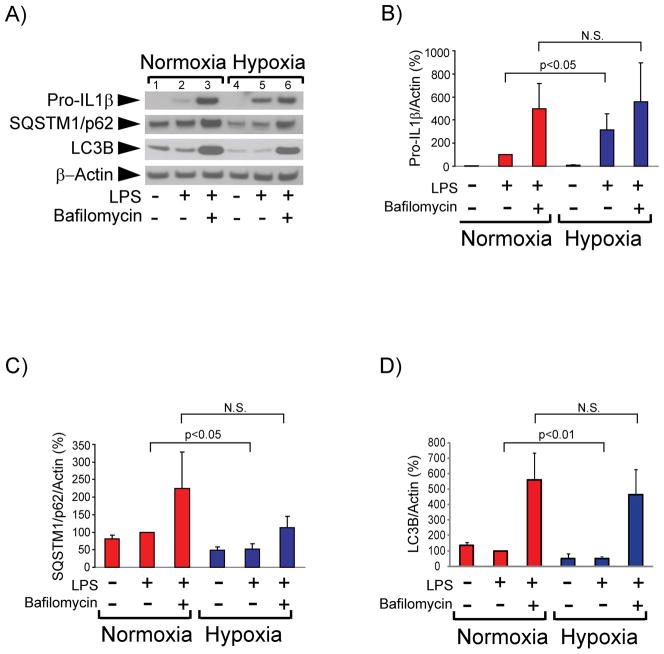
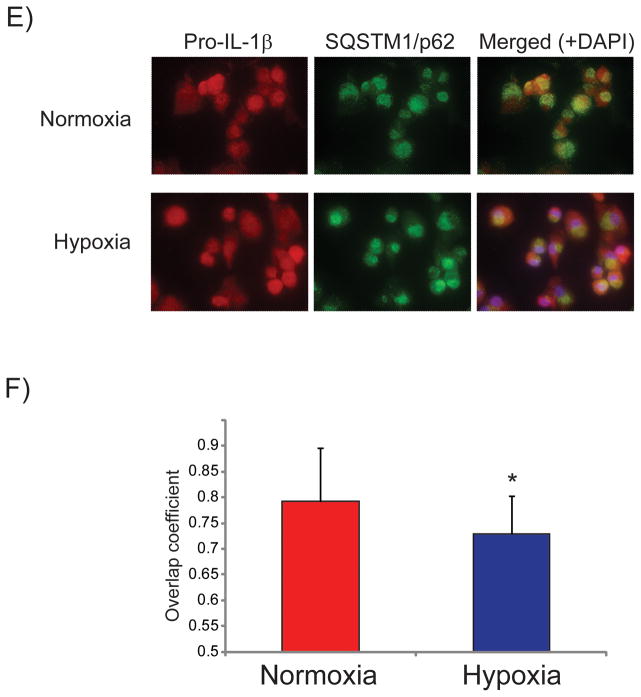
Harris et al. recently reported that autophagic degradation controls intracellular levels of pro-IL1β in immortalized mouse BMDMs.38 We therefore evaluated the involvement of autophagy in regulating pro-IL-1β concentration in primary cultures of human macrophages, and investigated whether hypoxia affects pro-IL-1β targeting to the autophagic pathway. The autophagy inhibitor bafilomycin A1 — a vacuolar H+ ATPase inhibitor that prevents fusion of autophagosomes with lysosomes — strongly augmented pro-IL-1β accumulation in normoxic cells, indicating that autophagy controls pro-IL-1β levels in human macrophages (Figure 3A, lanes 2 and 3, and Figure 3B). Hypoxic macrophages accumulated substantially higher amounts of pro-IL-1β after LPS stimulation than did normoxic macrophages (Figures 1, 3A, lanes 2 and 5; and 3B). Bafilomycin A1 mitigated this differential accumulation of pro-IL-1β in hypoxic cells compared to normoxic cells (Figure 3A, lanes 3 and 6, and Figure 3B), indicating that the lower concentrations of pro-IL-1β observed in normoxic cells stemmed from a higher rate of autophagic degradation. This observation is surprising, considering that moderate hypoxia generally augments autophagy39 and it would therefore likely accelerate rather than retard the degradation of a substrate for autophagy such as pro-IL1β. We affirmed that hypoxia indeed induced autophagy in our experiments by examining the steady-state levels of SQSTM1/p62 and LC3B, well-characterized substrates of the autophagic pathway, whose abundance correlates inversely with the extent of autophagy.40 Hypoxic cells contained lower concentrations of SQSTM1/p62 and of LC3B than normoxic cells (Figures 3A, lanes 1, 2, 4 and 5, and Figures 3C and 3D) and bafilomycin A1 caused SQSTM1/p62 and LC3B accumulation, confirming that hypoxia induced autophagy under conditions of our experiments (Figures 3A, 3C, and 3D). Pro-IL-1β colocalized to a higher extent with SQSTM1/p62 – a cargo receptor that targets proteins to the autophagic