Abstract
Objectives/Hypothesis
Three surgical approaches: cochleostomy (C), round window (RW), and extended round window (ERW); and two electrodes types: lateral wall (LW) and perimodiolar (PM), account for the vast majority of cochlear implantations. The goal of this study was to analyze the relationship between surgical approach and electrode type with final intracochlear position of the electrode array and subsequent hearing outcomes.
Study Design
Comparative longitudinal study.
Methods
One hundred postlingually implanted adult patients were enrolled in the study. From the postoperative scan, intracochlear electrode location was determined and using rigid registration, transformed back to the preoperative computed tomography which had intracochlear anatomy (scala tympani and scala vestibuli) specified using a statistical shape model based on 10 microCT scans of human cadaveric cochleae. Likelihood ratio chi-square statistics were used to evaluate for differences in electrode placement with respect to surgical approach (C, RW, ERW) and type of electrode (LW, PM).
Results
Electrode placement completely within the scala tympani (ST) was more common for LW than were PM designs (89% vs. 58%; P < 0.001). RW and ERW approaches were associated with lower rates of electrode placement outside the ST than was the cochleostomy approach (9%, 16%, and 63%, respectively; P < 0.001). This pattern held true regardless of whether the implant was LW or PM. When examining electrode placement and hearing outcome, those with electrode residing completely within the ST had better consonant-nucleus-consonant word scores than did patients with any number of electrodes located outside the ST (P = 0.045).
Conclusion
These data suggest that RW and ERW approaches and LW electrodes are associated with an increased likelihood of successful ST placement. Furthermore, electrode position entirely within the ST confers superior audiological outcomes.
Level Of Evidence
2b.
Keywords: Cochlear implant, electrode, cochleostomy, round window, sensorineural hearing loss
INTRODUCTION
In 1985, the United States Food and Drug Administration (FDA) approved multichannel cochlear implants (CIs) for adults with profound hearing loss; and in 1990, implantation was approved for children. Since then, this procedure has become the standard of care for patients with severe-to-profound sensorineural hearing loss. Successful outcomes are dependent not only on extrinsic factors, but also on intrinsic factors that cannot always be modified by the CI team. Significant predictive factors for hearing outcomes in patients with CIs have been previously reported.1–3 These include, but are not limited to, duration of deafness,4 level of preimplant speech recognition,5 pre/postlingual status,6 and the coupling of device electrodes.7,8 Recipient age does not appear to have a significant impact on hearing outcomes in elderly candidates.9,10
Earlier studies by Shepherd et al. reported that, because of its dimensions, the scala tympani (ST) is the preferred location for CI electrode placement.11 A number of recent studies have proposed that intraoperative factors may be important determinants of electrode location and possibly of audiological outcome. Preliminary reports suggest that intracochlear electrode position—specifically, placement within the ST—is associated with improved audiological outcomes.12–16 Additionally, different surgical techniques have been proposed to minimize trauma during electrode insertion and to increase the likelihood of placement within the ST. However, to the authors’ knowledge, no clinical study has investigated the relationship between surgical insertion technique, electrode type, final intracochlear electrode location, and subsequent hearing outcomes.
The aim of our study was to correlate electrode type and surgical approach with intracochlear electrode location and audiological performance.
MATERIALS AND METHODS
After obtaining institutional review board approval, post-lingually deafened adult patients who had preoperative temporal-bone computed tomography (CT) and CIs placed at the authors’ institution were offered enrollment in the study. The study period analyzed was from 2009 through 2013. After consent was obtained, postoperative temporal bone CT scans were obtained via either flat panel, volumetric computerized tomography (fpVCT) using a Xoran XCAT scanner (Xoran Industries, Ann Arbor, MI) or a traditional multislice CT scanner. Patient demographics, cause of deafness, length of auditory deprivation, type of implant, and surgical approach—as well as postoperative audiometric performance—were recorded. Three types of insertion technique were employed: anteroinferior cochleostomy (C); extended round window (ERW), defined as opening the round window membrane and enlarging it by drilling its anterior–inferior margin; and round window (RW), defined as removing the round window bony overhang when necessary and opening the RW membrane directly without enlargement. Four surgeons, who each have performed several hundred implants to date, implanted 98% of the CIs evaluated in the study. The determination of surgical approach was left to the discretion of the surgeon. Over the period of time analyzed (2009–2013), there was a gradual evolution in preference of surgical approach among all four surgeon; the RW and ERW approach has been utilized more frequently in recent years. Although it has been shown that there is tremendous variation in C techniques nationwide,17 the four surgeons in the current study routinely utilized anteroinferior C placement when a C approach was chosen.
