Abstract
Mounting evidence indicates that alcohol-induced neuropathology may result from multicellular responses in which microglia cells play a prominent role. Purinergic receptor signaling plays a key role in regulating microglial function and, more importantly, mediates alcohol-induced effects. Our findings demonstrate that alcohol increases expression of P2X4 receptor (P2X4R), which alters the function of microglia, including calcium mobilization, migration and phagocytosis. Our results show a significant up-regulation of P2X4 gene expression as analyzed by real-time qPCR (***p<0.002) and protein expression as analyzed by flow cytometry (**p<0.004) in embryonic stem cell-derived microglial cells (ESdM) after 48 hours of alcohol treatment, as compared to untreated controls. Calcium mobilization in ethanol treated ESdM cells was found to be P2X4R dependent using 5-BDBD, a P2X4R selective antagonist. Alcohol decreased migration of microglia towards fractalkine (CX3CL1) by 75% following 48 hours of treatment compared to control (***p<0.001). CX3CL1-dependent migration was confirmed to be P2X4 receptor-dependent using the antagonist 5-BDBD, which reversed the effects as compared to alcohol alone (***p<0.001). Similarly, 48 hours of alcohol treatment significantly decreased phagocytosis of microglia by 15% compared to control (*p<0.05). 5-BDBD pre-treatment prior to alcohol treatment significantly increased microglial phagocytosis (***p<0.001). Blocking P2X4R signaling with 5-BDBD decreased the level of calcium mobilization compared to ethanol treatment alone. These findings demonstrate that P2X4 receptor may play a role in modulating microglial function in the context of alcohol abuse.
Keywords: Microglia, Alcohol, Purinergic Receptor X4
Introduction
Alcohol abuse and alcoholism represent substantial problems that affect a large portion of the general public1. Alcohol is known to cause structural and functional abnormalities to the brain1-6 including the significant risk associated with chronic alcohol abuse, particularly disorders of the central nervous system (CNS)7. Alcohol is known to attenuate phagocytosis3, 8, 9, proliferation4, expression of neurotrophic factor brain-derived neurotrophic factor (BDNF) in hippocampus10 and apoptosis in microglia11. A hallmark of alcohol-induced neuropathology is activation of microglia12. As the immune effector cells of the CNS, microglia are vital for surveillance, maintenance and clearance of foreign material and debris13. Microglia express various cell surface ionotropic P2X purinergic receptors that are known to modulate microglial function14, 15 and to play a unique role in integrating neuronal and glial cellular circuits16.
P2X receptors in recent years have emerged in the spotlight and have been shown to play a pivotal role in regulating neuro-inflammatory and degenerative processes16-20. Several signaling pathways are coupled to P2 receptors in the CNS including the MAPK/ERK pathway, NGF expression, and calcium mobilization17, 21-25. P2X receptors are ubiquitously expressed ligand-gated cation channels that mediate a remarkable variety of physiological and pathophysiological reactions, especially in microglia16-20, 26. More recently, purinergic receptors have been implicated in alcohol abuse disorders, affecting signaling in the CNS27, 28. Studies have shown that alcohol acts as an allosteric regulator inhibiting P2X4R function most likely operating through interactions with the TM domains or hydrophobic regions within the channel structure29. Several animal studies have demonstrated a link between alcohol and P2X4R. Rats treated with ethanol showed a dependence on the drug through the P2X4R, which was blocked by Ivermectin, a P2X4R agonist30. Ethanol inhibits ATP-activated currents in rat and mouse neurons, suggesting a role for P2X4R in ethanol-related behaviors. While building evidence supports a role for P2X4R in the action of alcohol28, their role in modulating the functional activities of microglia is unknown.
In the current study we sought to determine the role of the P2X4 receptor in mediating EtOH-induced effects on microglial function. Our results indicate that alcohol increases P2X4R expression altering microglia functions. Furthermore, pharmacological blockade with a selective P2X4R antagonist could reverse the action, suggesting that P2X4R may play a role in mediating alcohol-induced effects on microglia.
Materials and Methods
Cell culture
Embryonic stem cell derived microglia (ESdM), were a generous gift from Dr. Harald Neumann (University of Bonn Germany; Bonn, Germany). In brief, ESdM cells were cultured in DMEM F12 50:50 (Cellgro; Manassas, VA) containing N2 supplement (Invitrogen; Carlsbad, CA) with 0.048 mM L-glutamine (Cellgro; Manassas, VA), 1% D-glucose (Sigma; St. Louis, MO) and 1% Pen/strep (Cellgro). The ESdM are stable proliferating cells with most characteristics of primary microglia and are a suitable tool to study microglial function in vitro 31, 32.
