Abstract
Rationale
Modulation of the endocannabinoid system has been found to interfere with opiate withdrawal. The potential of activation and blockade of the endocannabinoid system to prevent the aversive-affective state of naloxone-precipitated morphine withdrawal (MWD) was investigated in a one-trial conditioned place aversion (CPA) paradigm.
Objective
CPA provides a sensitive measure of the motivational effects of acute MWD. The potential of the fatty acid amide hydrolase (FAAH) inhibitors, URB597 and PF-3845, the CB1 antagonist/inverse agonist, AM251, and the neutral CB1 antagonists, AM4113 and AM6527 (oral), to interfere with establishment of a MWD-induced CPA was investigated. As well, the potential of AM251 and AM4113 to interfere with reinstatement of a previously established MWD-induced CPA was investigated.
Materials and methods
Using a one-trial place conditioning paradigm, rats were administered naloxone (1 mg/kg, subcutaneous (sc)) 24 h after receiving a high dose of morphine (20 mg/kg, sc) and were placed on the conditioning floor. To determine the effect of each pretreatment drug on the establishment of the MWD-induced CPA, URB597 (0.3 mg/kg, intraperitoneally (ip)), PF-3845 (10 mg/kg, ip), AM251 (1 or 2.5 mg/kg, ip), AM4113 (1 or 2.5 mg/kg, ip), and AM6527 (5 mg/kg, oral) were administered prior to conditioning.
Results
AM251 (2.5, but not 1 mg/k), AM4113, and AM6527, but not URB597 or PF-3845, interfered with the establishment of the MWD-induced CPA. AM251 and AM4113 did not prevent reinstatement of the CPA.
Conclusions
Neutral antagonism of the CB1 receptor reduces the aversive affective properties of morphine withdrawal.
Keywords: Conditioning, Addiction, Dependence, Aversion, URB597, AM251, AM4113, FAAH, CB1 antagonist, Naloxone, Morphine withdrawal
Introduction
Of growing interest has been the involvement of the endocannabinoid system in the etiology and maintenance of opiate addiction. Considerable evidence suggests that CB1 receptor antagonists/inverse agonists (SR141716 or AM251) attenuate the rewarding effects of opioids in the self-administration (Caille and Parsons 2003; De Vries et al. 2003; Navarro et al. 2001; Solinas et al. 2003) and place conditioning paradigms (Chaperon et al. 1998; Mas-Nieto et al. 2001; Navarro et al. 2001; Singh et al. 2004). Furthermore, SR141716 dose-dependently (0.3–3 mg/kg) blocks reinstatement of previously extinguished heroin-seeking in rats following a priming dose of heroin (Fattore et al. 2003) and heroin-associated cues (De Vries et al. 2003). Consequently, it has been suggested that pharmacological blockade of the CB1 receptor with antagonists/inverse agonists may also have therapeutic value in the treatment of opiate addiction.
However, opiate addiction is a disorder characterized by both positive and negative reinforcement exerted through physiological and psychological processes (Koob and Le Moal 2001), and the role of manipulations of the endocannabinoid system on the aversive effects of morphine withdrawal (MWD) has been less well-investigated. The intensity of naloxone-precipitated MWD symptoms are significantly reduced in CB1 receptor knockout mice (Ledent et al. 1999; Lichtman et al. 2001). In agreement, chronic pharmacological blockade of the CB1 receptor with SR141716 during the concurrent development of morphine dependence reduced naloxone-precipitated MWD while having no effect on morphine analgesia (Mas-Nieto et al. 2001; Rubino et al. 2000). Somewhat paradoxically, however, THC (Bhargava 1976), anandamide (Vela et al. 1995), and 2-AG (Yamaguchi et al. 2001) have also been reported to reduce naloxone-precipitated MWD in opiate-dependent rodents. Most recently, Ramesh et al. (2011) reported that elevation of anandamide, by the fatty acid amide hydrolase (FAAH) inhibitor, PF-3845 (10 mg/kg, ip), attenuated symptoms of naloxone-precipitated MWD such as jumps and paw flutters (but not diarrhea or weight loss) in mice. All effects were reversed by SR141716. Therefore, the potential of CB1 agonists versus antagonists to reverse naloxone-precipitated MWD effects is somewhat controversial.
An alternative to the investigation of the effect of endocannabinoid manipulations on somatic symptoms of MWD is the investigation of the affective motivational symptoms of opiate withdrawal using the place conditioning paradigm. MWD symptoms may be precipitated by an opioid antagonist after one to two experiences with a high dose of morphine in humans (Heishman et al. 1990; June et al. 1995) or in rats (Meyer and Sparber 1977; McDonald et al. 1997; Parker and Joshi 1998; Parker et al. 2002). Indeed, when rats are injected with a single high dose of morphine (20 mg/kg subcutaneous (sc)), but not saline, 24–48 h prior to naloxone (1 mg/kg, sc), they display a robust conditioned place aversion (CPA; Parker et al. 2002). That is, although naloxone alone did not produce a CPA, naloxone 24–48 h after a prime with a high dose of morphine did produce a strong CPA. The CPA produced by acute naloxone-precipitated MWD is highly resistant to extinction (Manwell et al. 2009; McCallum et al. 2010), but once extinguished, the CPA can be reinstated by priming with naloxone-precipitated morphine withdrawal (Li et al. 2007; McCallum et al. 2010). Therefore, the paradigm lends itself to the investigation of the effects of manipulations of the endocannabinoid system on the establishment and reinstatement of the aversive effects of MWD.