machinery40 – in normoxic macrophages than it did in hypoxic macrophages, as assessed by immunostaining of cells treated with LPS and bafilomycin A1 and quantification of the fluorescent label colocalization (Figures 3E and 3F). Taken together, these results indicate that moderate hypoxia augments the accumulation of pro-IL-1β by limiting its selective targeting to the autophagic machinery.
Figure 3. Hypoxia reduces autophagic degradation of pro-IL-1β, but not of SQSTM1/p62 or LC3B, in LPS-stimulated human macrophages.
Cells were incubated with 5 ng/ml LPS for 7 hours in the presence or absence of 1 μM bafilomycin A1, as indicated. A) Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to IL-1β, SQSTM1/p62, LC3B, and β-actin. B), C), and D) Densitometric analyses of the bands corresponding to pro-IL-1β (B), SQSTM1/p62 (C), and LC3B (D). β-actin served as an internal control for adjustment between samples. Data are expressed in means ± SD (N = 4 donors) relative to the values in normoxic cells stimulated with LPS, defined as 100%. E) and F) Colocalization of pro-IL-1β and SQSTM1/p62 in macrophages treated with LPS and bafilomycin A1 for 7 hours. E) Representative immunostaining for pro-IL-1β (red) and SQSTM1/p62 (green). Nuclei were stained with DAPI. F) Degree of overlap of red and green staining in experiments similar to the one shown in E). N=9 donors, 2–3 fields analyzed for each condition.
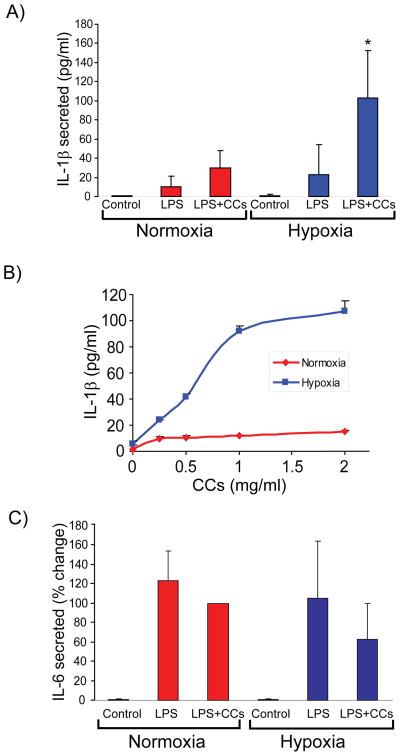
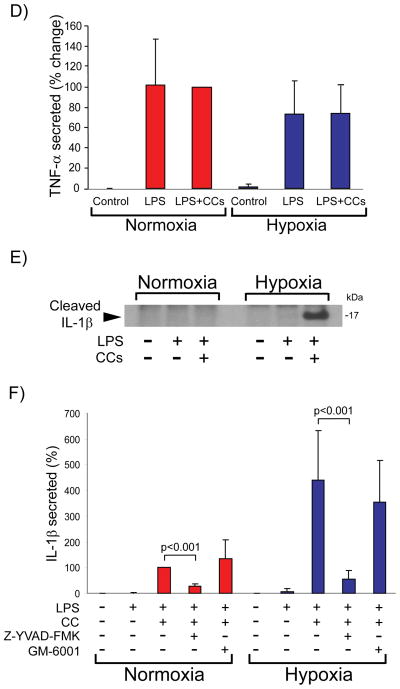
Hypoxia enhances IL-1β secretion induced by LPS and cholesterol crystals in human macrophages
Atheromata contain cholesterol crystals (CCs).41 Recent work has shown that CCs elicit release of mature IL-1β from mononuclear phagocytes by activating the NRLP3 inflammasome, providing a link between cholesterol metabolism and inflammation.5, 34 To examine the effect of hypoxia on IL-1β secretion, we exposed human macrophages to hypoxia for 24 hours, primed them with lipopolysaccharide (LPS) for 2 hours, and incubated them for 6 hours in the presence or absence of CCs. Compared with their normoxic