Antibodies and Reagents
Antibodies were purchased from the following sources: P2X4 antibody (Alomone; Jerusalem, Israel), CD11b (Abcam; Cambridge, England), 7-AAD (BioLegend; San Diego, CA), Fluo4, pHrodo, and Calcein AM were purchased from (Life Technologies; Carlsbad, CA). Ethanol 200 proof (Pharmaco-Aaper), ATP, Poly-L-lysine (PLL), Lipopolysaccharide (LPS), and cytochalasin D (Cyto D) were purchased from Sigma Aldrich; St. Louis, MO, USA, 5-BDBD was purchased from Tocris (Bristol, UK). Cell viability was determined by LIVE/DEAD assay (Invitrogen, Carlsbad, CA), and showed that ethanol (EtOH) 5-200 mM concentration had no toxic effects on microglia after 48 h of exposure (data not shown). The concentration of EtOH (100 mM) used in the present study is similar to other published studies29, 33-36. Blood alcohol concentration (BAC) and the effects of drinking alcohol significantly vary from person to person. Variables include body weight, alcohol percentage in each drink, and each person's ability to tolerate alcohol. The concentration of EtOH (100 mM) equivalent to 454 mg/dl, used in our current study is consistent with published in vitro studies that investigate alcohol-induced effects on glial and neuronal cells. As reviewed by Angela Dolganiuc and Gyongyi Szabo37 for in vitro studies the 10-100 mmol/L ethanol range is considered physiological, with 25 mmol/L ethanol being close to 0.08% BAL achieved in vivo after 4-5 drink equivalents. Few examples of in vitro studies using 100mM EtOH relevant to our study are: 1) Asatryan et al.,29; 2) Popp et al.,38; 3) Jian Y. Zou and Fulton T. Crews.,39; 4) Boyadjieva et al.,40; 5) Fernandez-Lizarbe et al.,41. Optimal concentrations of ATP (25 mM), cytocholasin D (5 μM), fractalkine (CX3CL1, 10 ng/mL), LPS (1 μg/ml), pHrodo (40 μg/ml), Fluo-4 (10 mM), Calcein (5 μM), 5-BDBD (1 nM/ml) were determined from dose and time dependent response studies.
RNA extraction and Real-Time qPCR
The gene expression profile for purinergic receptor P2X4 in ESdM cells was performed by Real-Time PCR. ESdM cells were seeded at a density of 2x105 cells per well in 6 well plates following treatment with 100 mM EtOH for 48 hours. Total RNA was isolated using the RNeasy Mini Kit as per manufacturer's instructions (Qiagen; Valencia, CA). RNA purity and concentration was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Fair Lawn, NJ). Conversion to cDNA was performed by reverse transcription using [1 μg of total RNA] the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Primers were designed for P2X4 forward primer 5’ TCATCCTGGCTTACGTCATTGGGT-3’, reverse primer 5’-CCACACCTTTGGCTTTGGTTGTCA-3’, GAPDH forward primer 5’CAT GGC CTT CCG TGT TCC TA-3’, reverse primer 5’-ACC TGG TCC TCA GTG TAG CCC-3’. cDNA (diluted 1:20) along with forward and reverse primers were mixed with SYBRgreen Mastermix (Fermentas; Hanover, MD). qPCR was performed on an Applied Biosystems StepOnePlus Real-Time PCR System (Applied Biosystems). The PCR conditions consisted of an initial melting cycle at 95°C for 15 minutes, followed by 40 cycles of amplification at 95°C for 15 s (denaturation), 60°C for 30 s (annealing), and 72°C for 30 s (extension). Raw data was analyzed using the Ct method (relative quantification). Results were expressed in relative gene expression levels (fold change) compared to the untreated control.
Flow Cytometric analysis
Analysis of P2X4 expression in ESdM was determined by flow cytometry. Briefly, following treatment with 100 mM EtOH, cells were washed and stained in 2% BSA buffer containing fluorochromeconjugated antibody CD11b (Alexa Fluor [AF]-647, anti rabbit polyclonal P2X4, conjugated to Alexa Fluor [AF]-488 (Life Technologies and Alomone Lab, Jerusalem, Israel) and fixed in 2% paraformaldehyde prior to analysis. Isotype-matched antibodies were used as negative controls. A total of 10,000 events were acquired on a BD FACS LSR II with Diva Software (version 6.1.3; BD Biosciences, San Jose, CA, USA). Percentage of cells expressing P2X4R and Median Fluorescence Intensity (MdFI) were analyzed using FlowJo Software v 8.7.
Immunofluorescent staining
ESdM cells (250,000 cells/well) were plated on coverslips coated with 0.01% PLL in a 6-well dish. Cells were fixed in 4% paraformaldehyde for 30 min followed by staining for purinergic receptor P2X4 (AB-1:200; Alomone,) overnight at 4°C. Secondary staining with anti-rabbit Alexa 488 IgG (1:800) (Life Technologies) was used to determine expression. Samples were washed and mounted on glass slides using ProLong Gold+Dapi reagent (Life Technologies, Carlsbad, CA). Immunoflourescence labeled ESdM cells were imaged at 40X. All immunostained cells were observed with an Eclipse I-80 Microscope (Nikon, Melville, NY) fitted with a CoolSnap-EZ digital camera (Photometrics, Tucson, AZ). Image acquisition analysis was performed using NIS Elements R (Nikon) imaging software.
Calcium Mobilization
ESdM cells (200,000 cells/well) were affixed to 0.01% PLL (Sigma) coated 1.5 mm MatTek (Ashland, MA) cell culture dishes. ESdM cells were loaded with 10 mM Fluo-4/AM (Life Technologies, Carlsbad, CA) in extracellular medium (ECM) containing 2.0% BSA at 37°C for 30 min on a shaker. Cells were washed and 0.25% BSA running buffer was added to each dish. After 1 min of baseline recording, an image was acquired every 2.5 s using the Carl Zeiss 510 Meta confocal microscopy system (Carl Zeiss Microimaging; Thornwood, NY) at 488nm excitation. Concentrations of ATP (25 mM) and EtOH (100 mM) were used to examine calcium mobilization. Treatment with 5-BDBD (20 μM) and EGTA (5 mM) were used to reverse alcohol's effect on calcium mobilization. The images were analyzed with AxioVision version 4.7 software and with National Institutes of Health Image J version 1.42 software (http://rsbweb.nih.gov/ij/)42.