Because of the controversial role of cannabinoid agonists and/or antagonists in the relief of somatic symptoms of naloxone-precipitated opiate withdrawal, the following experiments examined the potential of the FAAH inhibitors, URB597 and PF-3845, and CB1 antagonists to prevent the establishment and reinstatement of a place aversion. First, we evaluated the potential of the FAAH inhibitors URB597 and PF-3845, which neither produce place conditioning (Gobbi et al. 2005) nor increase the reinforcing effects of the opiate heroin (Solinas et al. 2005), to interfere with the affectively aversive effects of naloxone-precipitated MWD. Since FAAH inhibition failed to interfere with the CPA, the potential of the CB1 inverse agonist/antagonist AM251 to interfere with a single-cycle morphine withdrawal CPA was evaluated. Since CB1 inverse agonists/antagonists (SR141716 and AM251) have been shown to have adverse clinical side effects of nausea, anxiety, and depression (Bergman et al. 2008; Christensen et al. 2007; Sink et al. 2010), the neutral CB1 receptor antagonist, AM4113, was also assessed for its potential to interfere with the establishment of a CPA produced by naloxone-precipitated MWD. Finding both AM251 and AM4113 were successful in preventing establishment of the CPA, their potential to interfere with the reinstatement of an extinguished CPA by a naloxone-precipitated morphine prime was investigated, but such an effect was not revealed. To extend the clinical relevance of the findings, an orally bio-available CB1 neutral antagonist, AM6527, was evaluated for its potential to prevent establishment of a naloxone-precipitated MWD-induced CPA.
Materials and methods
Subjects
Subjects were 207 male Sprague-Dawley rats. Animals were housed individually in opaque shoebox cages with food and water ad libitum. They were maintained on a 12/12 h reverse light/dark schedule (lights off at 7 a.m.) with experiments being conducted during the dark cycle. The colony room in which the rats were held was kept at an ambient temperature of 21 °C. All animal procedures were approved by the Animal Care Committee of the University of Guelph and adhere to the guidelines of the Canadian Council of Animal Care.
Drugs
Morphine and naloxone were prepared with saline at a concentration of 20 and 1 mg/ml, respectively, and administered subcutaneously (sc) at a volume of 1 ml/kg. All cannabinoid-related compounds were dissolved in a vehicle mixture of ethanol, Tween 80, and physiological saline in a 1:1:18 ratio. The drugs were first dissolved in ethanol, then Tween 80 was added to the solution, and the ethanol was evaporated off with a nitrogen stream; after which, the saline was added. The final vehicle (VEH) consisted of 2:9 (Tween/saline). URB597 (0.3 mg/ml), PF-3845 (10 mg/ml; Ramesh et al. 2011), AM251 (1 and 2.5 mg/ml), and AM4113 (1 and 2.5 mg/ml) were administered intraperitoneally (ip) at a volume of 1 ml/kg. The doses of URB597 (0.3 mg/kg) and PF-3845 (10 mg/kg) were chosen on the basis of their ability to provide maximal inhibition of FAAH and concomitant elevation of AEA in rats when administered 2 h prior (Ahn et al. 2009; Fegley et al. 2005; Kathuria et al. 2003). AM6527 (2.5 mg/ml) was administered by oral gavage at a volume of 2 ml/kg (5 mg/kg). The dose of AM6527 was selected on the basis of a report by Sink et al. (2010) that when delivered orally, AM6527 was approximately half as potent as when delivered ip.
Apparatus
The conditioning boxes were rectangular (60×25×25 cm3) and made of black Plexiglas with a wire mesh lid (as previously described in Parker et al. 2004). During conditioning, single black metal floors made of a grid or hole pattern were used as contextual cues. During pretest, test, and reinstatement trials, split black metal floors equally divided into a half grid/half hole pattern were used. A camera mounted (1.5 m) directly over top of the boxes and fire-wired to a computer recorded their movement. EthoVision software was used to define box perimeters and assign a neutral floor zone for pretest, test, and reinstatement trials. All movement was tracked using EthoVision software.
Procedure
Prior to all experiments, rats received a 15-min pretest using the split grid/hole floor to detect any floor biases. EthoVision software tracked their movement and measured the time spent on each floor for the duration of the pretest. Rats were then assigned to a pretreatment drug group and drug floor (grid, hole; being the floor which would be paired with withdrawal) matched on the basis of initial pretest preferences. Rats with a bias of more than 250 s for either floor were removed.
Experiment 1: effect of URB597 (1A) and PF-3845 (1B) on the establishment of an acute naloxone-precipitated MWD-induced CPA
A 3-day conditioning cycle was used to obtain the naloxone-precipitated MWD-induced CPA. The rats received the appropriate pretreatment injection 2 h prior to both the saline conditioning trial (day 1) and the naloxone-precipitated morphine withdrawal conditioning trial (day 3) to ensure any difference in the amount of time spent on the drug floor at test was not due to any rewarding or aversive effects of the pretreatment drug itself. On the first day, the floor opposite the assigned drug floor was paired with a sc saline injection. Rats were administered VEH, 0.3 mg/kg URB597, or 10 mg/kg PF-3845 by ip injection 2 h prior to 1 ml/kg sc saline. Ten minutes later, each rat was placed on the saline-paired floor for 45 min in the conditioning box, and EthoVision recorded their movements. On the second day, 24 h post-saline injection, all rats received a high dose of morphine (20 mg/kg, sc) and were placed in an empty shoebox cage. The rats were monitored for signs of respiratory distress and returned to their home cage once fully ambulatory. On the third day, 24 h post-morphine, the floor assigned as the MWD floor was paired with a sc naloxone injection. As on the saline trial, rats received VEH, URB597, or PF-3845 by ip injection 2 h prior to 1 mg/kg sc naloxone. Ten minutes later, all rats were placed on the MWD-paired floor for 45 min, and EthoVision tracked their movement. The final groups were as follows: experiment 1A: VEH (n=12), URB597 (n=12); experiment 1B: VEH (n=12), PF-3845 (n=12). Five days later, all rats were given daily 15-min test trials with the split grid/hole floor for 3 days. On each occasion, rats received a sc injection of saline in the conditioning room 10 min prior to test. EthoVision software tracked their movement and measured the time spent on each floor for the duration of the test.
