Abstract
Background
Mixed cannabinoid CB1/CB2 agonists such as Δ9-tetrahydrocannabinol (Δ9-THC) can produce tolerance, physical withdrawal, and unwanted CB1-mediated central nervous system side effects. Whether repeated systemic administration of a CB2-preferring agonist engages CB1 receptors or produces CB1-mediated side effects is unknown.
Methods
We evaluated anti-allodynic efficacy, possible tolerance, and cannabimimetic side effects of repeated dosing with a CB2-preferring agonist AM1710 in a model of chemotherapy-induced neuropathy produced by paclitaxel using CB1KO, CB2KO, and WT mice. Comparisons were made with the prototypic classical cannabinoid Δ9-THC. We also explored the site and possible mechanism of action of AM1710.
Results
Paclitaxel-induced mechanical and cold allodynia developed equivalently in CB1KO, CB2KO, and WT mice. Both AM1710 and Δ9-THC suppressed established paclitaxel-induced allodynia in WT mice. Unlike Δ9-THC, chronic AM1710 did not engage CB1 activity or produce antinociceptive tolerance, CB1-mediated cannabinoid withdrawal, hypothermia, or motor dysfunction. Anti-allodynic efficacy of systemic AM1710 was absent in CB2KO mice or WT mice receiving the CB2 antagonist AM630, administered either systemically or intrathecally. Intrathecal AM1710 also attenuated paclitaxel-induced allodynia in WT but not CB2KO mice, implicating a possible role for spinal CB2 receptors in AM1710 anti-allodynic efficacy. Finally, both acute and chronic treatment with AM1710 decreased mRNA levels of tumor necrosis factor alpha and monocyte chemoattractant protein-1 in lumbar spinal cord of paclitaxel-treated WT mice.
Conclusions
Our results highlight the potential of prolonged use of CB2 agonists for managing chemotherapy-induced allodynia with a favorable therapeutic ratio marked by sustained efficacy and absence of tolerance, physical withdrawal, or CB1-mediated side effects.
Keywords: Cannabinoid CB2, chemotherapy-induced neuropathic pain, knockout mouse, tolerance, precipitated withdrawal, side effect
Introduction
Cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC), the psychoactive component of cannabis, are used clinically to treat neuropathic pain and chemotherapy-induced nausea and vomiting (1, 2). However, unwanted psychotropic side effects limit widespread therapeutic use (1). These side effects (e.g. psychoactivity, dizziness, physical dependence) are centrally mediated by cannabinoid CB1 receptors (3, 4). A preferable strategy that avoids safety and efficacy concerns while preserving antinociceptive property is to target cannabinoid CB2 receptors.
CB2 receptors are found predominantly in immune cells and tissues and also occur at low levels, relative to CB1, in the central nervous system (CNS) (5, 6). In preclinical studies, CB2-preferring agonists promote neuroprotection (7–9) and produce antinociception (10–19). However, CB2-preferring agonists often have significant affinity at CB1 receptors. Given the high abundance of CB1 in the CNS, even low-level CB1-occupancy by CB2-preferring agonists could eliminate the benefits of receptor selectivity and/or produce adverse side effects following chronic treatment (2). Whether it is possible to obtain therapeutic benefits from repeated systemic administration of CB2-preferring agonists without engaging CB1 receptors or producing unwanted CB1-mediated side effects remains poorly understood.
Dose-limiting peripheral neuropathy can develop in cancer patients receiving chemotherapeutic agents (paclitaxel, cisplatin, vincristine, etc.) (20). Side effects and limited efficacy of clinically available medications make this neuropathy difficult to manage (21). Thus, there is a significant need to identify novel analgesics for treating chemotherapy-evoked neuropathic pain. CB2-preferring agonists exhibit antinociceptive properties in animal models of chemotherapy-induced neuropathy (22–27). However, the site of action and mechanism by which CB2 receptors modulate chemotherapy-induced neuropathy are not yet clear. Several proinflammatory cytokines (e.g. tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1β), interleukin 6 (IL-6)) and downstream chemokines (e.g. monocyte chemoattractant protein-1 (MCP-1)) are implicated in mechanisms of neuropathic pain (28–35) and CB2-mediated actions (36). The potential contributions of such cytokines and chemokines in the antinociceptive action of CB2 agonist on chemotherapy-induced neuropathy remain unknown.
Here, we characterized antinociceptive efficacy of the CB2-preferring agonist AM1710 in a model of paclitaxel-induced neuropathy using CB2 knockout (CB2KO), CB1 knockout (CB1KO), and wildtype (WT) mice. We evaluated whether repeated administration of AM1710 would produce antinociceptive tolerance or CB1-mediated side effects (i.e. physical withdrawal, motor ataxia, and hypothermia). In addition, we investigated the site of action and the impact of AM1710 on mRNA levels of pro-inflammatory cytokines and chemokine in lumbar spinal cords of paclitaxel-treated mice.
Methods and Materials
Subjects
Adult CB2KO (B6.129P2-CNR2(tm1Dgen/J), Jackson, ME, USA) and WT littermates (Jackson) on C57BL/6J background, and CB1KO (generated as previously described (4)) and WT littermates (Charles River, MA, USA) on CD1 background, weighing 25–33g and of both sexes, were used in these experiments. Mice were periodically backcrossed to maintain genetic integrity. Animals were single-housed in a temperature-controlled facility (73±2 °F, 45% humidity, 12h light/dark cycle, lights on at 7am), with food and water ad libitum provided. All experimental procedures were approved by Bloomington Institutional Animal Care and Use Committee of Indiana University and followed guidelines of the International Association for the Study of Pain (37).
Drugs and chemicals
Paclitaxel (Tecoland, NJ, USA) was dissolved in cremophor-vehicle (1:1:18 ratio of cremophor® EL (Sigma-Aldrich, MO, USA)/ethanol (Sigma-Aldrich)/saline (Aqualite System, IL, USA)). AM1710 (Makriyannis lab), AM630 (Cayman, MI, USA) and rimonabant (SR141716A, National Institute on Drug Abuse (NIDA), MD, USA) were dissolved in vehicle (5:2:2:16 ratio of dimethyl sulfoxide (DMSO, Sigma-Aldrich)/alkamuls® EL-620 (Rhodia, NJ, USA)/ethanol/saline). Δ9-THC (NIDA) was dissolved in vehicle (1:1:18 ratio of ethanol/cremophor/saline). Drugs were administered intraperitoneally (i.p.) to mice in a volume of 5 ml/kg. AM1710 and AM630 were also dissolved in vehicle (1:1:1:17 ratio of DMSO/alkamuls/ethanol/saline) and administered intrathecally (i.t.) to animals in a volume of 5 μl (38).
General experimental protocol
All experiments were conducted double-blinded with mice randomly assigned to experimental conditions. Prior to paclitaxel treatment, no genotype or gender differences were detected in any dependent measure (P>0.26 for all comparison). Paclitaxel (4 mg/kg i.p.) was administered four times on alternate days (cumulative dose: 16 mg/kg i.p.) to induce neuropathy (39). Controls received an equal volume of cremophor-vehicle. Development of paclitaxel-induced allodynia was assessed every two days.