Phagocytosis Assay
To assess phagocytosis, ESdM cells (250,000 cells per well in 6-well dishes) were incubated with pH-sensitive pHrodo-conjugated E. coli bioparticles (Life Technologies). Appropriate wells were treated for 48 hr with EtOH (100 mM) either alone or in the presence of 5-BDBD (1nM/ml). LPS (1 μg/ml) treated for 24 hr, and Cyto D (5 μM) treated for 1 hour were used as controls in separate wells. Briefly, following treatment, cells were incubated for 1.5 h with pHrodo green bioparticles (40 μg/ml) at 37°C in 5% CO2. Immediately after incubation, cells were rinsed with cold phosphate buffered saline, scraped, and washed with FACS buffer (2% BSA in PBS) before being re-suspended in 2% paraformaldehyde and subjected to flow cytometric analysis by BD Canto II (BD Biosciences). Phagocytosis by microglia (FITC+) was quantified and cell viability was determined by 7-AAD staining. Analysis was carried out using FACS DiVa software (Becton Dickinson; Franklin Lake, NJ) and FlowJo Software v 8.7.
Migration assay
Quantitative migration assays were carried out using 8 micron pore Fluoroblock migration plates (Calbiochem; Darmstadt, Germany) as described previously 43. ESdM cells were loaded with 5 mM Calcein (Life Technologies) for 45 minutes at 37 °C, washed prior to seeding at 50,000 cells/well in the upper chamber of the tissue culture insert. CX3CL1 (10 ng/ml) was added to the lower chamber to stimulate migration. The optimal concentration of CX3CL1 was established via dose response of CX3CL1 to microglial migration32. The number of migrated cells were counted using an inverted fluorescence live cell imaging system (Carl Zeiss MicroImaging, Thornwood, NY). Each experiment was performed in triplicate and each experimental well was imaged 5 times in different locations, and the results were expressed as an average of the total number of migrated cells in response to chemoattractant under each experimental condition. The images were analyzed with AxioVision version 4.7software (Carl Zeiss Microimaging) and with National Institutes of Health ImageJ version 1.42 software (http://rsbweb.nih.gov/ij/) as described44
Statistical analysis
Data were analyzed using either the Student's t-test for independent means or a one-way analysis of variance (ANOVA) followed by post hoc Student Newman Keuls test to determine which conditions were significantly different from each other, and a Tukey post-test for multiple comparisons. Results were expressed as mean values (±SD), with standard errors deemed statistically significant when p<0.05 (marked in the figures as *p,0.05; **p,0.01; *** p,0.001).
Results
Alcohol increases P2X4R mRNA and protein expression in microglia
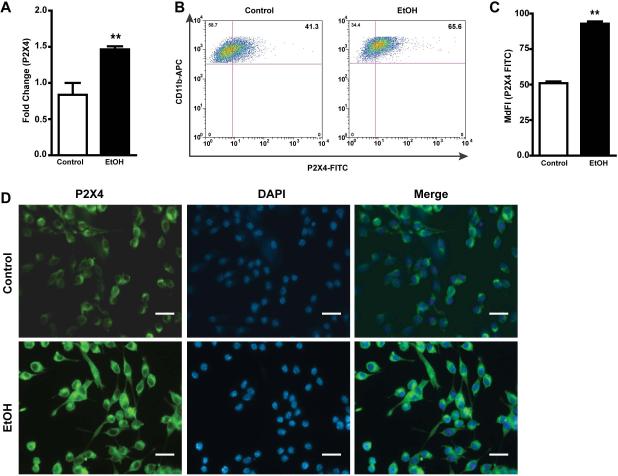
Purinergic receptors widely expressed in the brain are important mediators of many cellular functions 18, 45. P2X4 receptors (P2X4Rs), the most abundantly expressed purinergic receptor, are markedly up-regulated in dorsal horn microglia and critical for the pathogenesis of pain hypersensitivity caused by injury to peripheral nerves 46. Interestingly, the ATP-gated purinergic P2X4R is the most alcohol-sensitive P2XR subtype47. To evaluate the effects of EtOH on these receptors in microglial cells, we examined the mRNA and protein expression of P2X4R in ESdM exposed to EtOH (100 mM) for 48 hr. EtOH treated cells showed a statistically significant increase in P2X4R mRNA expression (Fig 1a) as compared to control (**p<0.002). Flow cytometric analysis showed up-regulation of P2X4R expression in EtOH-treated microglia (*p<0.05) as compared to control (Fig 1b), the median fluorescent intensity (MedF) demonstrated a dramatic increase in FITC labeled cells treated with EtOH as compared to control (**p<0.004) (Fig 1c). Similarly, immunocytochemistry revealed up-regulation of P2X4R expression (Fig 1d) in the cell membrane when exposed to EtOH, similar to those shown within microglia in vivo48 Together, these results suggest that EtOH modulates expression of P2X4R in microglial cells.