Experiment 2: effect of AM251 and AM4113 on the establishment of an acute naloxone-precipitated MWD-induced CPA
As in experiment 1, a 3-day conditioning cycle was used to obtain the naloxone-precipitated morphine withdrawal CPA. On days 1 and 3, the rats received the appropriate pretreatment drug 30 min prior to the saline or naloxone injection. The pretreatment conditions were as follows: VEH (n=10), 1 mg/kg AM251 (n=12), 2.5 mg/kg AM251 (n=8), 1 mg/kg AM4113 (n=12), or 2.5 mg/kg AM4113 (n=9). As in experiment 1, EthoVision software tracked the total distance moved during each conditioning trial. Beginning 5 days after conditioning, rats received daily 15-min test trials with the split grid/hole floor for 3 days as described in experiment 1.
Experiment 3: effect of AM251 and AM4113 on reinstatement of a CPA by a naloxone-precipitated MWD prime
As in experiment 1, a 3-day conditioning cycle was used to obtain the naloxone-precipitated morphine withdrawal CPA; however, no pretreatment drugs were administered during conditioning. Beginning 5 days after conditioning, rats received daily 15-min test trials with the split grid/hole floor until the place aversion extinguished (6 days). On each occasion, rats received a sc injection of saline in the conditioning room 10 min prior to test. Since the experiment aimed to evaluate the potential of the CB1 antagonist to interfere with reinstatement of a previously established CPA, rats with an aversion of less than 120 s for the MWD-paired floor on the first test trial were removed from the experiment. Test trials continued until the CPA extinguished (defined by lack of significant (p>0.05) paired t tests) for two consecutive days.
A week following the last extinction trial, the rats were tested for reinstatement of the CPA. On reinstatement day 1, they received a saline prime test. On day 2, they were injected sc with 20 mg/kg morphine in their home cage. On day 3, they received the naloxone-precipitated MWD prime test (1 mg/kg naloxone, sc). On both day 1 and day 3, the rats were injected ip with VEH (n=17), 2.5 mg/kg AM251 (n=15), or 2.5 mg/kg AM4113 (n=16) 30 min prior to saline or naloxone which was given 10 min prior to the 15-min test. EthoVision software tracked their total distance moved and the time spent on each floor for the duration of the test.
Experiment 4: effect of AM6527 on establishment of a CPA
As in experiment 1, a 3-day conditioning cycle was used to obtain the naloxone-precipitated morphine withdrawal CPA. Rats received the appropriate pretreatment drug (VEH, 5 mg/kg AM6527) 1 h prior to both the saline conditioning trial (day 1) and the naloxone-precipitated morphine withdrawal conditioning trial (day 3). EthoVision software tracked the total distance moved during each trial. The groups were as follows: VEH (n=11) and AM6527 (n=12). Beginning 5 days after conditioning, rats received daily 15-min test trials with the split grid/hole floor for 3 days as described in experiment 1.
Data analysis
For each experiment aimed at assessing the effect of the pretreatment drug on the establishment of naloxone-precipitated MWD-induced CPA, the number of seconds spent by each rat on the saline-paired floor and on the MWD-paired floor was entered into a mixed factor analysis of variance (ANOVA) with the within group factor of floor (saline/MWD) and the between groups factor of drug (VEH and appropriate compound (s)) and test trial (1–3). Subsequent main effects analyses were conducted as appropriate. Statistical significance was set at p<0.05.
Results
Experiment 1: effect of URB597 (experiment 1A) or PF-3845 (experiment 1B) on the establishment of a naloxone-precipitated MWD-induced CPA
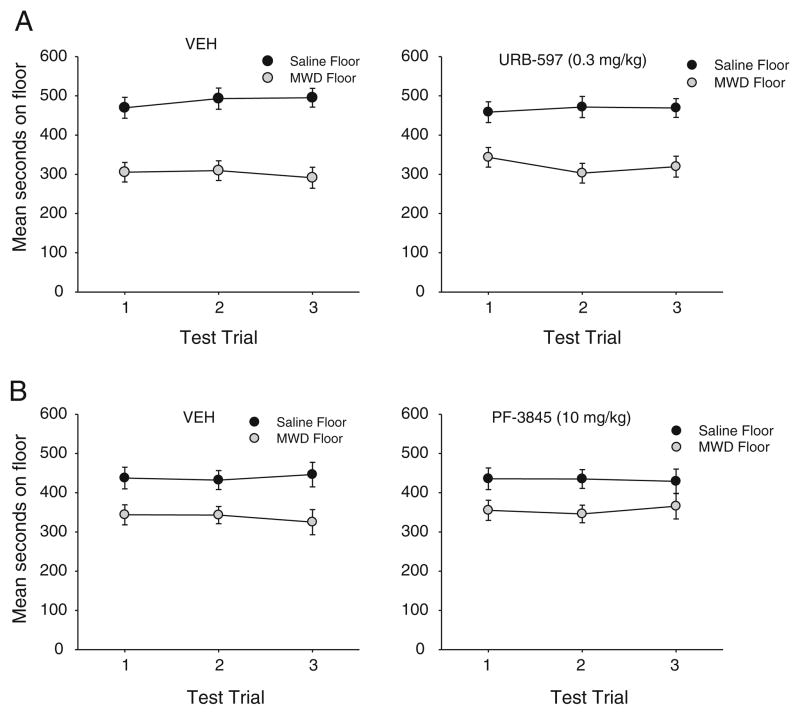
FAAH inhibition did not modify the strength of a naloxone-precipitated MWD-induced place aversion. As is evident in Fig. 1a, b, neither URB597 nor PF-3845 prevented the CPA. A 2×2×3 mixed factors ANOVA for experiment 1A with URB597 and for experiment 1B with PF-3845 revealed only a significant main effect of floor (experiment 1A: F (1, 22)=30.4; p<0.001; experiment 1B: F (1, 22)=7.1; p=0.014), but no significant floor by drug interactions. Overall, the rats displayed a CPA following a single pairing with naloxone-precipitated MWD, but FAAH inhibition did not attenuate that aversion.