Implants from all three FDA-approved device manufacturers (MED-EL [ME] GmbH Innsbruck Austria; Cochlear Americas [CA] Corporation Englewood, Colorado; and Advanced Bionics [AB] Corporation Valencia, California) were used. All electrode models were designated either perimodiolar (PM) or lateral wall (LW) according to manufacturer specifications.
In order to determine the location of the electrodes in relation to the ST and scala vestibuli (SV), an automated, highly accurate algorithm was utilized. This technique employs a nonrigid, atlas-based registration18 that has been previously validated using cadaveric models (Fig. 1).19
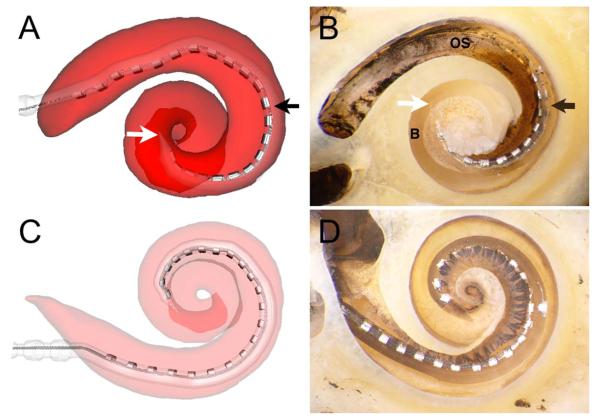
Fig. 1.
(A) Software-generated, three-dimensional reconstructed image and (B) microdissection demonstrating an electrode array entering the scala tympani (ST); after approximately 180° (black arrow indicates the array moves from ST through the basilar membrane and enters scala vestibuli [SV]), the electrode traverses the interscalar partition to enter the SV (white arrow indicates the clear silicone tip of the array, which is above the basilar membrane on the middle turn). (C) Three-dimensional reconstructed image. (D) Microdissection revealing an electrode completely residing within the ST without violation of the osseous spiral lamina or basilar membrane.19
The image analysis sequence involves three steps: First, the intracochlear anatomy is localized in a preimplantation CT. Second, the electrode array is localized in a postimplantation CT. Third, the two results are merged by bringing both sequences into global alignment. Anatomical structures are localized in preimplantation rather than postimplantation CT because the CI array creates a significant amount of metal-related artifact in the postimplantation image, which makes identification of intracochlear structures difficult.
Localization of anatomical structures in preimplantation CT involves the use of a shape model of intracochlear anatomy, created using microCT scans of 10 cadaveric specimens in which the ST and SV were manually delineated. The model consists of a surface representation of the average shape of the ST and SV, and it also describes how the shapes of the cochleae in the training set vary nonrigidly from the mean shape. This so-called statistical shape model can then be deformed to localize the cochlea in a new patient’s preimplantation CT. In this process, the external walls of the cochlea are used as landmarks, and the statistical shape model is fit to these landmarks to estimate the location of the rest of the intracochlear structures based on natural shape deformations in the ST and SV learned from the training set. This approach both accounts for nonrigid variations in cochlear anatomy across individuals and permits accurate localization of internal cochlear structures that are not visible in the clinical images. One limitation with this approach is that it is only capable of estimating nonrigid shape deformations represented in the training set. However, in our original validation study, we showed that using this model, which at the time was constructed from a more limited training set of six specimens, resulted in average surface-localization errors of 0.2 mm (less than half the length of a voxel diagonal).18
Next, the electrode array is localized in the postimplantation CT using algorithms previously reported by Noble et al.20 In this approach, a curve is fit to the image in order to estimate the centerline of the electrode array, after which a surface model of the electrode array is mapped to the extracted centerline. Recent work, which has not yet been published, has shown that the position of the contacts can be estimated with an average localization error of 0.1 mm. The final step is to merge results from the electrode and anatomical localization processes by bringing the two images into global alignment using well-known rigid image-registration techniques.21 Determination of electrode location was performed blinded to the type of surgical approach.