controls, hypoxic cells secreted threefold to fourfold higher amounts of IL-1β in response to CCs, as assessed by ELISA of cell-conditioned media (Figure 4A). The response depended on CC concentration, reaching maximal values at 2 mg/ml (Figure 4B). Hypoxia did not augment secretion of IL-6 (Figure 4C) or tumor necrosis factor alpha (TNF-α) under the same conditions (Figure 4D). Metabolic labeling of macrophages treated as described above with [35S] methionine and [35S] cysteine, followed by IL-1β immunoprecipitation from cell-conditioned media, verified that hypoxic cells synthesized de novo and secreted a ~17 kDa polypeptide that corresponds to cleaved, mature IL-1β, which was not detectable under normoxic conditions (Figure 4E). The caspase-1 inhibitor Z-YVAD-FMK — but not the pan-MMP inhibitor GM-6001— limited hypoxia-induced IL-1β secretion, indicating that caspase-1 participates in this process (Figure 4F).
Figure 4. Hypoxia potentiates IL-1β secretion induced by LPS and CCs in human macrophages.
Cells were primed with 5 ng/ml LPS for 2 hours, and subsequently were stimulated with CCs (2 mg/ml in A, C, D, E, and F, or various concentrations in B) for 6 hours. ELISA determined the concentration of the indicated cytokines in cell supernatants. A) IL-1β (N = 11 donors; * denotes p < 0.001 versus LPS + CCs in normoxia). B) IL-1β (N = 3 donors). C) IL-6 (N = 8 donors). D) TNF-α (N = 8 donors). Data are expressed in means ± S.D. In C and D, data are relative to the values of treatment with LPS + CCs in normoxic cells, defined as 100%. E) Macrophages were primed with 5 ng/ml LPS for 3 hours, and subsequently were radiolabeled with [35S] Met and [35S] Cys in the presence or absence of CCs for 4 hours. IL-1β immunoprecipitates from culture supernatants were analyzed by SDS-PAGE and fluorography. F) Cells were treated as in A, in the presence or absence of the indicated protease inhibitors. ELISA determined IL-1β concentration in cell supernatants. Data are expressed in means ± S.D (N = 4 donors) relative to the values in normoxic cells stimulated with LPS + CCs, defined as 100%.