Effects of pharmacological manipulations were assessed at 30 min post drug administration during the maintenance phase of paclitaxel-induced neuropathy (day 15 post initial paclitaxel injection). In Experiment #1, we assessed the dose responses of acute AM1710 on mechanical and cold allodynia in paclitaxel-treated WT (C57BL/6J) animals. In Experiment #2, we examined anti-allodynic efficacy and possible side effects of chronic AM1710 (5 mg/kg/day i.p. × 9 days) in paclitaxel-treated CB2KO, CB1KO, and respective WT littermates. Effects of chronic Δ9-THC (5 or 10 mg/kg/day i.p. × 9 days) in paclitaxel-treated WT (C57BL/6J) animals were also evaluated. Responsiveness to mechanical and cold stimulation was evaluated on treatment days 1, 4 and 8. Motor performance and rectal temperature were measured on treatment days 2 and 7. We also assessed whether chronic AM1710 would activate CB1 receptors sufficiently to produce CB1-dependent withdrawal symptoms following treatment with a CB1 antagonist. Thus, after the last injection of AM1710 (treatment day 9), we challenged CB2KO and WT mice with the CB1 antagonist rimonabant (10 mg/kg i.p.) to precipitate CB1-mediated withdrawal. We also challenged CB1KO and WT mice receiving chronic AM1710 with the CB2 antagonist AM630 (5 mg/kg i.p.) to determine if this treatment elicits behavioral signs reminiscent of CB1 or opioid receptor-mediated withdrawal. In Experiment #3, we examined pharmacological specificity of AM1710 in paclitaxel-treated WT or CB1KO mice that received vehicle, AM1710 (5 mg/kg/day i.p. × 8 days) alone or co-administered with AM630 (5 mg/kg/day i.p. × 8 days). In Experiment #4, we investigated the site of action of AM1710. We evaluated whether antagonism of spinal CB2 receptors by AM630 (5μg i.t.) would block the anti-allodynic effects of systemic AM1710 (5 mg/kg i.p.) in paclitaxel-treated WT mice. We also examined effects of intrathecal AM1710 (5μg i.t.) on paclitaxel-evoked allodynia in CB2KO and WT mice. In Experiment #5, we explored the impact of paclitaxel and AM1710 on spinal mRNA levels of pro-inflammatory cytokines (TNFα, IL-1β, IL-6), chemokine (MCP-1), and markers of the endocannabinoid system (CB1, CB2, fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MGL)) in WT (C57BL/6J) mice.
Assessment of mechanical allodynia
Withdrawal thresholds (g) to mechanical stimulation were measured in duplicate for each paw using electronic von Frey anesthesiometer supplied with 90-gram probe (IITC, CA, USA) (25). See Supplementary Material.
Assessment of cold allodynia
Response time (s) spent attending to (i.e. elevating, licking, biting, or shaking) the paw stimulated with acetone (Sigma-Aldrich) was measured in triplicate for each paw to assess cold allodynia (39). See Supplementary Material.
Evaluation of cannabinoid CB1 withdrawal symptoms
WT (C57BL/6J) mice receiving vehicle or Δ9-THC (5 or 10 mg/kg/day i.p. × 9 days) were challenged with vehicle or rimonabant (10 mg/kg i.p.). CB2KO and WT littermates receiving vehicle or AM1710 (5 mg/kg/day i.p. × 9 days) were challenged with rimonabant (10 mg/kg i.p.). CB1KO and WT mice receiving vehicle or AM1710 (5 mg/kg/day i.p. × 9 days) were challenged with AM630 (5 mg/kg i.p.). Challenge compounds were given 45 min post final injection. Mice were videoed and the number of paw tremors, headshakes, and scratching bouts were scored over 30 min following challenge (40).
Rotarod test
Motor performance was assessed using an accelerating rotarod (IITC) (4–40 rpm with cut-off time of 300 s) (41). See Supplementary Material.
Rectal temperature
Rectal temperature (°C) was measured using a thermometer (Physitemp, NJ, USA) with mouse rectal probe (Braintree, MA, USA).
RNA extraction and qRT-PCR
Total RNAs were extracted from lumbar spinal cords (42). One-step quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using PowerSYBR green PCR kit (Applied Biosystems, CA, USA) to quantify mRNA levels (43). The quantified mRNA levels were expressed as fold induction relative to control. Primer sequences (Table S1) see Supplementary Material.
Statistical analyses
The dose-response curves and ED50 values for AM1710 were determined using GraphPad Prism (CA, USA). Analysis of variance (ANOVA) for repeated measures was used to determine time course of paclitaxel-induced allodynia and drug effects. One-way ANOVA was used to identify the source of significant interactions at each timepoint and compare post-injection responses with baselines, followed by Bonferroni post hoc tests or two-tailed t-tests, as appropriate. Impact of paclitaxel or AM1710 on mRNA levels was analyzed using two-tailed t-tests or one-way ANOVA, respectively. Statistical analyses were performed using IBM-SPSS Statistics V21.0 (IL, USA). P<0.05 was considered significant.
Results
Paclitaxel-induced allodynia developed similarly in WT, CB2KO and CB1KO mice
In both CB2KO and WT mice, paclitaxel decreased mechanical thresholds (F3,20=519.03, P<0.0001, Figure 1A) and increased response time to cold stimulation (F3,20=553.78, P<0.0001, Figure 1B). Similarly, paclitaxel induced mechanical (F3,20=426.66, P<0.0001, Figure 1C) and cold (F3,20=707.28, P<0.0001, Figure 1D) allodynia in CB1KO and WT littermates. Mechanical and cold allodynia were present in paclitaxel-treated CB2KO, CB1KO, and WT mice relative to cremophor-vehicle since day 4 (P<0.0001). Responsiveness to paclitaxel did not differ between CB2KO and WT mice, or between CB1KO and WT mice (P=1.000).
Figure 1. Paclitaxel produced hypersensitivities to mechanical and cold stimulation.
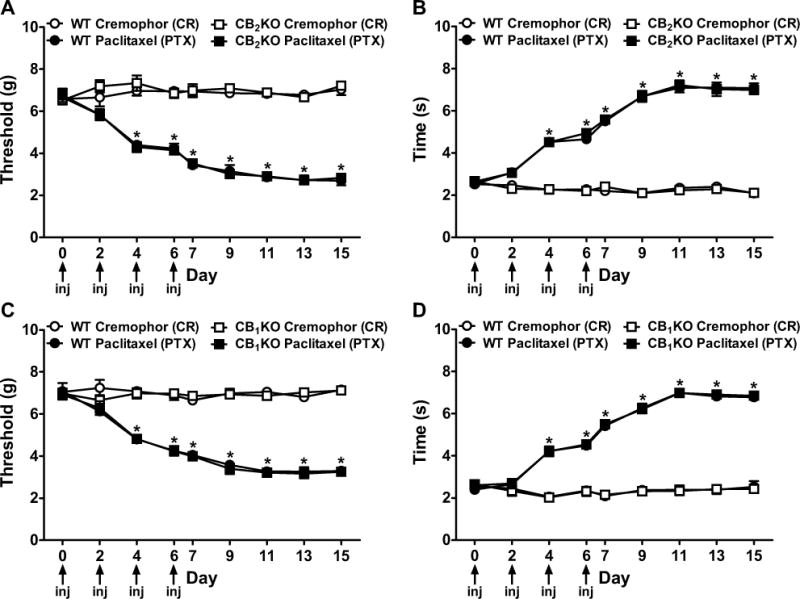
(A, C) Mechanical and (B, D) cold allodynia developed equivalently in (A, B) CB2KO, (C, D) CB1KO, and corresponding WT littermates following paclitaxel treatment. Non-chemotherapy controls received cremophor-vehicle in lieu of paclitaxel. Arrows show timing of paclitaxel or cremophor injections (inj). Data are expressed as mean ± SEM (n=6 per group). *P<0.05 vs. control, repeated measures ANOVA and one-way ANOVA at each timepoint.
Effects of Δ9-THC in paclitaxel-treated WT mice
In WT mice, Δ9-THC (5 or 10 mg/kg/day i.p.) suppressed paclitaxel-evoked mechanical (F2,14=26.57, P<0.0001) and cold allodynia (F2,14=13.58, P<0.002) relative to vehicle in a dose-and time-dependent manner (F8,56=27.97, P<0.0001 mechanical, F8,56=24.44, P<0.0001 cold, Figure 2A–B). The high dose of Δ9-THC (10 mg/kg/day i.p.) produced greater antinociceptive effects than the low dose (5 mg/kg/day i.p.) (P<0.01 mechanical, P<0.03 cold) and normalized responses to pre-paclitaxel levels (P=0.13 mechanical, P=0.07 cold) on treatment day 1. Tolerance developed more rapidly to the high dose of Δ9-THC. The high (P=1.00 day 4 and 8) and low (P<0.0001 day 4, P=1.00 day 8) doses of Δ9-THC failed to produce antinociception relative to vehicle after 4 or 8 days of injections, respectively. Both doses of Δ9-THC decreased motor performance and produced hypothermia in paclitaxel-treated WT mice relative to vehicle on day 2 (P<0.04), but not day 7 (P>0.11), of chronic dosing (Figure 2C–D). Thus, over 8 days of Δ9-THC (5 or 10 mg/kg/day i.p.) administration, tolerance developed to antinociceptive efficacy, motor ataxia, and hypothermia in paclitaxel-treated animals.