Descriptive statistics were used to summarize patient and surgical characteristics. Mean and standard deviations were used to summarize normally distributed data. Skewed data were summarized using the median and 25th to 75th interquartile range (IQR), representing the middle 50% of the data values. The likelihood ratio chi-square statistic was used to test for differences in the patterns of electrode placement among the types of surgery (C, RW, ERW) and category of implants (LW, PM), as well as rates of ST placement by type of surgery within each implant type. Logistic regressions generated odds ratios (OR) and 95% confidence intervals (c.i.) for the ORs. Spearman correlations were used to assess the association of time since surgery with hearing performance scores. To account for this dependency of observation, general linear modeling that adjusted the standard errors using generalized estimating equations were used to test for the effect of electrode placement on hearing performance assessed at least 1 year following surgery. Finally, Kruskal-Wallis tests were used to evaluate audiometric outcomes. A probability of ≤0.05 was used for determining statistical significance.
RESULTS
One hundred postlingually deafened adult patients (116 implants) were included, for which complete information about surgical approach, electrode location, and electrode type was available. There were 54 (54%) males and 46 (46%) females, with a mean age of 61.1 years. The etiology of hearing loss was documented in 50 patients: Fourteen (28.0%) patients had autoimmune hearing loss; eight (16.0%) patients had a congenital etiology; in five (10%) patients, an infectious cause was attributed; and in 23 (46%) patients, the cause of deafness was unknown. The median for duration of deafness was 1 year (IQR % 1.1).
Forty-seven (40.5%) of the implants were LW, and 69 (59.5%) of the implants were PM. Cochleostomy was performed in 38 (32.8%) ears; ERW was performed in 43 (37.1%) ears; and RW was performed in 35 (30.2%) ears. Eighty-two (70.7%) of the electrode arrays were fully located within the ST (Fig. 2 and Fig. 3). Twenty-seven (23.3%) electrodes crossed from the ST to the SV (Fig. 4 and Fig. 5), and seven (6%) electrodes were fully inserted into the SV. All patients were implanted with the most current device models available at the time of surgery. Twenty-three (19.8%) ears received an AB CI; 77 (66.4%) ears received a CA device; and 16 (13.8%) implants were from MED-EL GmbH.
Fig. 2.
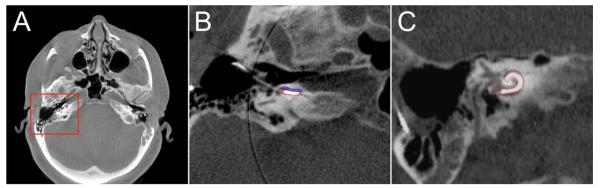
Computed tomography of a right temporal bone following cochlear implant electrode insertion. (A) Axial, (B) magnified axial, and (C) magnified coronal views showing the cochlear implant electrode situated completely within the scala tympani (ST). The ST is outlined in red and the scala vestibuli is outlined in blue.
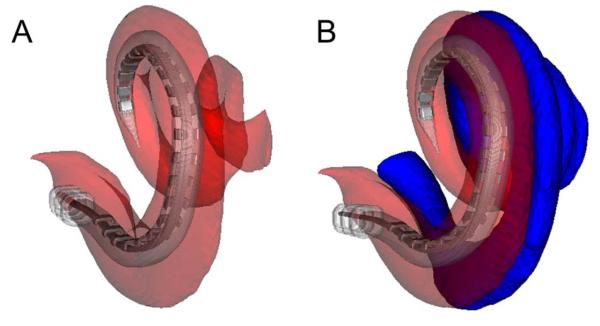
Fig. 3.