Fig. 1.
Mean (±sem) time spent in seconds on the saline-paired floor and the MWD-paired floor for experiment 1A (VEH, 0.3 mg/kg URB597) and experiment 1B (VEH, 10 mg/kg PF-3845) during each 15-min test trial
Experiment 2: effect of AM251 and AM4113 on the establishment of a naloxone-precipitated MWD-induced CPA
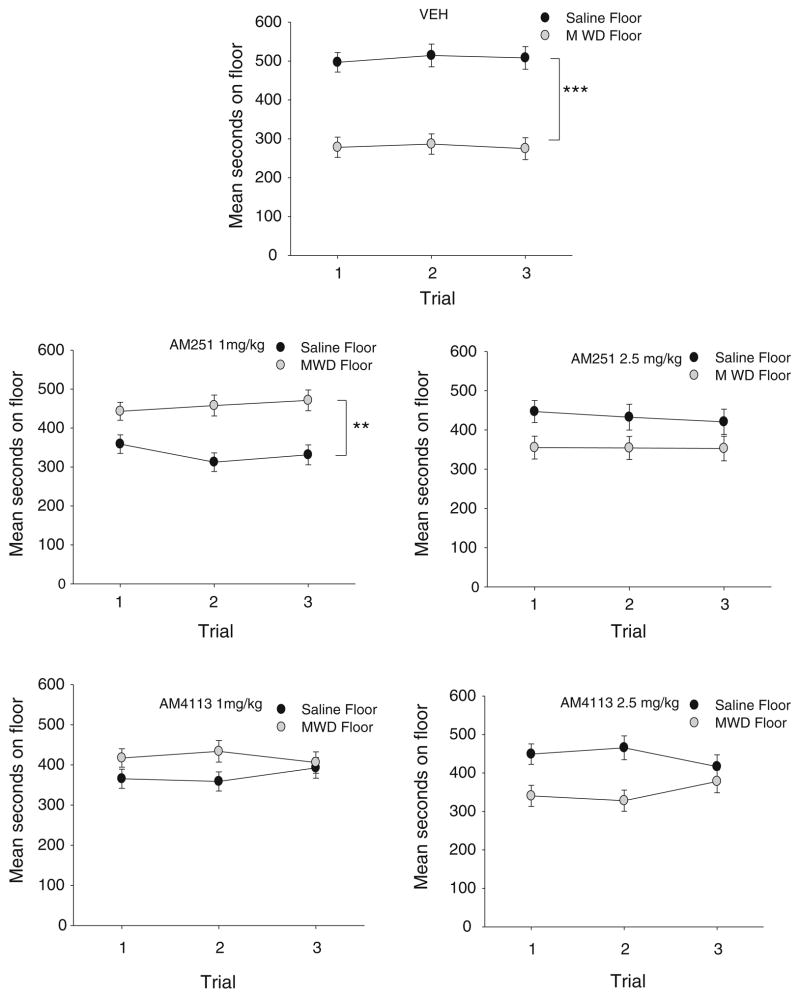
AM251 (at 2.5 mg/kg, but not 1 mg/kg) and AM4113 (at both 1 and 2.5 mg/kg) interfered with the establishment of the naloxone-precipitated MWD-induced CPA. Figure 2 presents the mean (±sem) number of seconds spent on the saline-paired and the MWD-paired floor during each test trial for each pretreatment group. The 5×2×3 mixed factor ANOVA with the between group factors of pretreatment drug (VEH, 1 mg/kg AM251, 2.5 mg/kg AM251, 1 mg/kg AM4113, 2.5 mg/kg AM251) and the within group factors of floor and trials revealed only significant effects of floor, F (1, 46)=38.5; p<0.001, and a drug by floor interaction F (4, 46)=2.9; p=0.032. Because of the lack of an effect of trials (or interaction with any other factor), the pooled number of seconds on the MWD-paired floor and on the saline-paired floor for each drug condition was analyzed as a paired t test. Overall, rats pretreated with VEH (p<0.001) and 1 mg/kg AM251 (p<0.01) spent significantly less time on the MWD-paired floor than the saline-paired floor; however, the rats pretreated with 2.5 mg/kg AM251, 1 mg/kg AM4113, or 2.5 mg/kg AM4113 did not show a CPA. Evaluation of the total distance moved on the saline conditioning trial revealed no significant differences in motor activity between the pre-treatment drugs.
Fig. 2.
Mean (±sem) time spent in seconds on the saline-paired floor and the MWD-paired floor for each pretreatment drug (VEH, 1 mg/kg AM251, 2.5 mg/kg AM251, 1 mg/kg AM4113, 2.5 mg/kg AM4113) during each 15-min test trial in experiment 2. Asterisks indicate a significant difference between the saline- and morphine withdrawal-paired floors. ***p<0.001
Experiment 3: effect of AM251 and AM4113 on reinstatement of the CPA by a naloxone-precipitated MWD prime
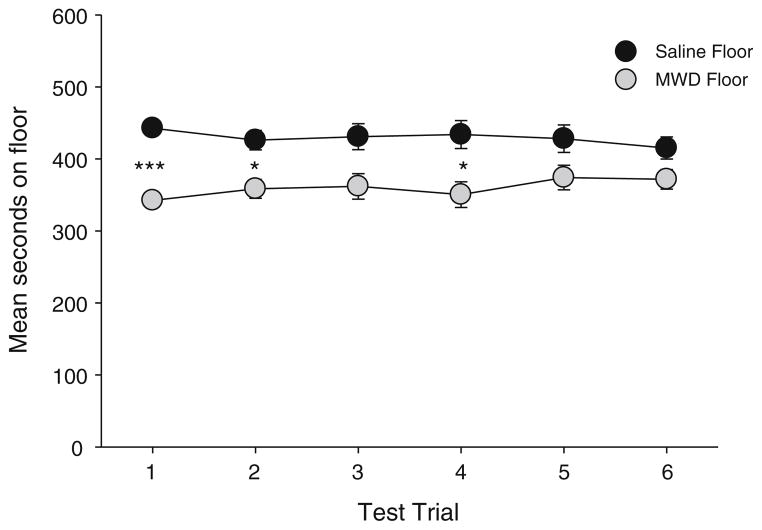
The naloxone-precipitated MWD-induced CPAwas reinstated by the prime following extinction; however, neither AM251 nor AM4113 interfered with or potentiated the reinstatement of the CPA. Figure 3 presents the floor aversion on the test trials for the naloxone-precipitated MWD-induced CPA. The rats displayed a significant CPA as assessed by paired t tests on test days 1 (p<0.001), 2 (p=0.013), and 4 (p=0.027), but not on days 3, 5, or 6.