Figure 1. Ethanol increases P2X4 purinergic receptor mRNA and protein expression in microglia.
a. Fold change of P2X4 receptor after 48 hr EtOH (100 mM) treatment in microglial cells as compared to control. Results are expressed as mean values ± SEM (n=6); **p<0.002 compared with control. b. Microglia were treated with 100 mM EtOH for 48 hr and then co-stained with P2X4 (1:200) and anti-rabbit 488 secondary and CD11b-APC conjugated antibody. Dot plot represents P2X4 and CD11b positive cells, and an increase of P2X4 receptor in microglia as depicted by the double positive cells after treatment of EtOH for 48 hr. c. Median fluorescent intensity of FITC in ESdM cells treated with EtOH as compared to control. Results are expressed as median fluorescent values (n=3) **p<0.004. d. Immunofluorescent staining of ESdM cells show a difference in protein expression of P2X4 receptor after 48 hr of EtOH (100 mM) treatment as compared to control cells without ethanol treatment.
P2X4R antagonist reverses EtOH-triggered calcium mobilization in microglia
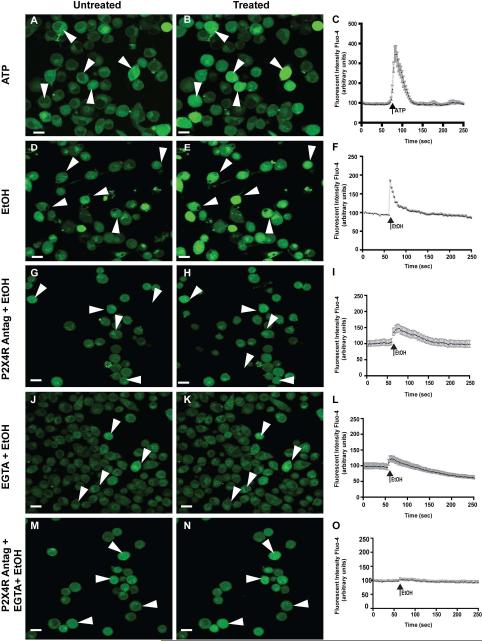
Second messenger calcium is important for many cellular responses, including signaling of immune response 49. Since P2X4 receptors are known to display dynamic regulation of calcium, we sought to estimate the changes in calcium levels trigged by EtOH. After an initial 1 min baseline reading, changes in calcium [Ca2+] levels were monitored by the calcium indicator dye, Fluo-4. We used ATP (25 mM) as a positive experimental control (Fig 2a-c). Stimulation of microglial cells with 100 mM EtOH induced a rapid increase in [Ca2+] (Fig 2d-f). Pretreatment with 5-BDBD (20 μM), a P2X4R selective antagonist, prior to EtOH treatment significantly decreased the [Ca2+] level as compared to ethanol alone (Fig 2g-i), suggesting possible involvement of P2X4 receptor in EtOH-induced calcium mobilization. To determine if P2X4R is primarily involved in EtOH-induced calcium affects, we treated the cells with EGTA (5 mM), a calcium chelating agent, to determine that no free floating calcium had an additive effect with EtOH (Fig 2j-l). Pretreatment with P2X4 antagonist and EGTA in combination prior to EtOH treatment decreased calcium mobilization as detected by the fluorescent intensity of fluo-4 (Fig 2m-o). Statistical analysis showed that calcium mobilization, when cells were pretreated with P2X4 antagonist, was significantly less compared to EtOH treatment alone (*p<0.02).
Figure 2. Increased calcium mobilization in microglia exposed to ethanol can be reversed with P2X4R antagonist.
ESdM cells were affixed to PLL-coated MatTek 1.5 mm cell culture dishes loaded with the cytosolic calcium indicator Fluo-4/AM (10 mM)42. After 1 min of baseline recording, microglia cells were treated with ATP (25 mM), EtOH (100 mM), and P2X4 antagonist (20 μM) or in combination with EGTA (5 mM) and the P2X4R antagonist and EtOH. a. Microglia cells pre-treatment; baseline. b. Microglia cells treated with 25 mM ATP. c. Graphical representation of Fluo4 fluorescent intensity of ATP treated cells. d. Microglia cells pre-treatment; baseline e. Microglia cells treated with 100 mM EtOH. f. Graphical representation of Fluo4 fluorescent intensity of EtOH-treated cells. g. Microglia cells pre-treatment P2X4 antagonist (20 μM) for 30 min; baseline h. Microglia cells treated with EtOH (100 mM). i. Graphical representation of Fluo4 fluorescent intensity of treated cells. j. Microglia cells pre-treatment with EGTA (5 mM) for 5 min; baseline. h. Microglia cells treated with EtOH (100 mM). i. Graphical representation of Fluo4 fluorescent intensity of treated cells. m. Microglia cells pre-treatment with P2X4 antagonist (20 μM) for 30minutes and EGTA (5 mM) for 5min; baseline. n. Microglia cells treated with EtOH (100mM). Graphical representation of Fluo4 fluorescent intensity of ESdM treated cells. Microglia cells treated with P2X4 antagonist prior to EtOH treatment were found to have a statistically significant decrease in calcium mobilization as compared to cells treated with EtOH alone, (*p<0.02).