Figure 2. Effects of Δ9-THC in paclitaxel-treated WT mice.
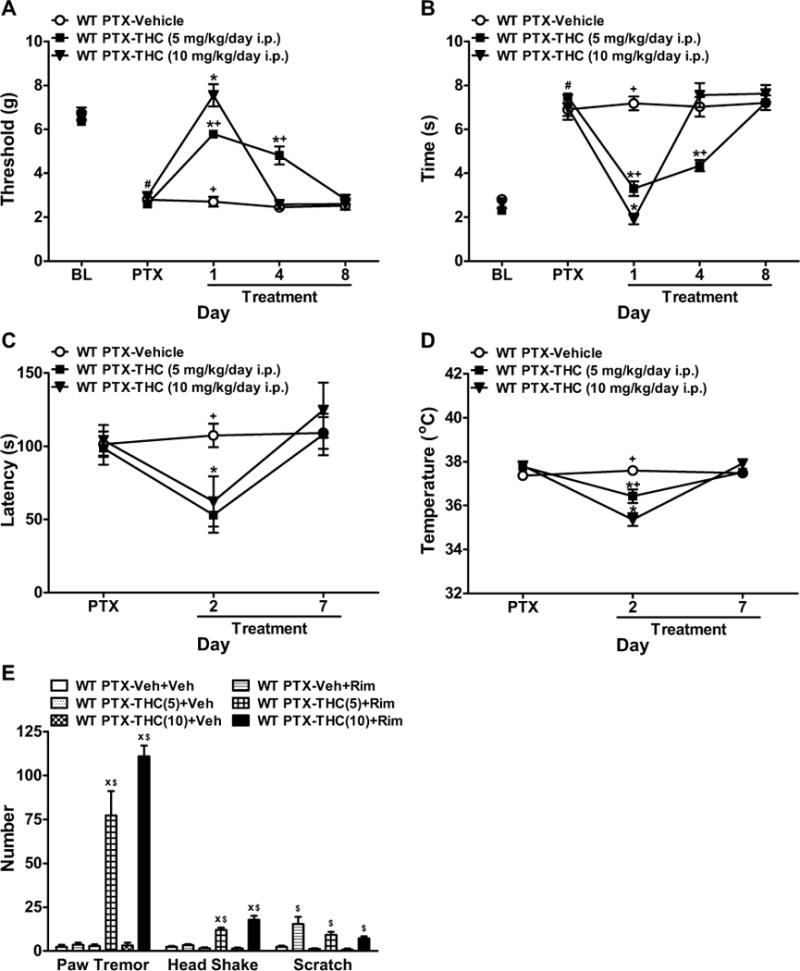
(A, B) Δ9-THC (5 or 10 mg/kg/day i.p.) attenuated paclitaxel-induced (A) mechanical and (B) cold allodynia in WT (C57BL/6J) mice in a dose- and time-dependent manner. (C, D) Δ9-THC (5 or 10 mg/kg/day i.p.) decreased (C) motor performance and (D) body temperature in paclitaxel-treated WT mice relative to vehicle on treatment day 2, but not day 7. (E) Δ9-THC (5 or 10 mg/kg/day i.p.) produced withdrawal symptoms when challenged with the CB1 antagonist rimonabant. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline. Data are expressed as mean ± SEM (n=5–6 per group). *P<0.05 vs. vehicle, +P<0.05 vs. Δ9-THC (10 mg/kg/day i.p.), xP<0.05 vs. Veh+Rim (chronic vehicle and challenge by rimonabant), $P<0.05 vs. Veh+Veh (chronic vehicle and challenge by vehicle), one-way ANOVA followed by Bonferroni post hoc test or two-tailed t-test. #P<0.05 vs. BL, repeated measures ANOVA.
In paclitaxel-treated WT mice, chronic Δ9-IHC (5 or 10 mg/kg/day i.p.) produced cannabinoid withdrawal signs following rimonabant (10 mg/kg i.p.) challenge, characterized by paw tremors (F5,26=65.60, P<0.0001) and headshakes (F5,26=38.13, P<0.0001) relative to vehicle (P<0.0001, Figure 2E). Rimonabant, but not vehicle, produced scratching behaviors (F5,26=10.34, P<0.0001) in animals receiving chronic vehicle or Δ9-THC (Figure 2E).
Effects of acute AM1710 in paclitaxel-treated WT mice
In WT mice, acute systemic AM1710 dose-dependently suppressed paclitaxel-induced mechanical (ED50: 1.14±0.07 mg/kg i.p.) and cold (ED50: 1.49±0.06 mg/kg i.p.) allodynia (Figure S1). AM1710 (5 mg/kg i.p.) produced maximal anti-allodynic efficacy and was used for chronic dosing.
Chronic AM1710 suppressed paclitaxel-induced allodynia in WT but not CB2KO mice
In WT mice, chronic AM1710 (5 mg/kg/day i.p.) suppressed paclitaxel-induced mechanical (F1,13=98.97, P<0.0001) and cold (F1,13=249.03, P<0.0001) hypersensitivities relative to vehicle (P<0.0001) and pre-injection levels (F4,52=67.12, P<0.0001 mechanical, F4,52=62.04, P<0.0001 cold, Figure 3A–B). AM1710 anti-allodynic efficacy was stable throughout the chronic dosing paradigm (P=0.75 mechanical, P=1.00 cold). AM1710 fully reversed paclitaxel-induced allodynia and normalized responses to pre-paclitaxel baselines (P=0.86 mechanical, P=0.46 cold, Figure 3A–B).
Figure 3. Chronic systemic administration of AM1710 suppressed paclitaxel-induced neuropathy in WT but not CB2KO mice.