Three-dimensional reconstructed images showing a cochlear implant completely within the scala tympani (ST). (A) Medial-to-lateral view with the ST is shown in semitransparent red. (B) The scala vestibuli is shown in opaque blue.
Fig. 4.
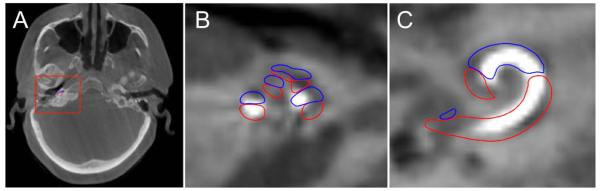
Computed tomography of a right temporal bone following cochlear implant electrode insertion. (A) Axial, (B) magnified oblique axial, and (C) magnified oblique coronal views demonstrating the implant beginning in the scala tympani (outlined in blue) and crossing over into the scala vestibuli (outlined in red) at approximately 180°.
Fig. 5.
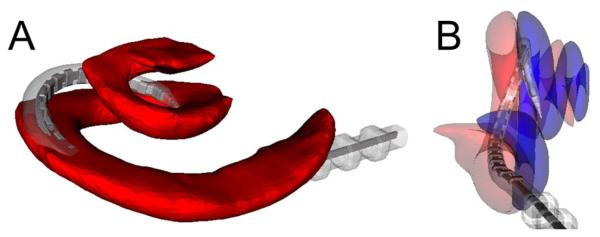
Three-dimensional reconstructed images demonstrating scala crossover from the scala tympani (ST) to the scala vestibuli (SV). (A) Inferior-to-superior, medial-to-lateral view of the ST (red) demonstrating the electrode array crossing the basilar membrane at approximately 180°. (B) View parallel to the basal turn shows electrode crossover from the ST (semitransparent red) to the SV (semitransparent blue).
Table I shows a summary of implant type with surgical approach and electrode location. There was no statistically significant difference in the rates of C, ERW, and RW approaches between LW and PM electrode designs. However, a complete ST insertion was successfully achieved more frequently with LW electrodes than with the PM designs (89% vs. 58%; OR = 6.09, 95% c.i. = 2.15–17.29, p < 0.001). Evaluating surgical approach and intracochlear electrode location, both ERW and RW procedures had higher rates of complete ST insertion than did C procedures (C: 36.8%; ERW: 83.7%, OR = 8.82, OR 95% c.i. = 3.10–25.04; RW: 91.4%, OR = 18.29, OR 95% c.i. = 4.72–70.86, p < 0.001). This pattern held true regardless of whether the electrode array was LW or PM (Table II). Furthermore, an evaluation of a possible confounding effect of surgical experience on electrode placement revealed no statistically significant effect (P = 0.657).
TABLE I.
Implant Type, Surgical Approach, and Electrode Location.
| Total |
Lateral Wall |
Perimodiolar |
||
|---|---|---|---|---|
| (N = 116) |
(n = 47) |
(n = 69) |
||
| N (%) | n (%) | n (%) | P Value | |
| Surgical approach | ||||
| Cochleostomy | 38 (32.8) | 11 (23.4) | 27 (39.1) | 0.200 |
| Extended round window |
43 (37.1) | 20 (42.6) | 23 (33.3) | |
| Round window | 35 (30.0) | 16 (34.0) | 19 (27.5) | |
| Completely within the scala tympani? |
||||
| Yes | 82 (70.7) | 42 (89.4) | 40 (58.0) | <0.001 |
| No | 34 (29.3) | 5 (10.6) | 29 (42.0) |
TABLE II.