Inhibition of P2X4R reverses EtOH effect on microglial phagocytosis
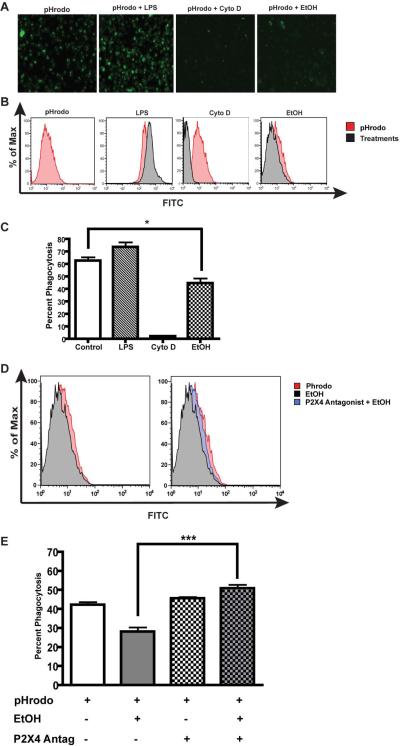
Alcohol is known to impair macrophage and microglial phagocytosis3. Since P2X4 receptors have a dynamic role in regulation of macrophage and microglial function, we investigated the possibility that P2X4R plays a role in altered microglial phagocytic function after EtOH treatment. Microglial phagocytic function was determined using pHrodo green E.coli bioparticles conjugates. pHrodo green-conjugated E. coli bioparticles are nonfluorescent outside the microglia at neutral pH, but fluoresce bright green at acidic pH such as in phagosomes. Experimental controls including activation of microglia by inflammatory stimulus, LPS (1 μg/ml) and treatment with Cyto D (5 μM), an inhibitor of cytoskeletal rearrangement showed increased or inhibition of microglia phagocytosis respectively. Treatment of ESdM cells with EtOH (100 mM) markedly decreased (*p<0.05) microglial phagocytic function compared to control when examined either by fluorescent microscopy (Fig. 3a and 3c) or flow cytometry (Fig 3b). Next, we addressed whether P2X4R plays a role in EtOH-induced suppression of microglial phagocytic function. As shown in the Fig. 3d and 3e, microglia pretreatment with 5-BDBD (1 nM/ml), a P2X4R selective antagonist prior to EtOH treatment significantly (**p<0.001) reversed the EtOH effect on microglial phagocytosis. Taken together, these data provide reasonable evidence of the involvement of P2X4R in EtOH induced microglial phagocytosis.
Figure 3. P2X4R antagonist reverses the effects of alcohol on microglial phagocytosis.
a. Representative images of microglial phagocytosis via pHrodo bioparticles after treatment with LPS (1 μg/ml), Cyto D (5 μM) or EtOH (100 mM). b. Representative histograms of microglia treatments compared to pHrodo control. c. Graphical representation of microglia phagocytosis after treatments. EtOH treated cells show a statistically significant decrease in phagocytosis as compared to the control, (*p<0.05). d. Representative histogram of microglia phagocytosis with P2X4 antagonist pretreatment (1 nM/mL) prior to EtOH treatment. Microglia cells treated with antagonist show an increase in phagocytosis (blue) as compared to EtOH treated (black) cells. e. Graphical representation of microglia phagocytosis of microglia pretreated with P2X4 antagonist prior to EtOH-treatment. Cells treated with antagonist show a 10% increase in phagocytosis as compared to ethanol treated cells alone, a one-way ANOVA was used to compare the two groups (Bonferonni post test) (***p<0.001).
EtOH effects on microglial migration towards CX3CL1 are reversed with a P2X4 selective antagonist
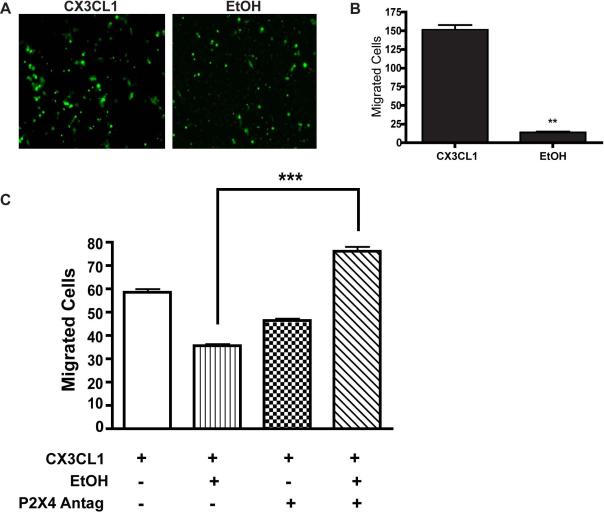
As the innate immune cell of the CNS, microglia constantly survey CNS parenchyma for pathogens and cellular stress signals. Hence, to explore the role P2X4R as a mediator of EtOH-induced effects on microglial migration, we used the transwell migration assay as described earlier50 and calculated microglial migration in response to the chemokine attractant, CX3CL1. Optimal concentration of CX3CL1 was determined by dose response experiments (data not shown). ESdM cells were loaded with 5 μM calcein AM, and then exposed to EtOH for 48 hr. The migration of microglial cells towards CX3CL1 was evaluated in presence or absence of the P2X4R antagonist, 5-BDBD. Migrated cells were imaged using an inverted fluorescent microscope (Zeiss) 10X zoom, and analyzed using Image J particle analysis to determine the exact number of migrated cells. EtOH (100 mM) significantly (p<0.02) suppressed the migration of microglia towards CX3CL1 (Fig 4a and 4b); this process was reversed (p<0.001) in the presence of the P2XR4 receptor antagonist 5-BDBD (1 nM/ml), suggesting that P2X4R critically contributes to EtOH-induced modulation of microglial migration.