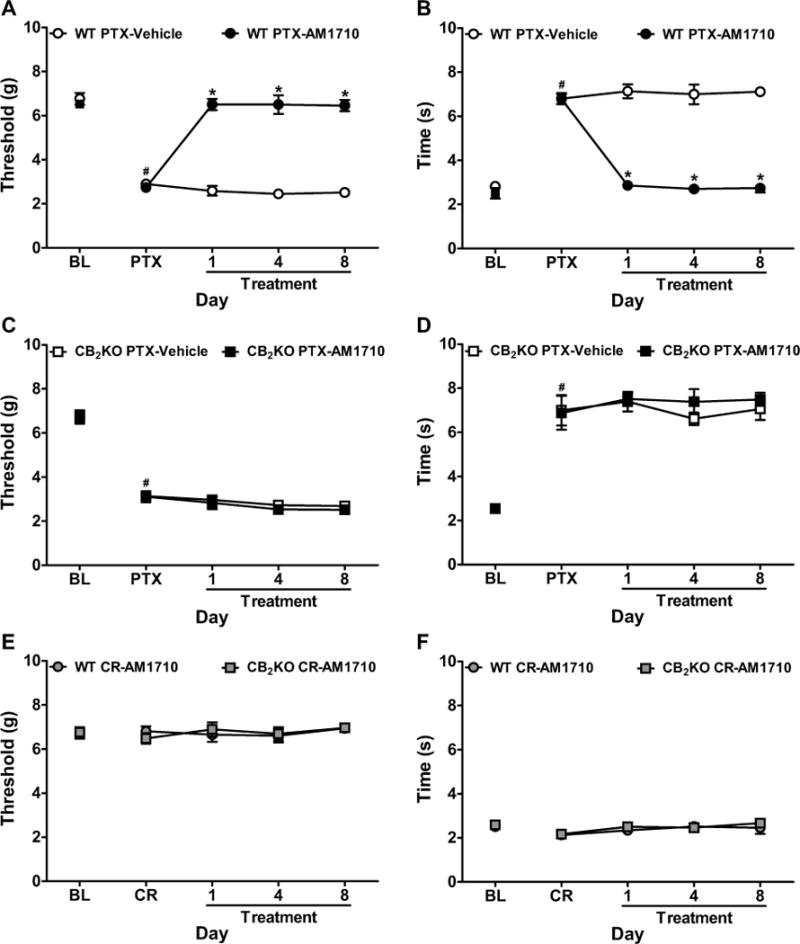
(A, B) AM1710 (5 mg/kg/day i.p. × 8 days) reversed paclitaxel-induced (A) mechanical and (B) cold allodynia in WT littermates. (C, D) AM1710 (5 mg/kg/day i.p. × 8 days) did not suppress paclitaxel-induced (C) mechanical or (D) cold allodynia in CB2KO mice. (E, F) AM1710 (5 mg/kg/day i.p. × 8 days) did not alter (E) mechanical or (F) cold responsiveness in cremophor-treated CB2KO or WT mice. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline; CR, post-cremophor baseline. Data are expressed as mean ± SEM (n=4–8 per group). *P<0.05 vs. vehicle, one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
By contrast, in CB2KO mice, AM1710 (5 mg/kg/day i.p.) failed to suppress paclitaxel-induced mechanical (P=0.22) or cold (P=0.79) allodynia relative to vehicle (P>0.20) on any day (P=1.00 mechanical, P=0.59 cold, Figure 3C–D). AM1710 did not alter responsiveness to mechanical (P=0.94) or cold (P=0.66) stimulation in CB2KO or WT littermates treated with cremophor-vehicle at any timepoint (P=0.84 mechanical, P=0.89 cold, Figure 3E–F).
Anti-allodynic effects of AM1710 were independent of CB1 signaling
In both CB1KO and WT littermates, AM1710 (5 mg/kg/day i.p.) reversed paclitaxel-induced mechanical (F3,17=112.37, P<0.0001) and cold (F3,17=29.24, P<0.0001) allodynia relative to vehicle (P<0.0001) and pre-injection levels (F12,68=17.04, P<0.0001 mechanical, F12,68=21.97, P<0.0001 cold, Figure 4A–B). AM1710-induced anti-allodynic effects were stable throughout the treatment paradigm (P=0.97 mechanical, P=0.12 cold). AM1710 fully reversed paclitaxel-induced mechanical (P>0.88) and cold (P>0.052) allodynia and normalized responses to pre-paclitaxel baselines in both CB1KO and WT littermates. Anti-allodynic efficacy of AM1710 did not differ between CB1KO and WT littermates at any timepoint (P>0.99, Figure 4A–B). AM1710 did not alter mechanical (P=0.72) or cold (P=0.11) responsiveness in CB1KO or WT littermates treated with cremophor-vehicle on any day (P=0.88 mechanical, P=0.53 cold, Figure 4C–D).
Figure 4. Chronic systemic administration of AM1710 reversed paclitaxel-induced neuropathic pain with similar efficacy in CB1KO and WT mice.
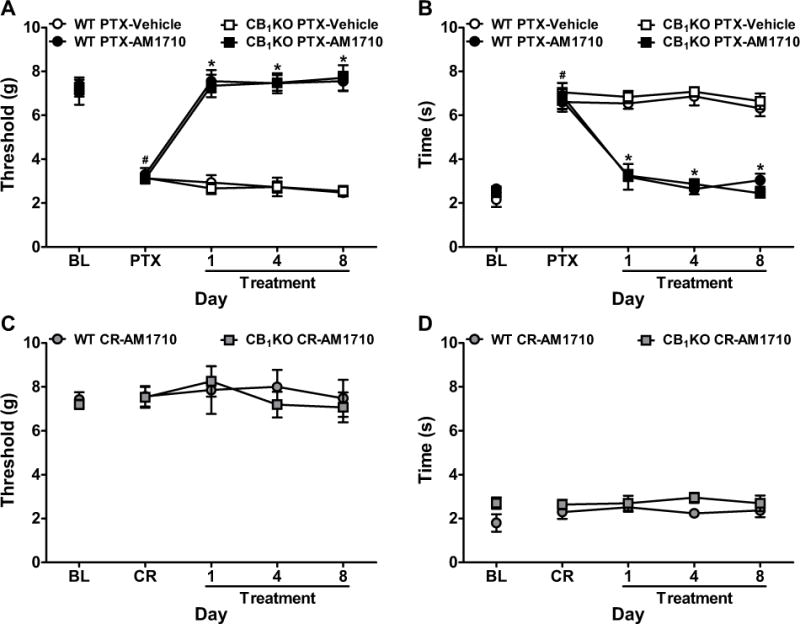
(A, B) AM1710 (5 mg/kg/day i.p. × 8 days) reversed paclitaxel-induced (A) mechanical and (B) cold allodynia in both CB1KO and WT littermates. (C, D) AM1710 (5 mg/kg/day i.p. × 8 days) did not alter (C) mechanical or (D) cold responsiveness in cremophor-treated CB1KO or WT mice. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline; CR, post-cremophor baseline. Data are expressed as mean ± SEM (n=4–8 per group). *P<0.05 vs. vehicle, one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
Anti-allodynic effects of AM1710 were mediated by CB2 receptors
In paclitaxel-treated WT (C57BL/6J) mice, AM1710 (5 mg/kg/day i.p.)-produced suppressions of mechanical (F3,19=65.57, P<0.0001) and cold (F3,19=95.35, P<0.0001) allodynia were blocked by the CB2 antagonist AM630 (5 mg/kg/day i.p.) at all timepoints (P<0.0001, Figure 5A–B). Identical results were obtained in WT (CD1) mice (data not shown).
Figure 5. Anti-allodynic effects of chronic systemic AM1710 were mediated by CB2 receptors.
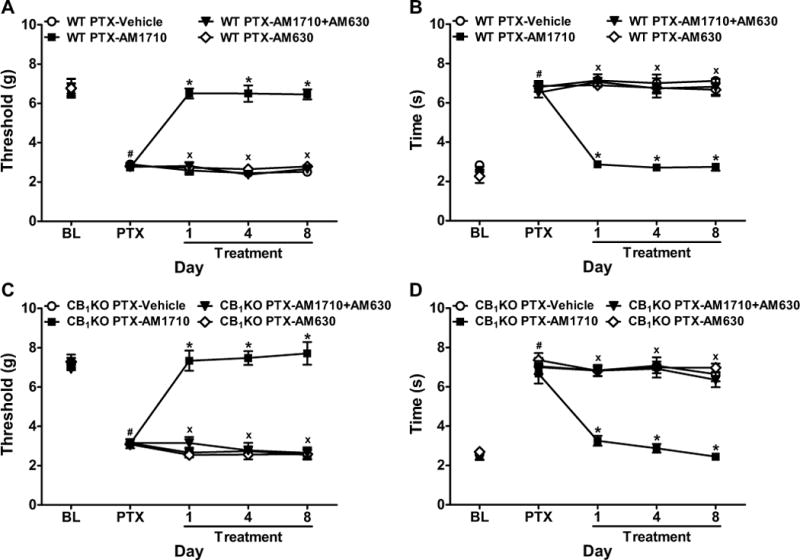
AM1710 (5 mg/kg/day i.p. × 8 days)-induced suppressions of paclitaxel-evoked (A, C) mechanical and (B, D) cold allodynia were blocked by the CB2 antagonist AM630 (5 mg/kg/day i.p. × 8 days) in both (A, B) WT (C57BL/6J) and (C, D) CB1KO mice. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline. Data are expressed as mean ± SEM (n=4–9 per group). *P<0.05 vs. vehicle, xP <0.05 vs. AM1710 (5 mg/kg i.p.), one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
In paclitaxel-treated CB1KO mice, the anti-allodynic effects of AM1710 (5 mg/kg/day i.p.) on mechanical (F3,16=111.06, P<0.0001) and cold (F3,16=37.02, P<0.0001) hypersensitivities were blocked by AM630 (5 mg/kg/day i.p.) at all timepoints (P<0.0001, Figure 5C–D). AM630 alone did not alter mechanical or cold responsiveness relative to vehicle in WT or CB1KO mice (P=1.00, Figure 5A–D).