Surgical Approach, Implant Type, and Electrode Location.
| Total |
Cochleostomy |
ERW |
RW |
||
|---|---|---|---|---|---|
| (N = 116) |
(n = 38) |
(n = 43) |
(n = 35) |
||
| N (%) | n (%) | n (%) | n (%) | P Value | |
| Completely in the scala tympani? |
|||||
| Yes | 82 (70.7) | 14 (36.8) | 36 (83.7) | 32 (91.4) | <0.001 |
| No | 34 (29.3) | 24 (63.2) | 7 (16.3) | 3 (8.6) | |
| Within type of implant |
|||||
| LW Implant (N = 47) in ST? |
(n = 11) | (n = 20) | (n = 16) | ||
| Yes | 42 (89.4) | 7 (63.6) | 19 (95.0) | 16 (100.0) | 0.009 |
| No | 5 (10.6) | 4 (36.4) | 1 (5.0) | 0 (0.0) | |
| PM Implant (N = 69) in ST? |
(n = 27) | (n = 23) | (n = 19) | ||
| Yes | 40 (58.0) | 7 (25.9) | 17 (73.9) | 16 (84.2) | <0.001 |
| No | 29 (42.0) | 20 (74.1) | 6 (26.1) | 3 (15.8) |
Abbreviations: ERW = extended round window; LW = lateral wall; PM = perimodiolar; RW = round window.
Evaluating intracochlear electrode location and audiological outcome, no statistically significant differences in performance were observed on the Hearing in Noise Test (HINT) or Arizona Biomedical Sentence test (AzBio), however, there was a statistically significant difference in performance on consonant-nucleus-consonant (CNC) word recognition. Specifically, the group with electrode placement completely within the ST had higher mean CNC scores than did the group with placement of at least one electrode outside the ST (48.9% vs. 36.1%; P < 0.045). No statistically significant differences were found between the three device manufacturers with respect to rate of complete ST-electrode insertion or the audiometric performance when comparing LW electrodes; company-specific outcomes comparing PM designs could not be performed secondary to the small number of Advanced Bionics PM devices included and because MED-EL does not offer PM arrays (Table III). No statistically significant difference regarding etiology of hearing loss, age at implantation, or duration of deafness were observed with hearing performance; however, duration of implant experience was positively associated with the HINT and CNC scores (rs = 0.31, p = 0.035 and rs = 0.24, p = 0.025 respectively). After adjusting for association of electrode placement with CNC word scores, the effect size remained similar (unadjusted: B = −12.86, SE = 6.41,95% c.i. = −25.43 to −0.30; adjusted: B = −11.77, SE = 6.42, 95% c.i. = −24.35 to 0.81). However, it was no longer statistically significant (P = 0.067) due to the decrease in statistical power.
TABLE III.
Device Manufacturer, Electrode Location, and Hearing Outcomes for Lateral Wall Electrode Designs.
| Advanced Bionics Corp |
Cochlear Corp |
MED-EL GmbH |
||
|---|---|---|---|---|
| (N = 21) | (N = 10) | (N = 16) | P Value | |
| Completely in the scala tympani? |
n (%) | n (%) | n (%) | |
| Yes | 18 (85.7) | 9 (90.0) | 15 (93.8) | 0.733 |
| No | 3 (14.3) | 1 (10.0) | 1 (6.2) | |
| Hearing performance |
N, median IQR |
N, median IQR |
N, median IQR |
P Value |
| CNC word score (%) |
11, 48.0 | 4, 64.0 | 5, 44.0 | 0.457 |
| 34,58 | 35,70 | 19,57 | ||
| HINT (%)* | 12, 0.8 | 0, – | 6, 0.7 | 1.000 |
| 0,1 | – | 0,1 | ||
| AzBio (%) | 3, 0.7 | 4, 0.9 | 1, 0.6 | 0.089 |
| 0,– | 0,1 | 0,1 |
No Cochlear Corp implants had HINT scores available for review.
Abbreviations: AzBio = Arizona Biomedical sentences; CNC = consonant-nucleus-consonant word tests; HINT = Hearing in Noise Test; IQR = interquartile range.
