Abstract
Childhood maltreatment is associated with adverse brain development and later life psychiatric disorders, with maltreatment from the caregiver inducing a particular vulnerability to later life psychopathologies. Here we review two complementary rodent models of early life abuse, which are used to examine the infant response to trauma within attachment and the developmental trajectories that lead to later life neurobehavioral deficits. These rodent models include being reared with an abusive mother, and a more controlled attachment-learning paradigm using odor-shock conditioning to produce a new maternal odor. In both of these rodent models, pups learn a strong attachment and preference to the maternal odor. However, both models produce similar enduring neurobehavioral deficits, which emerge with maturation. Importantly, cues associated with our models of abuse serve as paradoxical safety signals, by normalizing enduring neurobehavioral deficits following abuse. Here we review these models and explore implications for human interventions for early life maltreatment.
Keywords: rat, pup, maternal odor, abuse, stress, later life outcome, fear conditioning, amygdala, threat, predator odor, olfaction, safety signal
INTRODUCTION
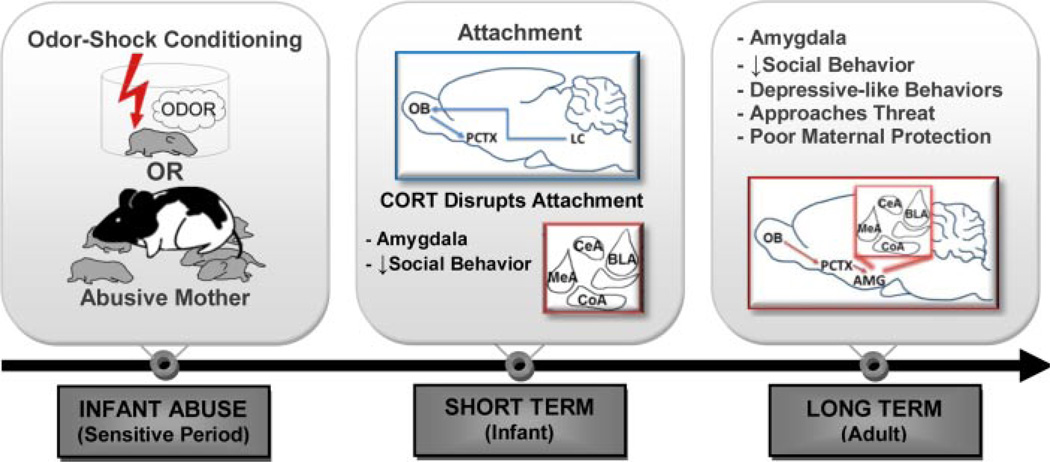
Childhood maltreatment is associated with later life psychiatric disorders and compromised brain development, with maltreatment from the caregiver inducing a particular vulnerability to later life psychopathologies. Paradoxically, maltreated children display strong attachment to their abusive caregivers (Bowlby, 1965; Stronach et al., 2011). However, abuse produces disrupted social behavior, altered emotionality and cognitive function, and an increased susceptibility for mental health disorders in later life (Cicchetti & Toth, 1995; McCrory, De Brito, & Viding, 2012; Pollak, Cicchetti, Hornung, & Reed, 2000). Both human and animal studies suggest that the emergence of abuse-related behaviors is due to compromised brain development, most notably in the limbic system, stress axis, and cerebellum (Bremner, 2003; Sanchez, Ladd, & Plotsky, 2001; Teicher et al., 2003). Here we review our rodent models of early life adversity within attachment to better understand the delayed emergence of abused-related neurobehavioral deficits following early life abuse, which is illustrated in Figure 1.
FIGURE 1.
Schematic of short- and long-term effects following infant abuse. Infant abuse during the sensitive period (until PN10), using our odor-shock conditioning or abusive mother paradigms of abuse, produces converging neurobehavioral deficits across development. Despite abuse, infants form a strong attachment to their caregiver, which is mediated by the olfactory bulb (OB), anterior piriform cortex (PCTX), and locus coeruleus (LC). However, in infants, corticosterone (CORT) uncovers the effects of early life abuse. Heightened CORT in infancy prematurely engages the amygdala, and produces disrupted interactions with the mother in abused pups. By adulthood, the long-term effects of infant abuse on amygdala-dependent behaviors include decreased social behavior, increased depressive-like behaviors, as well as increased approach to predator odor, and poor maternal protection in a threatening situation.