Chronic AM1710 did not produce motor dysfunction or hypothermia
Paclitaxel did not alter motor performance or body temperature in CB2KO, CB1KO or corresponding WT littermates relative to cremophor-vehicle (P>0.11, Figure S2). Moreover, AM1710 (5 mg/kg/day i.p.) did not produce motor dysfunction or hypothermia in either paclitaxel- or cremophor-treated groups in CB2KO, CB1KO, or WT littermates on treatment day 2 or 7 (P>0.95, Figure S2).
CB1 antagonism did not elicit classic cannabinoid withdrawal signs in mice receiving chronic AM1710
We asked whether the CB1 antagonist rimonabant would elicit cannabinoid CB1-dependent withdrawal symptoms in mice receiving chronic AM1710. In paclitaxel-treated WT mice that received chronic Δ9-THC (10 mg/kg/day i.p.), rimonabant (10 mg/kg i.p.) challenge produced paw tremors (F4,20=272.81, P<0.0001) and headshakes (F4,20=32.10, P<0.0001, Figure 6A). Rimonabant challenge did not elicit paw tremors or headshakes in CB2KO or WT mice receiving chronic AM1710 (5 mg/kg/day i.p.) relative to vehicle (P=1.00, Figure 6A, S3A). Neither Δ9-THC nor AM1710 treatment altered rimonabant-induced scratching (P=0.22) compared to vehicle (Figure 6A).
Figure 6. Chronic systemic AM1710 treatment did not produce cannabinoid CB1-dependent withdrawal signs.
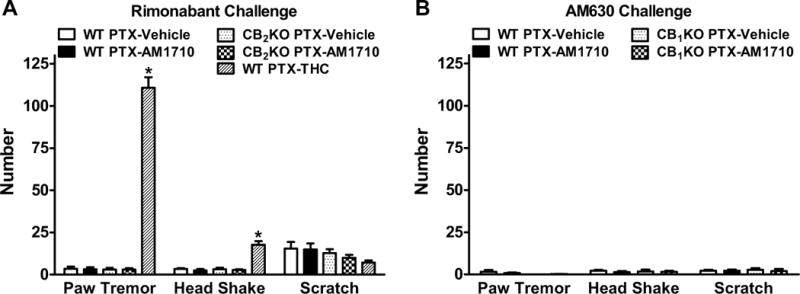
(A) AM1710 (5 mg/kg/day i.p. × 9 days) did not produce CB1-dependent withdrawal signs (i.e. paw tremors, headshakes) when precipitated with the CB1 antagonist rimonabant (10 mg/kg i.p.) in CB2KO or WT littermates. (B) Challenge with the CB2 antagonist AM630 (5 mg/kg i.p.) did not produce paw tremors, headshakes, or scratching behaviors in CB1KO or WT littermates treated chronically with AM1710 (5 mg/kg/day i.p. × 9 days). Data are expressed as mean ± SEM (n=4–5 per group). *P<0.05 vs. vehicle, one-way ANOVA followed by Bonferroni post hoc test.
We next asked whether the CB2 antagonist AM630 could precipitate paw tremors, headshakes and/or scratching behaviors in mice receiving chronic AM1710. AM630 (5 mg/kg i.p.) challenge did not elicit paw tremors (P=0.29), headshakes (P=0.88), or scratching (P=0.96) relative to vehicle in CB1KO or WT mice receiving chronic AM1710 (5 mg/kg/day i.p.) (Figure 6B, S3B). In addition, no autonomic signs (e.g. diarrhea, eyelid ptosis) or writhing behaviors were observed following AM630 challenge.
Spinal CB2 receptors were necessary for the anti-allodynic effect of systemic AM1710
In WT mice, anti-allodynic effects of AM1710 (5 mg/kg i.p.) on paclitaxel-induced mechanical (F3,20=16.51, P<0.0001) and cold (F3,20=30.93, P<0.0001) allodynia were blocked by intrathecal AM630 (5 μg i.t.) (P<0.0001, Figure 7). Intrathecal AM630 alone did not alter paclitaxel-evoked mechanical (P=1.00) or cold (P>0.72) allodynia relative to vehicle (Figure 7).
Figure 7. Antagonism of spinal CB2 receptors blocked anti-allodynic effects of systemic AM1710 in WT mice.
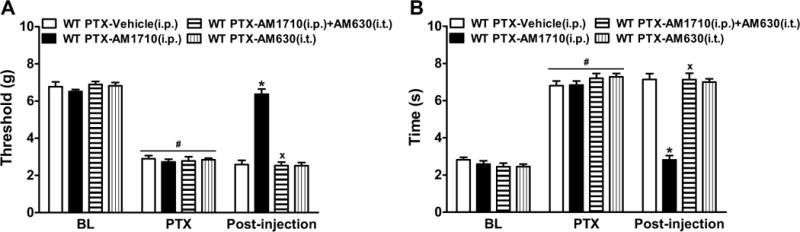
Intrathecal administration of the CB2 antagonist AM630 (5 μg i.t.) blocked AM1710 (5 mg/kg i.p.)-induced suppressions of (A) mechanical and (B) cold allodynia in paclitaxel-treated WT (C57BL/6J) mice. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline. Data are expressed as mean ± SEM (n=6 per group). *P<0.05 vs. vehicle, xP<0.05 vs. AM1710 (5 mg/kg i.p.), one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline, repeated measures ANOVA.
Intrathecal AM1710 suppressed paclitaxel-induced neuropathy in WT but not CB2KO mice
We asked whether activation of spinal CB2 receptors was sufficient to suppress paclitaxel-induced allodynia. In WT mice, intrathecal AM1710 (5 μg i.t.) suppressed paclitaxel-induced mechanical (F1,10=42.42, P<0.0001) and cold (F1,10=78.99, P<0.0001) allodynia compared to vehicle (P<0.0001); intrathecal AM1710 fully reversed paclitaxel-evoked allodynia and normalized responses to pre-paclitaxel levels (P=0.89 mechanical, P=0.87 cold, Figure 8A–B). By contrast, in CB2KO mice, AM1710 (5 μg i.t.) failed to attenuate paclitaxel-induced mechanical (P=0.85) or cold (P=0.46) allodynia relative to vehicle (Figure 8C–D).
Figure 8. Activation of spinal CB2 receptors suppressed paclitaxel-induced allodynia in WT but not CB2KO mice.
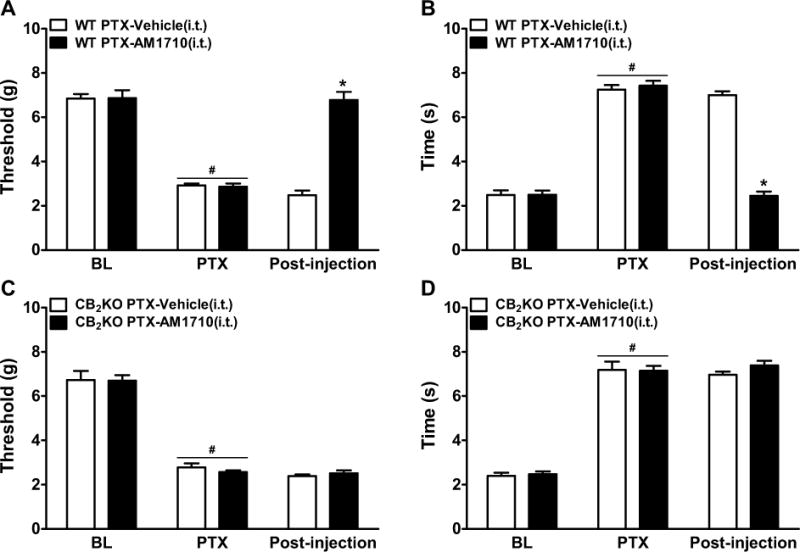
Intrathecal administration of AM1710 (5 μg i.t.) suppressed paclitaxel-induced (A, C) mechanical and (B, D) cold allodynia in (A, B) WT, but not (C, D) CB2KO mice. BL, pre-paclitaxel baseline; PTX, post-paclitaxel baseline. Data are expressed as mean ± SEM (n=6 per group). *P<0.05 vs. vehicle, one-way ANOVA followed by Bonferroni post hoc test. #P<0.05 vs. pre-paclitaxel baseline (BL), repeated measures ANOVA.