DISCUSSION
Although still not conclusively proven, most agree that minimizing trauma during CI electrode insertion will result in improved audiological performance. As such, extensive effort has been focused on minimizing the identified mechanisms of mechanical trauma during electrode insertion—including fracture of the osseous spiral lamina, injury to the modiolus, compression or tearing of vasculature, and interscalar excursion from ST to SV.22 Histological and radiological studies have shown that most electrodes demonstrate a relatively straight midscalar course in the basal turn of the cochlea. However, upon reaching the first turn, electrodes frequently abut the lateral wall and advance toward the basilar membrane. If continued forces are applied while the electrode array comes in contact with the outer wall, the electrodes can displace the basilar membrane, fracture the interscalar partition, and cross from ST to SV. The deeper that the insertion is, the narrower will be the radius of curvature, the smaller will be the scalar cross section, and the larger will be the chance of impingement along the lateral wall.23 Not only do such injuries risk destruction of residual acoustic hearing, but fracturing of the interscalar partition can injure those neurosensory elements that are necessary for electric stimulation.
Whereas the overall numbers are small, there is mounting evidence suggesting that electrodes residing fully within the ST are associated with improved audiological outcomes compared to those that are partially or entirely located within the SV.12–16 We hypothesized that surgical approach would have an impact on intracochlear electrode location. Gantz et al. has previously shown that the site of cochleostomy is critical in avoiding trauma.24 It has been proposed that a cochleostomy located 1-mm anterior and inferior to the round window carries the least risk to injury to the spiral ligament and the interscalar partition. In the early years of CI surgery, RW techniques were replaced by cochleostomy approaches, given the improved visualization and midscalar trajectory obtained by a separate inferior placed entrance.23 However, within the last half decade there has been a renewed interest in RW insertion with the aim of decreasing insertion trauma. Addressing a potential criticism of RW insertion, Roland et al. demonstrated that drilling the bony overhang of the RW may increase its visualization by up to three times and allow the surgeon to insert the electrode along the midscalar axis, avoiding modiolar trauma.25
To date, no clinical study has demonstrated a statistical advantage with respect to hearing preservation that compares RW to C with standard-length electrodes. Furthermore, to the best of our knowledge, there are no clinical studies that have assessed the correlations between surgical approach and electrode location in living subjects. This paucity of data is in part due to the fact that few techniques exist for precisely assessing intracochlear location in vivo. Manual identification from high-resolution CT scans is difficult at best. To predict the postoperative location of CI electrodes, we have utilized intracochlear atlases, obtained from micro-CT scans of cadaveric temporal bones. These statistical predictions of intracochlear anatomy are rigidly and nonrigidly registered to clinically applicable postoperative CT scans allowing for the highly accurate prediction of intracochlear anatomy.18 The accuracy of this technique has been validated using anatomic microdissection.19
Our data demonstrates that higher rates of electrode placement completely within the ST were observed for LW rather than for PM electrodes (89% vs. 58%, P < 0.001). Additionally, C was associated with higher rates of electrode placement outside the ST than were either ERW or RW insertions (63% vs. 16% and 9%, respectively; P < 0.001). The latter pattern held true regardless of whether the electrode was a LW or PW design. These findings might be explained by the fact that cochleostomy location is variable even among experienced surgeons—and by the tremendous anatomical variability that exists from one cochlea to the next.17,18,26–28 Although most surgeons agree on an anteroinferior cochleostomy, there is no consistent extracochlear reference point that reliably predicts intracochlear anatomy.29,30 In our practice, during the time of study all surgeons utilized an anteroinferior C. Furthermore, when we analyzed outcomes of electrode placement between surgeons, we found no statistically significant differences suggesting that divergent surgical techniques are unlikely to explain the less favorable outcomes in patients receiving a C approach. In the current study as in others, no single technique or electrode design has resulted in consistent ST insertion. To achieve this, in the future we will likely depend on tailored surgical strategies based on preoperative imaging as well as intraoperative image guidance.