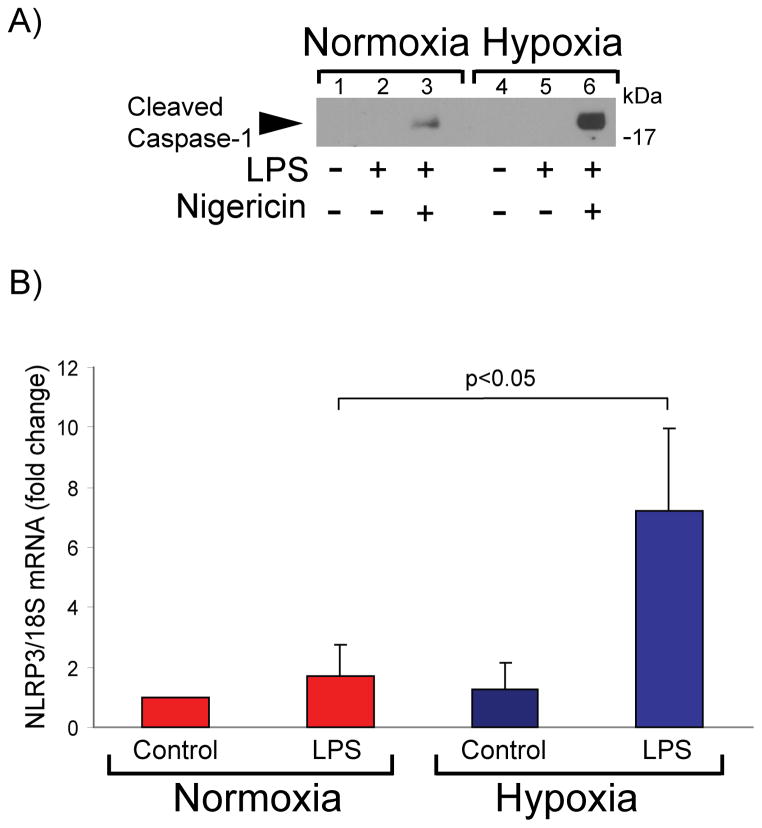
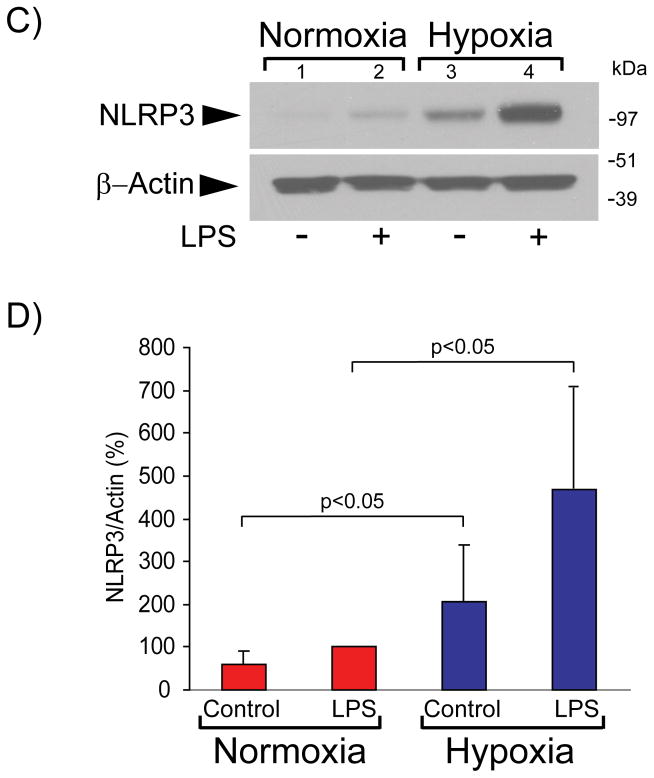
Hypoxia augments NLRP3 induction and inflammasome activation in LPS-primed human macrophages
Efflux of intracellular K+ is necessary and sufficient for NLRP3 inflammasome activation and caspase-1–dependent IL-1β secretion in LPS-primed mBMDMs.42 To test the effect of hypoxia on caspase-1 activation in human macrophages, we treated LPS-primed cells with the K+/H+ ionophore nigericin, a potent inflammasome activator.43 Cells exposed to hypoxia exhibited stronger caspase-1 activation than normoxic cells under these conditions, as assessed by the secretion of p20, a cleavage fragment of caspase-1 characteristic of the active enzyme (Figure 5A).
Figure 5. Hypoxia augments NLRP3 expression and caspase activity in human macrophages.
A) Cells were primed with 5 ng/ml LPS for 2 hours, and subsequently were stimulated with 10 μM nigericin for 3 hours. Cell supernatants were fractionated by SDS-PAGE and immunoblotted with antibodies to cleaved caspase-1. B) Cells were stimulated with 5 ng/ml LPS for 4 hours, followed by RNA extraction and determination of NLRP3 mRNA levels by RT-qPCR. mRNA levels of 18S served as an internal control for adjustment between samples. Data are expressed in means ± SD (N = 4 donors). C) Cells were incubated with 5 ng/ml LPS for 7 hours. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to NLRP3 (top) and β-actin (bottom). D) Densitometric analyses of the bands corresponding to NLRP3 in C). β-actin served as an internal control for adjustment between samples. Data are expressed in means ± SD (N = 6 donors) relative to the values in normoxic cells stimulated with LPS, defined as 100%.