Impact on spinal mRNA levels of markers of the endocannabinoid system, cytokines, and chemokine
In WT mice, paclitaxel increased MCP-1 (P<0.004), but not IL-1β (P=0.52), IL-6 (P=1.00), TNFα (P=0.83), CB1(P=0.34), CB2 (P=0.26), FAAH(P=0.28), or MGL (P=0.18) mRNA levels in spinal cords relative to cremophor-vehicle (Figure 9A, S4) during the maintenance phase of paclitaxel-induced neuropathy. In paclitaxel-treated WT mice, both acute and chronic (8 days) AM1710 (5 mg/kg/day i.p.) decreased TNFα (F2,9=19.52, P<0.002) and MCP-1 (F2,9=15.00, P<0.002), but not IL-1β (P=0.38) or IL-6 (P=0.68) spinal mRNA levels (Figure 9B).
Figure 9. Impact of paclitaxel and AM1710 on cytokine and chemokine mRNA levels in lumbar spinal cord.
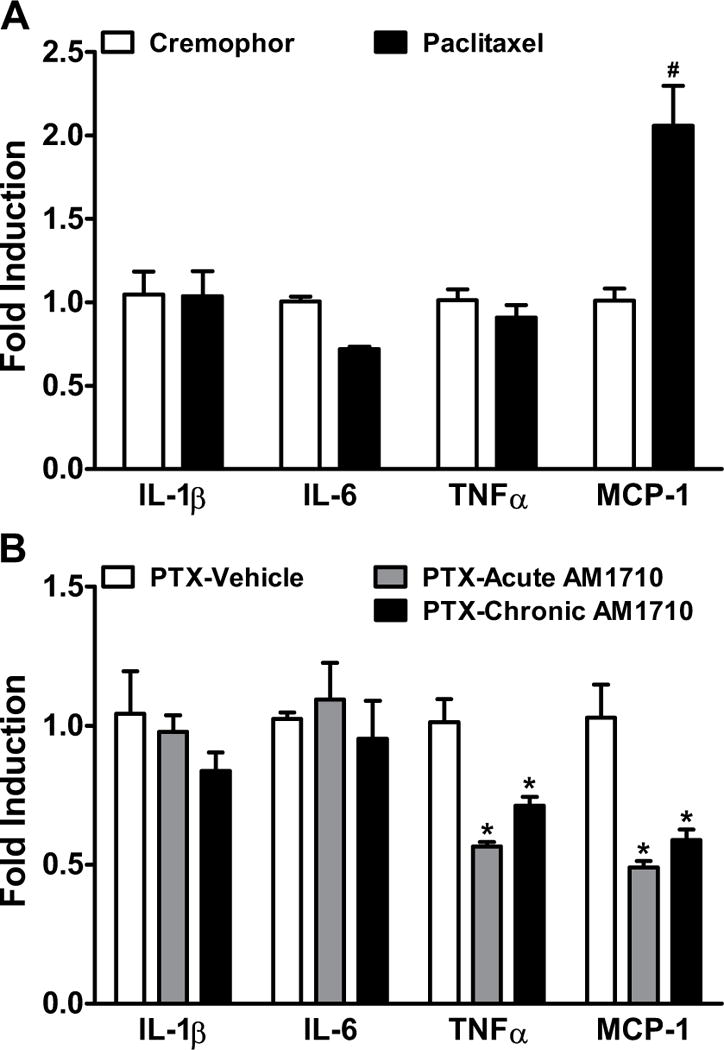
(A) Paclitaxel increased the spinal mRNA levels of MCP-1, but not IL-1β, IL-6, or TNFα relative to cremophor in WT mice (day 15 post initial paclitaxel dosing). (B) Both acute (once daily injections of vehicle × 7 days followed by a terminal injection of AM1710 (5 mg/kg i.p.) on the 8th day, grey bar) and chronic (5 mg/kg/day i.p. × 8 days, black bar) administrations of AM1710 decreased the spinal mRNA levels of TNFα and MCP-1, but not IL-1β or IL-6 relative to vehicle (once daily × 8 days, white bar) in paclitaxel-treated WT animals. IL-1β, interleukin-1 beta; IL-6, interleukin 6; TNFα, tumor necrosis factor alpha; MCP-1, monocyte chemoattractant protein-1. Data are expressed as mean ± SEM (n=4 per group). #P<0.05 vs. cremophor vehicle in lieu of paclitaxel, one-tailed t-test. *P<0.05 vs. vehicle in lieu of AM1710, one-way ANOVA followed by Bonferroni post hoc test.
Discussion
Drug development for neuropathic pain management has proved a challenge due in part to limited efficacy and troubling side-effect profiles. Indeed, these challenges also apply to potential therapeutic use of cannabinoids (44). Here, we showed that repeated systemic administration of the CB2-preferring agonist AM1710 suppressed chemotherapy-induced allodynia without tolerance or significant CB1 involvement (i.e. the absence of CB1 antagonist-precipitated withdrawal symptoms, motor ataxia, and hypothermia). We also confirmed a CB2-mediated mechanism of antinociceptive action for AM1710 both pharmacologically and through use of knockout mice. Moreover, we identified a spinal site of action of AM1710 and explored AM1710-mediated regulation of pro-inflammatory cytokines and chemokine mRNA levels following paclitaxel treatment.
CB2 receptors are implicated in pain mechanisms following sciatic nerve injury (45) and joint pain (46). In our study, neither the development nor the maintenance of paclitaxel-induced allodynia differed between CB2KO and WT mice. CB2 receptors are highly inducible and are expressed in spinal microglia upon inflammation (47) or neuropathic pain (48–52). However, we did not detect changes in CB2 or FAAH mRNA levels in lumbar spinal cords of WT animals following paclitaxel treatment. By contrast, cisplatin alters endocannabinoid tone (43, 53), highlighting distinct mechanisms underlying neuropathies produced by these two chemotherapeutic agents (20). More work is needed to understand the role of the endocannabinoid system in induction and maintenance of chemotherapy-induced neuropathy.
In our study, both acute and chronic systemic treatment with the CB2-preferring agonist AM1710 attenuated paclitaxel-induced allodynia in WT mice. Notably, deletion of CB2 receptors or pharmacological blockade with the CB2 antagonist AM630 prevented the anti-allodynic effects of AM1710. Thus, AM1710 suppressed chemotherapy-induced allodynia via CB2 receptor activation, consistent with previous observations on anti-allodynic efficacies of other CB2 agonists (14, 24, 26, 54) in neuropathic or inflammatory pain models. Taken together, these studies suggest therapeutic potential of CB2 agonists in managing a wide spectrum of pain states.
Most CB2 agonists identified to date exhibit low affinity for CB1 (2). Indeed, it has been speculated that antinociceptive therapeutic efficacy of CB2 agonists is mediated by CB1 receptors (2, 55). In our study, AM1710 fully reversed paclitaxel-induced allodynia with similar efficacy in both CB1KO and WT mice following either acute or chronic administration, consistent with a previous study showing that CB2 agonist AM1241 retained antinociceptive efficacy in CB1KO mice subjected to spinal nerve ligation (56). We also showed that antinociceptive effects of chronic AM1710 were blocked by a CB2 antagonist in CB1KO mice, further demonstrating that CB2, but not CB1, receptors mediate the anti-allodynic effects of the CB2 agonist AM1710 on paclitaxel-induced neuropathy.