Fig. 3.
Mean (±sem) time spent in seconds on the saline-paired floor and the MWD-paired floor during each 15-min test trial in experiment 3. Asterisks indicate a significant difference between the saline- and MWD-paired floors.*p<0.05, ***p<.001
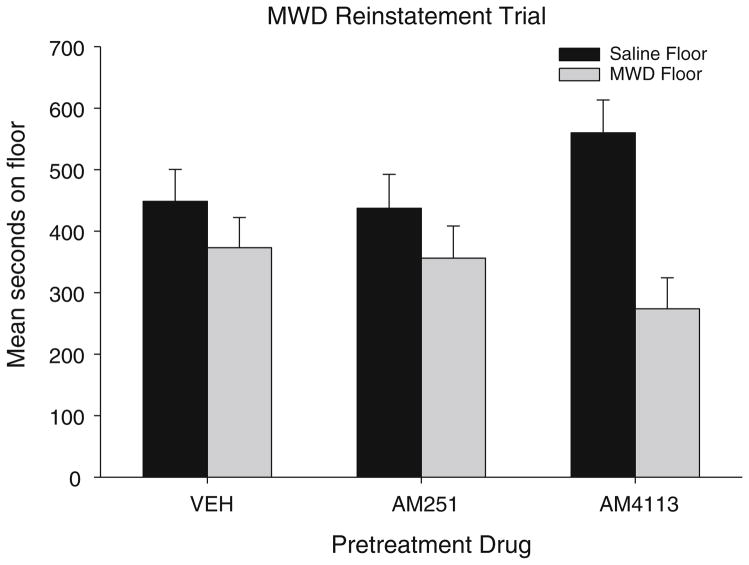
Figure 4 presents floor preferences on the naloxone-precipitated MWD reinstatement trial that occurred 9 days following the last extinction trial. On both the saline trial (not depicted) and MWD trial, the rats were pretreated with VEH, AM251, or AM4113 30 min before the test. On the saline trial, a 2×3 mixed factor ANOVA with the within group factor of floor (saline-paired, MWD-paired) and the between group factor of pretreatment drug (VEH, AM251, AM4113) revealed no significant differences. On the MWD reinstatement trial, the 2×3 mixed factor ANOVA revealed only a significant main effect of floor, F (1, 45)=6.1; p=0.02, but no interaction. Rats spent significantly less time on the MWD-paired floor than the saline-paired floor; however, this aversion was not modified by the pretreatment drug. Evaluation of the total distance moved on the saline reinstatement trial revealed no significant differences in motor activity between the pretreatment drugs.
Fig. 4.
Mean (±sem) time spent in seconds on the saline-paired floor and the MWD-paired floor for each pretreatment drug (VEH, 2.5 mg/kg AM251, 2.5 mg/kg AM4113) during the 15-min MWD reinstatement trial in experiment 3
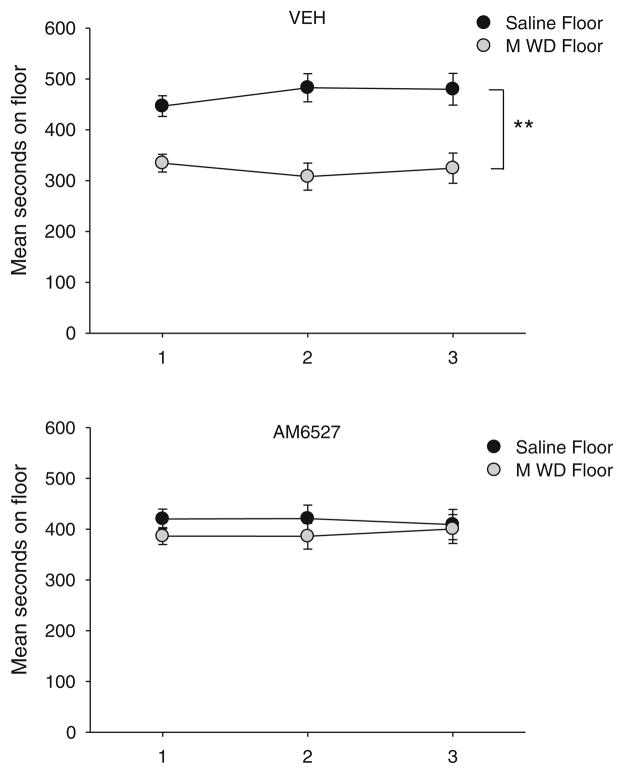
Experiment 4: effect of AM6527 on establishment of a naloxone-precipitated MWD-induced CPA
AM6527 interfered with the establishment of the naloxone-precipitated MWD-induced CPA. The floor preferences on the test trials for establishment of the naloxone-precipitated MWD-induced CPA are found in Fig. 5. The 2×2×3 mixed factor ANOVA with the between group factors of pretreatment drug (VEH, AM6527) and the within group factor of floor (saline-paired, MWD-paired) and trial revealed a significant main effect of floor, F (1, 21)=9.6; p=0.005, and a significant drug by floor interaction, F (1, 21)=4.7; p=0.04. To analyze the interaction, a paired t test pooled across trials revealed that rats pretreated with VEH (p<0.01), but not AM6527, spent significantly less time on the MWD-paired floor than the saline paired floor. Evaluation of the total distance moved on the saline conditioning trial and the MWD conditioning trial by an independent samples t test revealed a significant difference in motor activity between the pretreatment drugs on both the saline conditioning trial, t(21)=2.9; p<0.01 (mean (±sem) in centimeters: VEH=9,961 (±638); AM6527=6,749 (±162)) and on the MWD conditioning trial, t(21)=2.1; p=0.049 (mean (+sem) cm: VEH=5,346 (±385); AM6527=4,447 (±157)). Oral administration of 5 mg/kg of AM6527 not only interfered with the establishment of the CPA but also suppressed locomotor activity relative to VEH during conditioning.