NFκB-dependent regulation of NLRP3 expression controls inflammasome activation and caspase-1 activity in mBMDMs.44 Exposure of human macrophages to low oxygen tension augmented LPS-induced NLRP3 mRNA expression, compared to cells maintained under normal oxygen tension (Figure 5B). Concordantly, hypoxic LPS-treated cells exhibited higher levels of NLRP3 protein than normoxic LPS-treated cells (Figures 5C lanes 2 and 4, and 5D). Macrophages exposed to hypoxia but not treated with LPS also contained increased NLRP3 levels compared to their normoxic controls (Figures 5C lanes 1 and 3, and 5D). The addition of bafilomycin A1 did not alter NLRP3 levels, indicating that autophagy does not regulate NLRP3 protein concentration under these conditions (results not shown). Collectively, these results indicate that hypoxia regulates NLRP3 inflammasome activation at both NLRP3 mRNA and protein concentrations in human macrophages.
IL-1β localizes in macrophage-rich and potentially hypoxic areas of atherosclerotic lesions
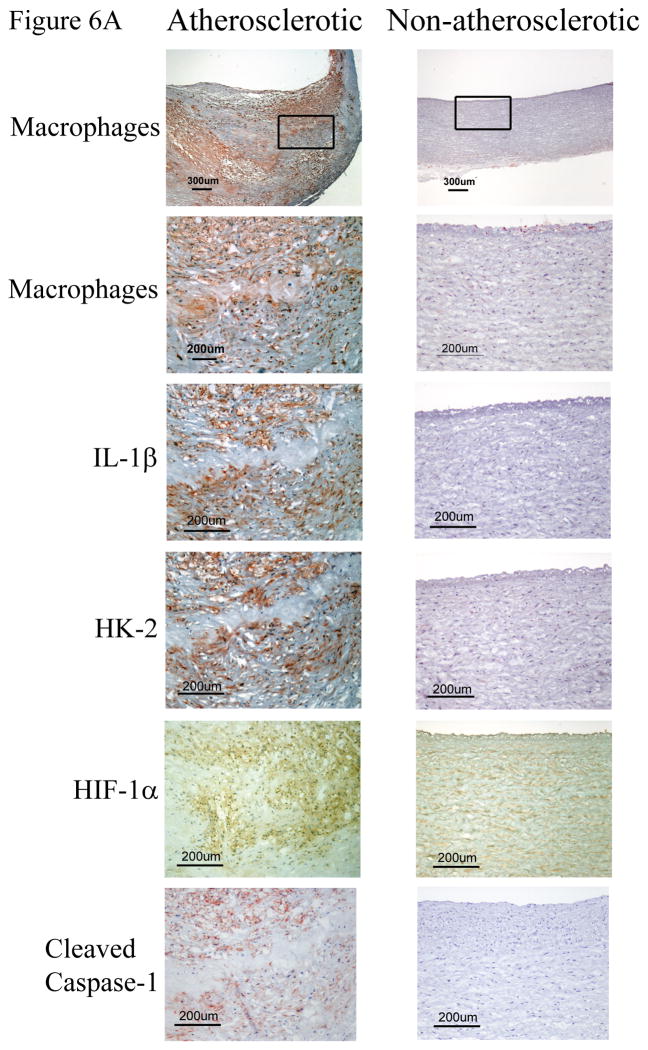
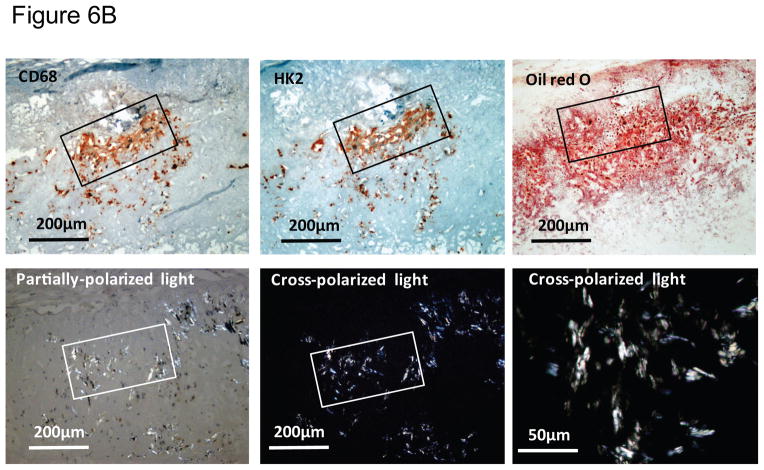
We recently reported the abundant expression of proteins involved in glucose utilization in macrophage-rich areas of human plaques characterized as hypoxic.28 Examination of IL-1β localization in atheromata revealed that this cytokine also localizes predominantly in macrophage-rich regions of plaques that express the hypoxia markers HIF-1α and HK-2, and activated caspase-1. Normal arteries did not contain detectable levels of hypoxia-regulated proteins, IL-1β, or activated caspase-1 (Figure 6A). These macrophage-rich, hypoxic regions of plaques are lipid-rich, as assessed by their staining with oil red O, and contain crystalline cholesterol, visualized by its birefringence under cross-polarized light microscopy (Figure 6B).36 These results indicate that the hypoxia-induced pro-inflammatory mechanisms described above may operate in atherosclerotic lesions.