Tolerance may limit an analgesic’s therapeutic use (57–59). It occurs following prolonged exposure of CB1 receptors to cannabinoids in preclinical (60–63) and clinical (44) studies. Here, we showed that chronic dosing over 4 to 8 days with Δ9-THC was sufficient to produce tolerance to both anti-allodynic efficacy and CB1-mediated side effects in the paclitaxel-induced neuropathy model. However, no decrement in anti-allodynic efficacy was observed in animals received daily administration of the maximally effective dose of AM1710 over 8 days. Our data are in line with previous works showing that intrathecal JWH015 (17) or systemic A-836339 (64) does not produce antinociception tolerance following traumatic nerve injury.
In binding assays, the CB2-preferring agonist AM1710 exhibits 54-fold selectivity for CB2 over CB1 receptors (65). This limited selectivity raises the possibility that a low level of CB1 occupancy by this compound could potentially activate CB1 receptors and translate into unwanted CB1-mediated side effects following chronic administration, negatively impacting its therapeutic ratio and hindering its clinical acceptance. We evaluated this possibility in two ways. The first was that in our study, chronic AM1710 did not result in motor deficits or hypothermia, hallmarks of CB1 agonists, consistent with previous observations with other CB2 agonists (11, 54, 64, 66–69). The second was to detect signs of CB1-mediated withdrawal. Physical dependence, quantified by signs of withdrawal following antagonist administration, has been reported after chronic cannabinoid (3, 58) and opioid (70–72) use. For example, challenge with the CB1 antagonist rimonabant elicits profound withdrawal symptoms in animals treated chronically with CB1 agonists (40, 72–74). However, no study has examined whether prolonged treatment with a CB2-preferring agonist results in a state where cannabinoid withdrawal signs through residual CB1 activity can be elicited. In theory, this would be a very sensitive way to detect low levels of sustained CB1 receptor activation. Here, we showed that unlike with chronic Δ9-THC, mice treated with chronic AM1710 did not exhibit signs of rimonabant-precipitated withdrawal. Importantly, we assessed withdrawal signs in a neuropathic pain model to mimic a common clinical scenario. Coupled with the observation that CB2 agonists show little intrinsic reward (75, 76), this class of compounds may lack drug abuse liability. These findings collectively support the clinical potential of prolonged use of CB2 agonists.
Whether withdrawal symptoms could be elicited by precipitation at CB2 receptors in animals receiving chronic CB2 agonists is an important question that has never been studied. Here, we evaluated behaviors (i.e. paw tremors, headshakes, scratching) that are signs common to withdrawal precipitated by CB1 or opioid receptor antagonists (40, 70–72). These behaviors were absent in AM1710-treated WT or CB1KO mice following CB2 antagonist AM630 challenge (CB1KO mice were used to avoid potential residual CB1-mediated component of AM1710). Interestingly, scratching was produced independent of withdrawal by rimonabant, but not AM630, consistent with pruritis as a common response to CB1 antagonists (77). More work is necessary to further investigate possible withdrawal signs at CB2 receptors.
Here, we reported the first evaluation of the site of action of a CB2 agonist in the chemotherapy-induced neuropathy model. We showed that anti-allodynic effects of systemic AM1710 were blocked by intrathecal administration of a CB2 antagonist. Moreover, a systemically inactive dose of AM1710 (5 μg/animal, equivalent to 0.16–0.2 mg/kg), administered intrathecally, produced robust antinociception in WT but not CB2KO mice. Thus, activation of spinal CB2 receptors by AM1710 is sufficient to reverse paclitaxel-induced allodynia. Peripheral (11, 12), spinal (14–17), or both peripheral and spinal (13, 18) sites of action are implicated in CB2 agonist efficacy in various preclinical pain models. The differences in site of action could be attributed to different functional properties of the CB2 agonists or distinct mechanisms produced by the specific pain state. Interestingly, in line with our results in chemotherapy-induced neuropathy, spinal site of CB2 agonist action has been implicated in models of traumatic nerve injury (13–17). Therefore, CB2 agonists may possess a shared mechanism of action in suppressing neuropathic pain through activation of spinal CB2 receptors.
To further explore the mechanism of CB2-mediated antinociception, we studied the impact of AM1710 on expression of cytokines and a chemokine in paclitaxel-induced neuropathy. Pro-inflammatory cytokines (e.g. IL-1β (28), IL-6 (29), TNFα (30–34)), and the chemokine MCP-1 (35) are implicated in mechanisms of neuropathic pain produced by traumatic nerve injury. Inflammatory processes are also generated by chemotherapy treatments (78–81). We did not detect alterations of spinal mRNA levels of IL-1β, IL-6 or TNFα during the maintenance phase of paclitaxel-induced allodynia. Transient upregulations of TNFα (81) or IL-6 (29) have been observed during the development of neuropathy induced by vincristine or nerve injury. It is possible that earlier timepoints during the development of paclitaxel-induced neuropathy would be sensitive to transient alterations in cytokine production. Nonetheless, AM1710 robustly decreased spinal mRNA levels of TNFα in paclitaxel-treated WT mice. Our results, along with published report on CB2 agonist JWH015-induced TNFα downregulation in vitro (36), suggest possible TNFα involvement in CB2 activity. We also observed that spinal MCP-1 mRNA levels were elevated by paclitaxel and were decreased by AM1710. Thus, suppression of MCP-1 may contribute to the mechanism of CB2-mediated anti-allodynic efficacy in chemotherapy-induced neuropathy (79, 80). In inflammatory and neuropathic pain, TNFα upregulates MCP-1 (82) and modulates central sensitization (83–85) and c-fiber responses (86, 87). CB2 agonists suppress central sensitization (88–91). More studies are necessary to identify the source of spinal TNFα and MCP-1, their regulation, and their potential roles in CB2-mediated suppression of central sensitization and chemotherapy-induced neuropathy.
In conclusion, chronic systemic treatment with the CB2 agonist AM1710 suppressed chemotherapy-induced allodynia without producing tolerance, CB1-mediated cannabinoid withdrawal or CNS side effects associated with CB1 activation. The observed anti-allodynic efficacy required activation of spinal CB2 receptors and was independent of CB1 signaling. Furthermore, the pro-inflammatory cytokine TNFα and chemokine MCP-1 are likely involved in CB2-mediated anti-allodynic efficacy. Together, our results support the therapeutic potential of prolonged use of CB2 agonists for managing toxic neuropathic pain without apparent adverse effects.
Supplementary Material
Acknowledgments
The authors wish to thank Vishnu Kodumuru for providing AM1710 and James Wager- Miller for designing and providing the RT-PCR primers.