Fig. 5.
Mean (±sem) time spent in seconds on the MWD-paired floor for each pretreatment drug (VEH, 5 mg/kg AM6527 po) during each 15-min test trial in experiment 4. Asterisks indicate a significant difference between the saline- and MWD-paired floors. **p<0.01
Discussion
The present findings are the first to show that antagonism of the CB1 receptor is capable of interfering with the acquisition of the motivationally aversive state of acute morphine dependence as quantified by the place conditioning paradigm. Specifically, rats having received AM251 (at 2.5, but not 1 mg/kg), AM4113 (at both 1 and 2.5 m/kg), or oral AM6527 (at 5 mg/kg) prior to conditioning did not show a one-trial naloxone-precipitated MWD-induced CPA. Only orally administered AM6527 also suppressed locomotor activity during conditioning. These findings are in agreement with prior studies demonstrating the ability of antagonism of the endocannabinoid system to attenuate opioid self-administration (Caille and Parsons 2003; De Vries et al. 2003; Navarro et al. 2001; Solinas et al. 2003) and conditioned place preference (Chaperon et al. 1998; Mas-Nieto et al. 2001; Navarro et al. 2001; Singh et al. 2004). Interestingly, however, although antagonism of the endocannabinoid system with the CB1 antagonist SR141716 has been shown to block reinstatement of opioid drug-seeking (De Vries et al. 2003; Fattore et al. 2003), the current findings suggest that this phenomenon may be exclusive to the rewarding properties of opioids. Indeed, following establishment and extinction of the CPA, none of the antagonists tested interfered with (or potentiated) reinstatement of the aversion. The apparent dissociations between reinstatement of CPP and CPA, and the establishment and reinstatement of the CPA found in the present study, suggest that each of these processes may be engaging distinct brain regions or a combination of distinct brain regions.
Although the manifestation of withdrawal is associated with changes in the cyclic adenosine monophosphate (cAMP) pathway (Nestler and Aghajanian 1997), it is unlikely that attenuation of the establishment of the CPA was mediated by an inhibition of intrinsic cellular activity and increased expression of cAMP since the inverse agonist, AM251, and the neutral antagonists, AM4113 and AM6527, were all effective in attenuating establishment of the CPA. As previously noted, neutral antagonists have been found to lack such effects on intrinsic cellular activity (Chambers et al. 2007). This suggests that the present findings may be attributed solely to the blockade of endocannabinoid binding, although the specific neurons and brain circuits involved in mediating these effects remain to be elucidated.
Somewhat surprisingly, although consistent with the present findings implicating the efficacy of CB1 receptor antagonism in preventing establishment of the morphine withdrawal CPA, the FAAH inhibitors, URB597 and PF-3845, did not interfere with establishment of the CPA. This finding is inconsistent with prior studies demonstrating the ability of FAAH inhibitors to block naloxone-precipitated somatic withdrawal symptoms in morphine-dependent mice (Ramesh et al. 2011). Several factors could contribute to these discrepant findings including the type of species used (mice vs. rats), precipitation from chronic vs. acute dependence, and the brain regions involved in the manifestation of physical vs. motivational morphine withdrawal. Indeed, a dissociation between the brain regions involved in mediating physical and motivational opiate dependence has been described with regions such as the periaqueductal gray (Wei et al. 1972, 1973), dorsal thalamus (Bozarth and Wise 1984), and locus coeruleus (Maldonado et al. 1992) responsible for mediating physical aspects of withdrawal, and the nucleus accumbens and amygdala (Stinus et al. 1990) identified as important in mediating the motivational or emotional aspects of withdrawal. Recently, Trang et al. (2006) also implicated the depletion of calcitonin-gene-related peptide in the spinal cord to the manifestation of opioid physical dependence, for which endocannabinoid tone was shown to help mediate. Therefore, it is possible that the brain regions involved in physical withdrawal are more sensitive to modulation of endocannabinoid tone than those implicated in motivational withdrawal.