Figure 6. IL-1β colocalizes with hypoxia markers and cleaved caspase-1 in macrophage-rich regions of human atherosclerotic lesions that contain cholesterol crystals.
A) Representative immunohistochemical staining of serial sections of human atherosclerotic carotid lesions (N = 8) for CD68 (macrophages), IL-1β, HK-2, HIF-1α, and cleaved caspase-1. B) Representative immunohistochemical staining of serial sections of human carotid artery atherosclerotic lesions (N = 5) for CD68 (macrophages), HK-2, and oil red O. Cholesterol crystals were visualized by oil red O (ORO) staining and cross-polarized light microscopy. The original magnification is indicated on each image.
DISCUSSION
The current study reveals that moderate hypoxia acts by two different and heretofore unrecognized mechanisms to augment IL-1β production in LPS-stimulated human macrophages: it limits the selective targeting of pro-IL-1β to autophagic degradation, and increases NLRP3 expression and caspase-1 activation. Tanahill et al. recently reported that hypoxia (1% O2) enhances IL-1β protein secretion in LPS-stimulated mouse macrophages, and attributed — but did not conclusively demonstrate — this effect to transcriptional induction of the IL-1β gene mediated by a HIF-1α –binding site in the IL-1β promoter.45 The reasons for the discrepancy between our work and that by Tanahill et al. remain unclear, but the different experimental approaches used in these two studies offers the simplest explanation: the present study used primary human macrophages subjected to moderate hypoxia (2% O2) and stimulated with LPS for 1–8 hours, versus mBMDMs exposed to severe hypoxia (1% O2) and treated with LPS for 24 hours in the study by Tanahill et al.45 In human macrophages, LPS stimulation requires a second signal (e.g. CCs) to elicit secretion of IL-1β, whereas LPS suffices to induce IL-1β secretion in mBMDMs,45 thus highlighting the differences between the experimental conditions used in these two studies.