Figure 4. P2X4R antagonist increases migration to CX3CL1 in the presence of ethanol in microglia.
In vitro migration was performed using the transwell migration assay. ESdM cells (5×104) were loaded with 5 mM Calcein AM. In the lower chamber of the transwell plate, 10 ng/mL of fractalkine (CX3CL1) was added to determine the number of migrating cells. a. Microglial cells after migration as seen under the microscope (40X magnification). Microglia treated with EtOH, did not migrate well as compared to control based on the fluorescent intensity. b. Graphical representation of the number of total cells migrated before and after EtOH treatment was analyzed using Image J software. The data represents a statistically significant decrease in migration towards CX3CL1 in EtOH-treated cells as compared to control, (**p<0.02) (t-test). c. Graphical representation of microglia pretreated with P2X4R antagonist (1 nM/mL) prior to EtOH treatment. The data shows pretreatment of microglia with P2X4R antagonist increased migration towards fractalkine as compared to EtOH-treatment alone, ***p<0.001 (ANOVA).
Discussion
As immune sentinels of the CNS, microglia respond to homeostatic changes and play a pivotal role in regulation of neuroinflammatory processes including neurodegenerative disease51. Amongst the plethora of well-described subsets of immune receptors a growing body of evidence now points to purinergic receptors on microglia as contributing to various neuropathologies 52, 53. Notably, ionotropic P2X receptor subtype P2X4R has emerged as a key regulator of microglial functions and has been implicated in neurodegenerative and neuroimmune disorders 45. While the deleterious effects of alcohol on the brain, including microglia1, 3-6, are well enumerated, much remains to be known about factors that operate in regulating microglial functional responses induced by EtOH. In light of the importance of P2X receptor role in regulating microglia functions17, the present study explores the functional relevance of the P2X4 receptor on microglia associated with alcohol-related immune dysfunction.
Recent evidence demonstrates that P2X4 is an EtOH sensitive receptor47 and is an important mediator of EtOH-induced effects. Expression of P2X4R mRNA (Fig 1a) and protein (Fig 1b and c) in the microglia was up-regulated in response to EtOH suggesting that purinergic signaling may be involved in modulating microglial responses. Up-regulation of P2X4R expression in microglia has been demonstrated in several in vitro studies48, 54. Similarly up-regulation of ionotropic P2X4R in activated microglia is evident from various animal models of microglia-mediated disorders55. Abundantly expressed in microglia56, de novo P2X4R expression is known to be stable in the lysosomes, and trafficking of P2X4R protein to the cell surface occurs upon stimulation48. More over, activation of P2X4 receptor has been linked to PI3K-AKT, MAPK, MAPK/ERK kinase, and MEK signaling cascades57. Whether EtOH-induced P2X4R up-regulation is at the transcriptional and translational levels or due to trafficking of P2X4R to the cell surface of microglia with lysosomal exocytosis58 without changing the total cellular level of P2X4R protein remains to be determined. The mechanism by which, EtOH affects P2X4 receptor expression and related downstream signaling pathways, is the focus of ongoing studies in our laboratory.
Calcium is a second messenger that activates many transcription factors and mediates cell signaling. Given that P2X4R are known to mobilize calcium20, we characterized purinergic-mediated changes in [Ca2+] levels in response to EtOH. ESdM cells loaded with Fluo-4, which stains cytosolic calcium, were exposed to EtOH in the absence and presence of P2X4R antagonist. Acute exposure to EtOH evoked a rapid increase in [Ca2+] levels (Fig 2f) a similar increase in [Ca2+] levels has been noted in gastric cells, where EtOH in a dose-dependent manner has been shown to modulate calcium59. Inhibition of P2X4R activation by 5-BDBD, a specific P2X4 antagonist, suppressed (Fig 2i) the EtOH-induced [Ca2+] response. Additionally, when P2X4R antagonist were applied in the extracellular [Ca2+] free environment, the EtOH-induced [Ca2+] levels were also decreased (Fig 2o) indicating that EtOH via the ionotropic P2X4R, increases [Ca2+] levels, probably by a mechanism independent of extracellular [Ca2+] influx15. Purinergic receptors are sensitive to calcium release and can activate other P2XR on microglia as well as other surrounding glia60. Indeed evidence indicates that calcium ion via P2X4R can transiently activate transcription factors61 not only in microglia but also in neurons and other glia in the brain. These data collectively suggest the role of P2X4R in EtOH-induced changes in [Ca2+] levels and functional processes in microglia
Regulation of microglia via P2XR serve as a modulatory factors in shaping cellular responses62. Hence, we investigated the effects of EtOH on phagocytic activity of microglia, since it is an important functional characteristic of microglia and necessary for CNS maintenance and clearance of debris. Microglial cells exposed to EtOH with and without P2X4R antagonist were incubated with opsonized pHrodo-conjugated E. coli bioparticles. Compared to control microglia, EtOH-treated microglia engulfed fewer E. coli particles, indicated by the leftward shift of the fluorescent spectrum (Fig 3b) and decreased mean fluorescence intensity in these cells (Fig 3d). Our data is similar to previous studies where macrophages treated with EtOH exhibit a decrease in phagocytosis3, 9.