Regarding the correlation between electrode location and audiological outcome, a statistically significant mean improvement of 12.8% on CNC word scores was observed in subjects with complete ST insertions compared to those with partial or whole SV electrode placement. This finding was unexpected based on earlier analysis with more limited patient numbers.31 Recently, Holden et al. evaluated 114 postlingually deafened adults and found a positive correlation between CNC score and electrode location within the ST, depth of electrode insertion, and “warping factor.”15 They attributed the poorer audiological performance among SV insertions to cross-turn stimulation, which can lead to pitch confusion and diminished speech recognition.14,15 Several other studies have corroborated these findings.12,13,16
Duration of deafness has been shown to have a strong negative correlation with hearing outcome.4,5,32 In the current study, no statistically significant association with duration of deafness was observed with respect to hearing performance. This might be explained by the fact that our population was very homogeneous regarding duration of deafness with a median of 1 year. Also, we did not find a statistically significant association between patient age and CI performance—a finding that is in agreement with other studies.9,10,32,33 Duration of CI experience was positively associated with HINT and CNC scores (rs = 0.31, p = 0.035 and rs = 0.24, p = 0.025 respectively), indicating improvement with continued device use.
More and more studies are demonstrating the importance of preserving residual hearing during cochlear implantation.24,34–36 Carlson et al. found that patients with hearing preservation had significantly better postoperative speech-perception performance in the CI-only condition compared to those who lost residual hearing.34 Gifford et al. recently showed that cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments.36 Finally, Gantz et al. has shown that preserving acoustic hearing offers advantages in background noise and music appreciation.24,35
In the current study, intracochlear trauma—as evidenced by ST–SV crossover—was more common with C and PM electrodes than with ERW or RW techniques and LW electrode designs. Combined with the finding that patients with full ST insertions have statistically significantly better CNC word scores, this leads to our conclusion that—in the authors’ experience—ERW and RW techniques and LW electrode designs are associated with superior audiological outcomes.
It also must be explicitly stated that other confounding variables might account for the observations. As the insertion technique has evolved and surgeons learn from experience, it is likely that higher rates of complete ST insertion and avoidance of intracochlear trauma would occur, although the statistical analysis did not identify such a trend. Additionally, although the study was blinded by its very methodology (a member of the engineering team, without knowledge of the surgical approach that was used, analyzed the CT scans; and a member of the clinical team identified the surgical approach from the operative notes), bias cannot be completely excluded.
CONCLUSION
In the current study, the authors investigated associations between surgical approach, type of implant, electrode scalar location, and audiometric outcomes. Using a semiautomated, highly accurate atlas-based algorithm to determine CI electrode position in reference to the interscalar partition, our data indicate that LW implants are associated with higher rates of ST insertion compared to PM electrodes, and C independently is associated with a higher risk of interscalar excursion than with either RW or ERW approaches. In agreement with prior studies, we noted a positive correlation between CI electrode position and postoperative CNC word scores. Based on the current data, we recommend use of LW electrodes inserted via either ERW or RW approaches to maximize full ST insertion and optimize audiological outcome.
Acknowledgments
This project was supported by the National Institute on Deafness and Other Communication Disorders (R01DC008408: r.f.l.; R21DC012620: j.h.n.; R01DC009404: r.h.g.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health. d.s.h. is a consultant for Cochlear Corporation, Advanced Bionics Corporation, and MED-EL GmbH. r.f.l. is a consultant for Ototronix Corporation.