Autophagy, a pathway that involves the lysosomal degradation of damaged organelles and cytoplasmic components, may play a protective role in atherosclerosis.46, 47 Limitation of supply of essential nutrients — including O2 — induces autophagy. In nutrient-rich conditions, autophagy operates at basal levels as a highly selective process that participates in the quality control of cellular organelles and in the regulation of the abundance of many cytoplasmic proteins, including pro-IL-1β. 38, 48, 49 Our results show that hypoxia prolongs pro-IL-1β’s half-life despite activating autophagy, indicating decreased targeting of pro-IL-1β to autophagic degradation under low O2 tension. A plausible explanation for this finding is that the half-life of pro-IL-1β likely depends on the intracellular availability of SQSTM1/p62, a cargo receptor that interacts with selected polyubiquitinated proteins and targets them to the autophagic machinery.50 Hypoxic cells have reduced levels of SQSTM1/p62 (Figure 3), and most likely a further reduced availability of this protein due to its diversion to mitophagy and other processes needed to maintain homeostasis under low O2 tension. Thus, in contrast to the anti-inflammatory nature of normal autophagy, hypoxia elicits a selective deficit in autophagy that results in augmented pro-IL-1β accumulation and therefore increased inflammatory potential in activated human macrophages. Such defective autophagy contributes critically to certain inflammatory diseases such as Crohn colitis51 and chronic granulomatous disease.52
The induction of mature IL-1β secretion by CCs in human monocytes and mouse macrophages depends on caspase-1.5 We show that the caspase-1 inhibitor Z-YVAD-FMK — but not the MMP inhibitor GM-6001 — limits hypoxia-induced IL-1β secretion in LPS-primed human macrophages treated with CCs, indicating the participation of caspase-1 in this process. The effect of hypoxia likely stems from its effect on NLRP3 expression, a limiting factor for caspase-1 activation triggered by the NLRP3 inflammasome.44 Of note, LPS-primed macrophages respond to various well-characterized activators of the NLRP3 inflammasome to vastly different extents under the experimental conditions used here. Indeed, IL-1β release induced by nigericin exceeds the secretion levels elicited by CCs or ATP by one order of magnitude. Consequently, our immunoblots detected secreted p20, a product of caspase-1 activation, upon cell stimulation with nigericin but not with CCs or ATP.
Collectively, our data indicate that levels of hypoxia highly relevant to atherosclerosis potentiate the generation of mature IL-1β in human macrophages, both in vitro and in the context of atheromata. These findings provide new evidence for the intersection of hypoxia and inflammation and disclose novel mechanisms by which low oxygen tension can aggravate atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Many pre-clinical studies support a role for the primordial pro-inflammatory cytokine, interleukin 1 (IL – 1), as a promoter of atherogenesis.
Like many proteins critical in biological control, IL-1 has multiple levels of regulation including a requirement for limited proteolysis of a precursor to produce its active form.
Caspase-1, a component of the inflammasome, activates pro-IL-1β to the mature cytokine in response to certain cytokines and danger signals including crystalline cholesterol, a component of atherosclerotic plaques.
What New Information Does This Article Contribute?
This study identifies moderate hypoxia, a condition that prevails within atherosclerotic plaques, as a novel activator of the inflammasome, hence contributing to the generation of active IL-1β.
This work further discovered a selective and unexpected defect in autophagy induced by moderate hypoxia as a contributor to a buildup of pro-IL-1β, a substrate for the inflammasome.
Thus, moderate hypoxia, a disease-related stimulus, augments IL-1β activity through dual mechanisms: 1) increased accumulation of the precursor and 2) a boost in its processing by caspase-1.
These results enhance our understanding of the molecular mechanisms of inflammation that operate in the local environment of the atherosclerotic plaque. They also have direct clinical relevance, as strategies that target IL-1β signaling can reduce biomarkers of cardiovascular risk. A large-scale clinical endpoint trial currently underway will evaluate the hypothesis that neutralization of IL-1β can improve outcomes in patients with established coronary artery disease.
Acknowledgments
We thank Eugenia Shvartz and Marc Belanger for technical assistance, Elena Maganto for the preparation of cholesterol crystals, and Sara Karwacki and Chelsea Swallom for editorial assistance.
SOURCES OF FUNDING
This work was in part supported by grants from the National Heart, Lung and Blood Institute (RO1 HL080472 and PO1 HL048743 to Dr. Libby)
Nonstandard Abbreviations and Acronyms
- IL
interleukin
- IL-1Ra
IL-1 receptor antagonist
- TNF-α
tumor necrosis factor alpha
- HIF-1α
hypoxia-inducible factor 1 alpha
- HK-2
hexokinase-2
- CC
cholesterol crystal
- ATP
adenosine triphosphate
- MMP
matrix metalloproteinase
- Cat
cathepsin
- LPS
lipopolysaccharide
- SQSTM1
sequestosome 1
Footnotes
In August, 2014, the average time from submission to first decision for all original research papers submitted to Circulation Research was 13.55 days.