To date, two brain regions have been identified as having a role in the manifestation of motivational opioid withdrawal, the nucleus accumbens (NAc) and the extended amygdala (Gracy et al. 2001; Koob 2009; Stinus et al. 1990; Valverde et al. 1996). It is well-known that increased dopamine within the NAc plays an important role in mediating the rewarding effects of a variety of drugs of abuse, including opiates. Specifically, opiates such as morphine bind to presynaptic mu-opioid receptors on GABAergic neurons in the ventral tegmental area (VTA) where they inhibit GABA release onto dopaminergic neurons that project to the NAc, resulting in an increased release of dopamine (Ford et al. 2006; Johnson and North 1992; Madhavan et al. 2010; Shoji et al. 1999). Similarly, just as elevation of dopamine in the NAc is responsible for the rewarding effects of morphine, disruption of the dopaminergic system may be responsible for mediating the aversive effects of morphine, termed a within-system neuroadaptation (Koob 2009). Within the mesolimbic dopamine system, cannabinoid receptors have been found to be located predominantly on presynaptic GABAergic and glutamatergic neurons where their activation inhibits neurotransmitter release (Maldonado et al. 2006). Through these mechanisms, endocannabinoids have been found to indirectly modulate dopamine transmission by inhibiting GABA release onto dopaminergic neurons in the VTA in a manner similar to morphine. Additionally, endocannabinoids also act on glutamatergic neurons within the NAc itself, inhibiting the release of glutamate projecting to GABAergic neurons regulating dopaminergic neuronal activity in the VTA (Maldonado et al. 2006). As a result, contrary to our findings, it would be expected that antagonism of the endocannabinoid system within the NAc would result in a decrease of dopamine release and the manifestation of an aversive affective state. Therefore, it is unlikely that the attenuation of a morphine withdrawal-induced CPA by the CB1 antagonist is mediated by its action within the NAc. Indeed, Hou et al. (2009) have reported that lesions to the NAc were unable to impair CPA in acute morphine-dependent rats. However, before endocannabinoid blockade within the NAc can be ruled out, it should be noted that there is evidence to suggest that CB1 and mu-opioid receptors within the NAc core may allosterically interact through G-protein coupled heterodimeric receptor complexes to block the effects of concurrent antagonist treatments (Schoffelmeer et al. 2006). Indeed, consistent with the present findings, Schoffelmeer et al. (2006) reported that SR141617 blocked the antagonistic effect of naloxone at mu-opioid receptors, and similarly, naloxone prevented the antagonistic action of SR141716 at CB1 receptors regulating inhibition of GABA and glutamate release from superfused NAc core slices. However, further studies are required in order to establish unequivocal evidence.
A second brain region attributed to mediating the aversive effects of opioid withdrawal is the extended amygdala. This entity comprising the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and a transition zone in the nucleus accumbens shell, has been implicated in mediating the negative effects on reward termed “antireward” which is based on the hypothesis that brain stress systems are recruited to limit reward function and maintain a state of hedonic homeostasis, an example of a between-system neuroadaptation (Koob and Le Moal 1997, 2001; Koob 2009). The central nucleus of the amygdala (CeA), in particular, has been identified as having a central role in the establishment of naloxone-precipitated morphine withdrawal-induced CPA, as lesions to this brain region result in impaired acquisition of the CPA (Watanabe et al. 2002; Xu et al. 2012). Furthermore, increases in c-Fos expression in the CeA have been shown to parallel the development of CPA in acute and chronic morphine-dependent rodents (Frenois et al. 2002, 2005; Ishida et al. 2008; Jin et al. 2004, 2005). Antagonism of the endocannabinoid system has also been found to attenuate the anxiogenic effects produced by systemic naloxone when administered directly to the CeA (Zarrindast et al. 2008), providing evidence not only for a role of the CeA in mediating the aversive effect of opioids but also for a role of blockade of the endocannabinoid system to ameliorate its occurrence. Finally, in agreement with the inability of CB1 antagonists to prevent reinstatement of a CPA, the involvement of the CeA in withdrawal-induced CPA has been limited to its acquisition, suggesting that reinstatement of the CPA may be mediated by a different brain region.
In conclusion, the present findings provide additional evidence for the ability of the cannabinoid system to modulate opiate addiction processes. Specifically, blockade of the CB1 receptor, but not an increase in anandamide through inhibition of FAAH, is able to prevent the motivationally aversive effects of acute MWD. Furthermore, the ability of AM4113 and AM6527 to effectively attenuate establishment of the naloxone-precipitated MWD-induced CPA suggests that neutral antagonism of the CB1 receptor is sufficient in mediating the effects, providing potential for neutral CB1 antagonists in the treatment of opiate dependence.
Acknowledgments
We would like to thank Dr. Roger Pertwee, University of Aberdeen, for helpful discussions about the data presented in the manuscript. KLW was supported by a CGS Scholarship from NSERC. The research was supported by research grants to LAP from NSERC (92057) and to A. Makriyannis from NIH.
Contributor Information
Kiri L. Wills, Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON N1G 2W1, Canada
Kiran Vemuri, Center for Drug Discovery, Northeastern University, Boston, MA, USA.
Alana Kalmar, Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON N1G 2W1, Canada.
Alan Lee, Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON N1G 2W1, Canada.
Cheryl L. Limebeer, Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON N1G 2W1, Canada
Alexandros Makriyannis, Center for Drug Discovery, Northeastern University, Boston, MA, USA.