Footnotes
This article received the Mosher Award and will be presented at the Triological Society Section Meeting at the Combined Otolaryngology Spring Meeting, Las Vegas, NV, U.S.A., May 15, 2014.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24:582–589. doi: 10.1097/00129492-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Shipp DB, Nedzelski JM. Prognostic indicators of speech recognition performance in adult cochlear implant users: a prospective analysis. Ann Otolo Rhinol Laryngol Suppl. 1995;166:194–196. [PubMed] [Google Scholar]
- 3.Summerfield AQ, Marshall DH. Preoperative predictors of outcomes from cochlear implantation in adults: performance and quality of life. Ann Otolo Rhinol Laryngol Suppl. 1995;166:105–108. [PubMed] [Google Scholar]
- 4.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurotol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20:445–452. [PubMed] [Google Scholar]
- 6.Tong YC, Busby PA, Clark GM. Perceptual studies on cochlear implant patients with early onset of profound hearing impairment prior to normal development of auditory, speech, and language skills. J Acoust Soc Am. 1988;84:951–962. doi: 10.1121/1.396664. [DOI] [PubMed] [Google Scholar]
- 7.Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: a crossover study. Otol Neurotol. 2005;26:957–964. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- 8.Pfingst BE, Franck KH, Xu L, Bauer EM, Zwolan TA. Effects of electrode configuration and place of stimulation on speech perception with cochlear prostheses. J Assoc Res Otolaryngol. 2001;2:87–103. doi: 10.1007/s101620010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson ML, Breen JT, Gifford RH, et al. Cochlear implantation in the octogenarian and nonagenarian. Otol Neurotol. 2010;31:1343–1349. doi: 10.1097/MAO.0b013e3181edb69d. [DOI] [PubMed] [Google Scholar]
- 10.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131:1049–1054. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- 12.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 13.Aschendorff A, Kubalek R, Turowski B, et al. Quality control after coch-lear implant surgery by means of rotational tomography. Otol Neurotol. 2005;26:34–37. doi: 10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34:342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otolo Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 17.Adunka OF, Buchman CA. Scala tympani cochleostomy I: results of a survey. Laryngoscope. 2007;117:2187–2194. doi: 10.1097/MLG.0b013e3181453a6c. [DOI] [PubMed] [Google Scholar]
- 18.Noble JH, Labadie RF, Majdani O, Dawant BM. Automatic segmentation of intracochlear anatomy in conventional CT. IEEE Trans Biomed Eng. 2011;58:2625–2632. doi: 10.1109/TBME.2011.2160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope. 2010;120:2277–2283. doi: 10.1002/lary.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble JH, Schuman A, Wright CG, Labadie RF, Dawant BM. Automatic identification of cochlear implant electrode arrays for post-operative assessment. Proc SPIE Med Imaging. 2011;796217:1–10. doi: 10.1117/12.878490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 22.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- 23.Roland JT., Jr. A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325–1339. doi: 10.1097/01.mlg.0000167993.05007.35. [DOI] [PubMed] [Google Scholar]
- 24.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 25.Roland PS, Wright CG, Isaacson B. Cochlear implant electrode insertion: the round window revisited. Laryngoscope. 2007;117:1397–1402. doi: 10.1097/MLG.0b013e318064e891. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos P, Muren C. Anatomic variations of the cochlea and relations to other temporal bone structures. Acta Radiol. 1990;31:439–444. [PubMed] [Google Scholar]
- 27.Erixon E, Hogstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol. 2009;30:14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- 28.Escude B, James C, Deguine O, Cochard N, Eter E, Fraysse B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol. 2006;11(suppl 1):27–33. doi: 10.1159/000095611. [DOI] [PubMed] [Google Scholar]
- 29.Pelosi S, Noble JH, Dawant BM, Labadie RF. Analysis of inter-subject variations in promontory and intracochlear anatomy for cochelar implantation. Otol Neurotol. 2013;34:1675–1680. doi: 10.1097/MAO.0b013e3182a1a7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noble JH, Labadie RF, Wanna GB, Dawant BM. Image guidance could aid performance of atraumatic cochlear implantation surgical techniques. Proc SPIE Med Imaging. 2013;86711:1–7. [Google Scholar]
- 31.Wanna GB, Noble JH, McRackan TR, et al. Assessment of electrode placement and audiological outcomes in bilateral cochlear implantation. Otol Neurotol. 2011;32:428–432. doi: 10.1097/MAO.0b013e3182096dc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green KM, Bhatt Y, Mawman DJ, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007;8:1–11. doi: 10.1179/cim.2007.8.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Budenz CL, Cosetti MK, Coelho DH, et al. The effects of cochlear implantation on speech perception in older adults. J Am Geriatr Soc. 2011;59:446–453. doi: 10.1111/j.1532-5415.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 34.Carlson ML, Driscoll CL, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32:962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hear Res. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013;34:413–25. doi: 10.1097/AUD.0b013e31827e8163. [DOI] [PMC free article] [PubMed] [Google Scholar]