Microglial processes such as polarization and convergence at the site of injury, is mediated via purinoreceptors P2X4R63. Responding to factors released from damaged cells, microglia are recruited towards the damaged or infected site where they are involved in degenerative and regenerative processes. In this study, we showed that P2X4R antagonist partially reversed the effects EtOH on microglial migration towards CX3CL1 (Fig 4c). Purinergic signaling can activate Rho to induce microglial cytoskeleton reorganization. The small GTPase Rho and their downstream effectors such as Rho-effector kinases (ROCKs) are known to mediate the reorganization of actin cytoskeleton during phagocytosis64. Exploring the mechanism that regulates microglia effector functions will further shed light on understanding EtOH mediate immune dysfunction perturbing normal microglia function.
In summary, our study demonstrated that EtOH alters P2X4 receptor expression, and modulates microglia effector functions in a receptor dependent manner suggesting a role of P2X4 in part attributable to the underlying mechanisms responsible for deregulation of microglial immune function. Deciphering the role of purinoreceptor signaling in EtOH-induced microglia dysregulation will provide important clues to the molecular basis alcohol impairment of immune function and as an attractive target for reversing alcohol-induced tissue damage.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Nancy L. Reichenbach for editing and Dr. Uma Sriram for critical reading of the manuscript, and Dr. Slava Rom for his expert help with the graphics.
This work was supported by NIH grant R01 DA031064 and Temple Development Grant to RP
References
- 1.Deehan GA, Jr., Brodie MS, Rodd ZA. What is in that Drink: The Biological Actions of Ethanol, Acetaldehyde, and Salsolinol. Current topics in behavioral neurosciences. 2013;13:163–184. doi: 10.1007/7854_2011_198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 3.Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cellular immunology. 2012;274:61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiology of disease. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suk K. Microglial signal transduction as a target of alcohol action in the brain. Current neurovascular research. 2007;4:131–142. doi: 10.2174/156720207780637261. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G. Alcohol's contribution to compromised immunity. Alcohol Health Res World. 1997;21:30–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson S, Kolls JK. Alcohol, host defence and society. Nature reviews Immunology. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 8.Rimland D, Hand WL. The effect of ethanol on adherence and phagocytosis by rabbit alveolar macrophages. The Journal of laboratory and clinical medicine. 1980;95:918–926. [PubMed] [Google Scholar]
- 9.Aroor AR, Baker RC. Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol. 1998;15:277–280. doi: 10.1016/s0741-8329(97)00129-8. [DOI] [PubMed] [Google Scholar]
- 10.Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacology, biochemistry, and behavior. 2011;100:253–258. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyadjieva NI, Sarkar DK. Role of microglia in ethanol's apoptotic action on hypothalamic neuronal cells in primary cultures. Alcoholism-Clinical and Experimental Research. 2010;34:1835–1842. doi: 10.1111/j.1530-0277.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of disease. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 14.Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Archiv : European journal of physiology. 2006;452:615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K. Purinergic systems in microglia. Cellular and molecular life sciences : CMLS. 2008;65:3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Molecular neurobiology. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 17.Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochemistry international. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Tsuda M. Purinergic systems, neuropathic pain and the role of microglia. Experimental neurology. 2012;234:293–301. doi: 10.1016/j.expneurol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 20.James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. European journal of pharmacology. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- 21.Mei L, Du W, Gao W, Mei QB. Purinergic signaling: a novel mechanism in immune surveillance. Acta pharmacologica Sinica. 2010;31:1149–1153. doi: 10.1038/aps.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Molecular pharmacology. 2000;58:936–945. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 23.Ko WH, Au CL, Yip CY. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life sciences. 2003;72:1519–1535. doi: 10.1016/s0024-3205(02)02410-4. [DOI] [PubMed] [Google Scholar]
- 24.Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic signalling. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLarnon JG, Choi HB, Lue LF, Walker DG, Kim SU. Perturbations in calcium-mediated signal transduction in microglia from Alzheimer's disease patients. Journal of neuroscience research. 2005;81:426–435. doi: 10.1002/jnr.20487. [DOI] [PubMed] [Google Scholar]
- 26.Dooley R, Mashukova A, Toetter B, Hatt H, Neuhaus EM. Purinergic receptor antagonists inhibit odorant-mediated CREB phosphorylation in sustentacular cells of mouse olfactory epithelium. BMC neuroscience. 2011;12 doi: 10.1186/1471-2202-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed Dar M, Davies DL, Choi DS. Implication of the purinergic system in alcohol use disorders. Alcoholism-Clinical and Experimental Research. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples RW, Alkana RL, Davies DL. Ethanol is a fast channel inhibitor of P2X4 receptors. The Journal of pharmacology and experimental therapeutics. 2011;337:171–179. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asatryan L, Popova M, Perkins D, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in purinergic P2X4 receptors. The Journal of pharmacology and experimental therapeutics. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosten TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacology, biochemistry, and behavior. 2011;98:533–538. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoli I, Kierdorf K, Neumann H. Microglial Precursors Derived from Mouse Embryonic Stem Cells. Glia. 2009;57:1660–1671. doi: 10.1002/glia.20878. [DOI] [PubMed] [Google Scholar]