DISCLOSURES
None.
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 5.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, Francis S. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–1403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isoda K, Shiigai M, Ishigami N, Matsuki T, Horai R, Nishikawa K, Kusuhara M, Nishida Y, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation. 2003;108:516–518. doi: 10.1161/01.CIR.0000085567.18648.21. [DOI] [PubMed] [Google Scholar]
- 9.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Olofsson PS, Sheikine Y, Jatta K, Ghaderi M, Samnegard A, Eriksson P, Sirsjo A. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta:interleukin-1 receptor antagonist balance in atherosclerosis. Circ J. 2009;73:1531–1536. doi: 10.1253/circj.cj-08-1150. [DOI] [PubMed] [Google Scholar]
- 11.Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 12.Kamari Y, Werman-Venkert R, Shaish A, Werman A, Harari A, Gonen A, Voronov E, Grosskopf I, Sharabi Y, Grossman E, Iwakura Y, Dinarello CA, Apte RN, Harats D. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996:97769–776. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 16.Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Heughan C, Niinikoski J, Hunt TK. Oxygen tensions in lesions of experimental atherosclerosis of rabbits. Atherosclerosis. 1973;17:361–367. doi: 10.1016/0021-9150(73)90027-0. [DOI] [PubMed] [Google Scholar]
- 19.Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–876. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- 20.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, Matsumura Y. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertens Res. 2005;28:837–845. doi: 10.1291/hypres.28.837. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Folco E. Tension in the plaque: hypoxia modulates metabolism in atheroma. Circ Res. 2011;109:1100–1102. doi: 10.1161/RES.0b013e31823bdb84. [DOI] [PubMed] [Google Scholar]
- 24.Marsch E, Sluimer JC, Daemen MJ. Hypoxia in atherosclerosis and inflammation. Curr Opin Lipidol. 2013;24:393–400. doi: 10.1097/MOL.0b013e32836484a4. [DOI] [PubMed] [Google Scholar]
- 25.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 26.Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, Olofsson SO, Hulten LM. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol. 2006;26:1871–1876. doi: 10.1161/01.ATV.0000229665.78997.0b. [DOI] [PubMed] [Google Scholar]
- 27.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 30.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deguchi JO, Yamazaki H, Aikawa E, Aikawa M. Chronic hypoxia activates the Akt and beta-catenin pathways in human macrophages. Arterioscler Thromb Vasc Biol. 2009;29:1664–1670. doi: 10.1161/ATVBAHA.109.194043. [DOI] [PubMed] [Google Scholar]
- 32.Kolev K, Skopal J, Simon L, Csonka E, Machovich R, Nagy Z. Matrix metalloproteinase-9 expression in post-hypoxic human brain capillary endothelial cells: H2O2 as a trigger and NF-kappaB as a signal transducer. Thromb Haemost. 2003;90:528–537. doi: 10.1160/TH03-02-0070. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 34.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folco EJ, Koren G. Degradation of the inducible cAMP early repressor (ICER) by the ubiquitin-proteasome pathway. Biochem J. 1997;328:37–43. doi: 10.1042/bj3280037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 37.Radwan M, Stiefvater R, Grunert T, Sharif O, Miller I, Marchetti-Deschmann M, Allmaier G, Gemeiner M, Knapp S, Kovarik P, Muller M, Strobl B. Tyrosine kinase 2 controls IL-1ss production at the translational level. J Immunol. 2010;185:3544–3553. doi: 10.4049/jimmunol.0904000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 42.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 44.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xavier RJ, Huett A, Rioux JD. Autophagy as an important process in gut homeostasis and Crohn’s disease pathogenesis. Gut. 2008;57:717–720. doi: 10.1136/gut.2007.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW, Chamilos G, Netea MG, Xavier RJ, Dinarello CA, Romani L, van de Veerdonk FL. PNAS. 2014;11:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.