Linda A. Parker, Email: parkerl@uoguelph.ca, Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON N1G 2W1, Canada
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16(4):411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93:666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN. Inhibition of naloxone-induced withdrawal in morphine dependent mice by 1-trans-Δ9-tetrahydrocannabinol. Eur J Pharmacol. 1976;36(1):259–262. doi: 10.1016/0014-2999(76)90283-1. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–3149. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri KV, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berlin) 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmer ANM. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology. 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3- carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313(1):352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Cador M, Caillé S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005;25(6):1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Manqieri R, Bortalato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain mono-aminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy NK, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place a version. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: effect of naloxone at 6 and 24 hr postmorphine. Pharmacol Biochem Behav. 1990;36:393–399. doi: 10.1016/0091-3057(90)90421-d. [DOI] [PubMed] [Google Scholar]
- Hou YY, Lu B, Li M, Liu Y, Chen J, Chi ZQ, Liu JG. Involvement of actin rearrangement within the amygdala and the dorsal hippocampus in aversive memories of drug withdrawal in acute morphine-dependent rats. J Neurosci. 2009;29(39):12244–12254. doi: 10.1523/JNEUROSCI.1970-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Shimosaka R, Kawasaki Y, Jin C, Kitamura Y, Araki H, Sendo T, Gomita Y. Involvement of the amygdala on place aversion induced by naloxone in single-dose morphine-treated rats. Yakugaku Zasshi. 2008;128(3):395–403. doi: 10.1248/yakushi.128.395. [DOI] [PubMed] [Google Scholar]
- Jin C, Araki H, Nagata M, Suemaru K, Shibata K, Kawasaki H, Hamamura T, Gomita Y. Withdrawal-induced c-Fos expression in the rat centromedial amygdala 24 h following a single morphine exposure. Psychopharmacology. 2004;175:428–435. doi: 10.1007/s00213-004-1844-4. [DOI] [PubMed] [Google Scholar]
- Jin C, Araki H, Nagata M, Shimosaka R, Shibata K, Suemaru K, Kawasaki H, Gomita Y. Expression of c-Fos in the rat central amygdala accompanies the acquisition but not expression of conditioned place aversion induced by withdrawal from acute morphine dependence. Behav Brain Res. 2005;161:107–112. doi: 10.1016/j.bbr.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Stitzer ML, Cone E. Acute physical dependence: time course and relation to human plasma morphine concentrations. Clin Pharmacol Ther. 1995;57:270–280. doi: 10.1016/0009-9236(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana GL, Calignano A, Guistino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu X, Chen H, Deng H, Xiang X, Chen H, Hao W. Development, extinction and reinstatement of morphine withdrawal-induced conditioned place aversion in rats. Addict Biol. 2007;12:470–477. doi: 10.1111/j.1369-1600.2007.00059.x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in Δ9-tetrahy-drocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- Madhavan A, Bonci A, Whistler JL. Opioid-induced GABA potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci. 2010;30:14029–14035. doi: 10.1523/JNEUROSCI.3366-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. FAAH inhibitor, URB- 597, promotes extinction and CB1 antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav. 2009;94:154–162. doi: 10.1016/j.pbb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, Roques BP, Noble F. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132(8):1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum AL, Limebeer CL, Parker LA. Reducing endocannabinoid metabolism with the fatty acid amide hydrolase inhibitor, URB597, fails to modify reinstatement of morphine-induced conditioned floor preference and naloxone-precipitated morphine withdrawal-induced conditioned floor avoidance. Pharmacol Biochem Behav. 2010;96:496–500. doi: 10.1016/j.pbb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- McDonald RV, Parker LA, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav. 1997;58:1003–1008. doi: 10.1016/s0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- Meyer DR, Sparber SB. Evidence of possible opiate dependence during the behavioral depressant action of a single dose of morphine. Life Sci. 1977;73:1087–1094. doi: 10.1016/0024-3205(77)90106-0. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gómez R, del Arco I, Villanúa MA, Maldonado R, Koob GF, Rodríguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Parker LA, Joshi A. Naloxone-precipitated morphine withdrawal induced place aversions: effects of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav. 1998;61(3):331–333. doi: 10.1016/s0091-3057(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Parker LA, Cyr JA, Santi AN, Burton PD. The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine: evaluation by taste and place conditioning. Pharmacol Biochem Behav. 2002:7287–7292. doi: 10.1016/s0091-3057(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of Δ9- tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology. 2004;175:360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339:173–186. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Massi P, Vigano’ D, Fuzio D, Parolaro D. Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrome. Life Sci. 2000;66(22):2213–2219. doi: 10.1016/s0024-3205(00)00547-6. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Delfs J, Williams JT. Presynaptic inhibition of GABA(B)-mediated synaptic potentials in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:2347–2355. doi: 10.1523/JNEUROSCI.19-06-02347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ME, Verty AN, McGregor IS, Mallet PE. A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004;1026:244–253. doi: 10.1016/j.brainres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, Markus EJ, Vemuri VK, Makriyannis A, Salamone JD. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol. 2010;20:112–122. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methylpyrazole-3- carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and the amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37(3):767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Trang T, Ma W, Chabot JG, Quirion R, Jhamandas K. Spinal modulation of calcitonin gene-related peptide by endocannabinoids in the development of opioid physical dependence. Pain. 2006;126:256–271. doi: 10.1016/j.pain.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Valverde O, Tzavara E, Hanoune J, Roques BP, Maldonado R. Protein kinases in the rat nucleus accumbens are involved in the aversive component of opiate Withdrawal. Eur J Neurosci. 1996;8:2671–2678. doi: 10.1111/j.1460-9568.1996.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Vela G, Ruiz-Gayo M, Fuentes JA. Anandamide decreases naloxone precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology. 1995;34(6):665–668. doi: 10.1016/0028-3908(95)00032-2. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M. Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res. 2002;958:423–428. doi: 10.1016/s0006-8993(02)03468-6. [DOI] [PubMed] [Google Scholar]
- Wei E, Loh HH, Way EL. Brain sites of precipitated abstinence in morphine dependent rats. J Pharmacol Exp Ther. 1972;185:108–115. [PubMed] [Google Scholar]
- Wei E, Loh HH, Way EL. Neuroanatomical correlates of wet shake behavior in the rat. Life Sci. 1973;12(Part II):489–496. doi: 10.1016/0024-3205(73)90342-1. [DOI] [PubMed] [Google Scholar]
- Xu W, Li YH, Tan BP, Luo XJ, Xiao L, Zheng XG, Yang XY, Sui N. Inhibition of the acquisition of conditioned place aversion by dopaminergic lesions of the central nucleus of the amygdala in morphine-treated rats. Physiol Res. 2012;61:437–442. doi: 10.33549/physiolres.932273. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hagiwara Y, Tanaka H, Sugiura T, Waku K, Shoyama Y, Watanabe S, Yamamoto T. Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res. 2001;909:121–126. doi: 10.1016/s0006-8993(01)02655-5. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A. Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behav Pharmacol. 2008;19:716–72. doi: 10.1097/FBP.0b013e3283123c83. [DOI] [PubMed] [Google Scholar]