- 32.Beutner C, Roy K, Linnartz B, Napoli I, Neumann H. Generation of microglial cells from mouse embryonic stem cells. Nature protocols. 2010;5:1481–1494. doi: 10.1038/nprot.2010.90. [DOI] [PubMed] [Google Scholar]
- 33.Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Davies DL, Asatryan L, Kuo ST, Woodward JJ, King BF, Alkana RL, Xiao C, Ye JH, Sun H, Zhang L, Hu XQ, Hayrapetyan V, Lovinger DM, Machu TK. Effects of ethanol on adenosine 5'-triphosphate-gated purinergic and 5-hydroxytryptamine receptors. Alcoholism, clinical and experimental research. 2006;30:349–358. doi: 10.1111/j.1530-0277.2006.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Hartog CR, Beckley JT, Smothers TC, Lench DH, Holseberg ZL, Fedarovich H, Gilstrap MJ, Homanics GE, Woodward JJ. Alterations in ethanol-induced behaviors and consumption in knock-in mice expressing ethanol-resistant NMDA receptors. PloS one. 2013;8:e80541. doi: 10.1371/journal.pone.0080541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asatryan L, Yardley MM, Khoja S, Trudell JR, Hyunh N, Louie SG, Petasis NA, Alkana RL, Davies DL. Avermectins differentially affect ethanol intake and receptor function: implications for developing new therapeutics for alcohol use disorders. Int J Neuropsychopharmacol. 2014:1–10. doi: 10.1017/S1461145713001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolganiuc A, Szabo G. In vitro and in vivo models of acute alcohol exposure. World journal of gastroenterology : WJG. 2009;15:1168–1177. doi: 10.3748/wjg.15.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popp RL, Dertien JS. Actin depolymerization contributes to ethanol inhibition of NMDA receptors in primary cultured cerebellar granule cells. Alcohol. 2008;42:525–539. doi: 10.1016/j.alcohol.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou JY, Crews FT. Release of neuronal HMGB1 by Ethanol through decreased HDAC activity activates brain neuroimmune signaling. PloS one. 2014;9:e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyadjieva NI, Sarkar DK. Role of microglia in ethanol's apoptotic action on hypothalamic neuronal cells in primary cultures. Alcoholism, clinical and experimental research. 2010;34:1835–1842. doi: 10.1111/j.1530-0277.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 42.Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, Morsey B, Brodie MR, Persidsky Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185:2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramirez SH, Fan S, Zhang M, Papugani A, Reichenbach N, Dykstra H, Mercer AJ, Tuma RF, Persidsky Y. Inhibition of glycogen synthase kinase 3beta (GSK3beta) decreases inflammatory responses in brain endothelial cells. The American journal of pathology. 2010;176:881–892. doi: 10.2353/ajpath.2010.090671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnstock G. Introduction to purinergic signalling in the brain. Advances in experimental medicine and biology. 2013;986:1–12. doi: 10.1007/978-94-007-4719-7_1. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. The Journal of pharmacology and experimental therapeutics. 2000;295:862–869. [PubMed] [Google Scholar]
- 47.Popova M, Trudell J, Li K, Alkana R, Davies D, Asatryan L. Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic signalling. 2013 doi: 10.1007/s11302-013-9373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toulme E, Khakh BS. Imaging P2X4 receptor lateral mobility in microglia: regulation by calcium and p38 MAPK. The Journal of biological chemistry. 2012;287:14734–14748. doi: 10.1074/jbc.M111.329334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moller T. Calcium signaling in microglial cells. Glia. 2002;40:184–194. doi: 10.1002/glia.10152. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez SH, Fan SS, Dykstra H, Reichenbach N, Del Valle L, Potula R, Phipps RP, Maggirwar SB, Persidsky Y. Dyad of CD40/CD40 Ligand Fosters Neuroinflammation at the Blood-Brain Barrier and Is Regulated via JNK Signaling: Implications for HIV-1 Encephalitis. Journal of Neuroscience. 2010;30:9454–9464. doi: 10.1523/JNEUROSCI.5796-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural brain research. 2013;236:270–282. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 52.Inoue K, Tsuda M. P2X4 receptors of microglia in neuropathic pain. CNS & neurological disorders drug targets. 2012;11:699–704. doi: 10.2174/187152712803581065. [DOI] [PubMed] [Google Scholar]
- 53.Trang T, Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinergic signalling. 2012;8:621–628. doi: 10.1007/s11302-012-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Vazquez-Villoldo N, Domercq M, Martin A, Llop J, Gomez-Vallejo V, Matute C. P2X4 receptors control the fate and survival of activated microglia. Glia. 2014;62:171–184. doi: 10.1002/glia.22596. [DOI] [PubMed] [Google Scholar]
- 56.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacology & therapeutics. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. Journal of cell science. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 59.Kokoska ER, Smith GS, Deshpande Y, Wolff AB, Rieckenberg C, Miller TA. Calcium accentuates injury induced by ethanol in human gastric cells. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 1999;3:308–318. doi: 10.1016/s1091-255x(99)80073-0. [DOI] [PubMed] [Google Scholar]
- 60.Glaser T, Resende RR, Ulrich H. Implications of purinergic receptor-mediated intracellular calcium transients in neural differentiation. Cell communication and signaling : CCS. 2013;11:12. doi: 10.1186/1478-811X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. The European journal of neuroscience. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 62.Ohsawa K, Kohsaka S. Dynamic motility of microglia: purinergic modulation of microglial movement in the normal and pathological brain. Glia. 2011;59:1793–1799. doi: 10.1002/glia.21238. [DOI] [PubMed] [Google Scholar]
- 63.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain : a journal of neurology. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chimini G, Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nature cell biology. 2000;2:E191–196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.