Financial Disclosures
Supported by DA021644 (AGH),DA037673 (AGH), DA011322 (KM), DA021696 (KM), DA3801, DA07215, DA09158 (AM) and DA035068 (KM and AGH). AM serves as a consultant for MAKScientific.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide
- ANOVA
analysis of variance
- BL
baseline
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CNS
central nervous system
- CR
cremophor
- DMSO
dimethyl sulfoxide
- Δ9-THC
Δ9-tetrahydrocannabinol
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- FAAH
fatty acid amide hydrolase
- i.p
intraperitoneal
- i.t
intrathecal
- IL-1β
interleukin-1 beta
- IL-6
interleukin 6
- KO
knock out
- MCP-1/CCL2
monocyte chemoattractant protein-1
- MGL
monoacylglycerol lipase
- NIDA
National Institute on Drug Abuse
- PTX
paclitaxel
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- TNFα
tumor necrosis factor alpha
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease–successes and failures. The FEBS journal. 2013;280:1918–1943. doi: 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:3353–3363. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms–a review of recent preclinical data. Psychopharmacology. 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 4.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 5.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS & neurological disorders drug targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Current neuropharmacology. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagredo O, Gonzalez S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington’s disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhaveri MD, Sagar DR, Elmes SJ, Kendall DA, Chapman V. Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain. Molecular neurobiology. 2007;36:26–35. doi: 10.1007/s12035-007-8007-7. [DOI] [PubMed] [Google Scholar]
- 11.Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 12.Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, et al. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, et al. Central and peripheral sites of action for CB(2) receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. British journal of pharmacology. 2011;162:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. European journal of pharmacology. 2008;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Landry RP, Martinez E, DeLeo JA, Romero-Sandoval EA. Spinal cannabinoid receptor type 2 agonist reduces mechanical allodynia and induces mitogen-activated protein kinase phosphatases in a rat model of neuropathic pain. The journal of pain : official journal of the American Pain Society. 2012;13:836–848. doi: 10.1016/j.jpain.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, et al. Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain. 2012;153:1091–1106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108:722–734. doi: 10.1097/ALN.0b013e318167af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curto-Reyes V, Llames S, Hidalgo A, Menendez L, Baamonde A. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. British journal of pharmacology. 2010;160:561–573. doi: 10.1111/j.1476-5381.2009.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 20.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the peripheral nervous system: JPNS. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 21.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clinical pharmacology and therapeutics. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 22.Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. The Journal of pharmacology and experimental therapeutics. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. British journal of pharmacology. 2008;155:1104–1116. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naguib M, Xu JJ, Diaz P, Brown DL, Cogdell D, Bie B, et al. Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesthesia and analgesia. 2012;114:1104–1120. doi: 10.1213/ANE.0b013e31824b0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, et al. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Molecular pain. 2012;8:71. doi: 10.1186/1744-8069-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu JJ, Diaz P, Astruc-Diaz F, Craig S, Munoz E, Naguib M. Pharmacological characterization of a novel cannabinoid ligand, MDA19, for treatment of neuropathic pain. Anesthesia and analgesia. 2010;111:99–109. doi: 10.1213/ANE.0b013e3181e0cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. British journal of pharmacology. 2007;152:765–777. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei XH, Na XD, Liao GJ, Chen QY, Cui Y, Chen FY, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Experimental neurology. 2013;241:159–168. doi: 10.1016/j.expneurol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 33.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Zhang H, Dougherty PM. Dynamic effects of TNF-alpha on synaptic transmission in mice over time following sciatic nerve chronic constriction injury. Journal of neurophysiology. 2013;110:1663–1671. doi: 10.1152/jn.01088.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. British journal of pharmacology. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 38.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 39.Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesthesia and analgesia. 2011;113:947–950. doi: 10.1213/ANE.0b013e3182283486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. The Journal of pharmacology and experimental therapeutics. 1998;285:1150–1156. [PubMed] [Google Scholar]
- 41.Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139:225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature medicine. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 43.Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Deutsches Arzteblatt international. 2012;109:495–501. doi: 10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, et al. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12125–12135. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.La Porta C, Bura SA, Aracil-Fernandez A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain. 2013;154:160–174. doi: 10.1016/j.pain.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. Journal of neurochemistry. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. The European journal of neuroscience. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 49.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, et al. 1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyraz ole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiology of disease. 2010;37:177–185. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Burgos E, Gomez-Nicola D, Pascual D, Martin MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. European journal of pharmacology. 2012;682:62–72. doi: 10.1016/j.ejphar.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Bishay P, Schmidt H, Marian C, Haussler A, Wijnvoord N, Ziebell S, et al. R-flurbiprofen reduces neuropathic pain in rodents by restoring endogenous cannabinoids. PloS one. 2010;5:e10628. doi: 10.1371/journal.pone.0010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7091–7101. doi: 10.1523/JNEUROSCI.0403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. British journal of pharmacology. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McQuay H. Opioids in pain management. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 58.Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handbook of experimental pharmacology. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacology, biochemistry, and behavior. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 60.De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behavioural pharmacology. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Welch SP. Characterization of anandamide-induced tolerance: comparison to delta 9-THC-induced interactions with dynorphinergic systems. Drug and alcohol dependence. 1997;45:39–45. doi: 10.1016/s0376-8716(97)01342-2. [DOI] [PubMed] [Google Scholar]
- 62.Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. The Journal of pharmacology and experimental therapeutics. 1994;271:1383–1390. [PubMed] [Google Scholar]
- 63.Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Delta(9)-tetrahydrocannabinol in mice. Drug and alcohol dependence. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- 64.Yao BB, Hsieh G, Daza AV, Fan Y, Grayson GK, Garrison TR, et al. Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2,2,3,3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)-4,5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. The Journal of pharmacology and experimental therapeutics. 2009;328:141–151. doi: 10.1124/jpet.108.145011. [DOI] [PubMed] [Google Scholar]
- 65.Khanolkar AD, Lu D, Ibrahim M, Duclos RI, Jr, Thakur GA, Malan TP, Jr, et al. Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity. Journal of medicinal chemistry. 2007;50:6493–6500. doi: 10.1021/jm070441u. [DOI] [PubMed] [Google Scholar]
- 66.Rahn EJ, Thakur GA, Wood JA, Zvonok AM, Makriyannis A, Hohmann AG. Pharmacological characterization of AM1710, a putative cannabinoid CB2 agonist from the cannabilactone class: antinociception without central nervous system side-effects. Pharmacology, biochemistry, and behavior. 2011;98:493–502. doi: 10.1016/j.pbb.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott MB, Tuma RF, Amenta PS, Barbe MF, Jallo JI. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. Journal of neurotrauma. 2011;28:973–981. doi: 10.1089/neu.2010.1672. [DOI] [PubMed] [Google Scholar]
- 69.Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. Journal of neuroscience research. 2012;90:2293–2305. doi: 10.1002/jnr.23114. [DOI] [PubMed] [Google Scholar]
- 70.Ozek M, Uresin Y, Gungor M. Comparison of the effects of specific and nonspecific inhibition of nitric oxide synthase on morphine analgesia, tolerance and dependence in mice. Life sciences. 2003;72:1943–1951. doi: 10.1016/s0024-3205(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 71.Vekovischeva OY, Zamanillo D, Echenko O, Seppala T, Uusi-Oukari M, Honkanen A, et al. Morphine-induced dependence and sensitization are altered in mice deficient in AMPA-type glutamate receptor-A subunits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:4451–4459. doi: 10.1523/JNEUROSCI.21-12-04451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. The Journal of pharmacology and experimental therapeutics. 2001;298:1007–1014. [PubMed] [Google Scholar]
- 73.Tsou K, Patrick SL, Walker JM. Physical withdrawal in rats tolerant to delta 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. European journal of pharmacology. 1995;280:R13–15. doi: 10.1016/0014-2999(95)00360-w. [DOI] [PubMed] [Google Scholar]
- 74.Aceto MD, Scates SM, Lowe JA, Martin BR. Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. The Journal of pharmacology and experimental therapeutics. 1996;278:1290–1295. [PubMed] [Google Scholar]
- 75.Gutierrez T, Crystal JD, Zvonok AM, Makriyannis A, Hohmann AG. Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain. 2011;152:1976–1987. doi: 10.1016/j.pain.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nature neuroscience. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, et al. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. International journal of obesity. 2010;34:1243–1254. doi: 10.1038/ijo.2010.38. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. The journal of pain : official journal of the American Pain Society. 2012;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, et al. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. The journal of pain : official journal of the American Pain Society. 2013;14:1031–1044. doi: 10.1016/j.jpain.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. The Journal of pharmacology and experimental therapeutics. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiguchi N, Maeda T, Kobayashi Y, Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neuroscience letters. 2008;445:140–143. doi: 10.1016/j.neulet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, et al. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–715. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 88.Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, et al. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PloS one. 2013;8:e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. Journal of neurophysiology. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 90.Sagar DR, Kelly S, Millns PJ, O’Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. The European journal of neuroscience. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 91.Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. The European journal of neuroscience. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