As outlined in this special issue of Developmental Psychobiology, researchers rely on a multitude of rodent models involving early life manipulations, in order to understand the effects of infant experience across the lifespan, as well as the underlying mechanisms (Maestripieri & Carroll, 1998; Raineki, Cortes, Belnoue, & Sullivan, 2012; Roth & Sullivan, 2005; Sanchez et al., 2001). In this manuscript, we highlight our lab’s rodent models of infant abuse, which focus on trauma within the attachment system. The first is a naturalistic model, which uses a stressed mother that maltreats and hurts her pups (Ivy, Brunson, Sandman, & Baram, 2008; Raineki, Moriceau, & Sullivan, 2010; Roth & Sullivan, 2005). The second model uses an olfactory-based classical conditioning paradigm to parallel learning processes evoked during abusive infant-caregiver interactions (odor-shock, with shock mimicking maltreatment) (Raineki et al., 2010). These two models of abuse induce similar neurobehavioral changes during development, with abuse-related deficits emerging with maturation, as we discuss below (Fig. 1). Here we review the developmental neurobehavioral effects of our models of abuse and implications for human interventions for early life abused individuals.
RODENT MODELS OF INFANT ABUSE
Here we present two complementary rodent models of early life abuse, which are used to examine the infant response to trauma within attachment and developmental trajectories that lead to later life neurobehavioral deficits. The first model uses a naturally abusive paradigm where the mother handles her pups roughly when provided with insufficient bedding for nest building (Ivy et al., 2008; Raineki et al., 2010; Roth & Sullivan, 2005). Specifically, rat mothers are stressed by providing them with insufficient bedding (100mL of shavings from PN5-9, PN7-9, or PN8-12) to build a nest, resulting in frequent nest building, trampling, and rough handling of pups, as well as decreased nursing (although typical weight gain occurs) (Raineki et al., 2010; Roth & Sullivan, 2005). This naturalistic paradigm reflects the complex dynamics of an abusive relationship experienced by children, and models the effects of a stressful environment as a risk factor for potentiating infant maltreatment, including in humans (Gunnar, 2003; Righthand, Kerr, & Drach, 2003). Despite multiple occurrences of abusive behaviors from their mother, pups continue to pursue contact with the mother and show approach to odors associated with abuse, even as an adult (Moriceau, Roth, & Sullivan, 2010; Sevelinges et al., 2011).
The second model uses an infant olfactory classical conditioning paradigm where odor-shock pairings (nine pairings of neutral peppermint odor with a .5mA shock to the tail or foot; ITI 4 min) paradoxically produce an odor preference in infant rat pups (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Hofer & Sullivan, 2001; Roth & Sullivan, 2005). More recent work has indicated that a neutral odor paired with shock acquires the same quality and value of the natural maternal odor, and is able to control mother-pup social behavior, despite the association of the odor with aversive shock presentations (Landers & Sullivan, 2012; Moriceau & Sullivan, 2006; Sullivan, Landers, Yeaman, & Wilson, 2000). Furthermore, associative learning of odor-shock pairings before postnatal Day (PN) 10 uses the same neural pathway the infant rat naturally uses to learn maternal odor. When modeling early life abuse, odor-shock conditioning provides a more controlled adverse environment, for the only aversive stimulation pups receive is shock. This allows the assessment of changes in the brain based exclusively on aversive stimulation, and capitalizes on the well-documented neural circuitry in adult and infant rats (Fanselow & LeDoux, 1999; Moriceau & Sullivan, 2006; Moriceau, Wilson, Levine, & Sullivan, 2006). Importantly, this olfactory-based classical conditioning paradigm of abuse also allows for the study of infant learning processes to be explored within the context of attachment.
SHORT-TERM BENEFITS: EARLY LIFE ATTACHMENT DESPITE ADVERSITY
In species that require parental care to survive, evolution has ensured that infants learn rapid and robust preferences to their caregiver, regardless of the quality of care that they receive (Hofer & Sullivan, 2001). Through the use of our lab’s olfactory-based classical conditioning paradigm of abuse, we have identified a sensitive period for attachment learning in infant rats, which provides insight into how attachment occurs in the face of adversity. During the first 10 days of the neonatal rat’s life (“sensitive period”), pups learn to approach and prefer a novel, artificial odor, even when it is paired with painful sensory stimuli, such as tail pinches or moderate (.5 mA) electric shocks (Camp & Rudy, 1988; Sullivan, Brake et al., 1986; Sullivan, Hofer et al., 1986; Sullivan et al., 2000). Pain-induced odor preference learning of rat pups is neither due to the pups’ inability to detect pain nor to differences in their pain threshold (Barr, 1995; Moriceau et al., 2010). Rather, it is dependent upon the unique neurobiological system that prevents pups’ ability to learn to avoid odors associated with painful stimuli. Specifically, the olfactory bulb, anterior piriform cortex, and hyperfunctioning noradrenergic locus coeruleus (LC) underlie heightened preference learning (Sullivan & Wilson, 1991; Sullivan, Wilson, & Leon, 1989; Sullivan, Zyzak, Skierkowski, & Wilson, 1992), while reduced corticosterone (CORT) and the hypofunctioning amygdala appear to underlie attenuated aversion learning (Barr et al., 2009; Moriceau et al., 2006; Sullivan et al., 2000).
Odor attachment learning may have evolved to prevent pups from learning to avoid or inhibit responses towards the mother, as brief painful stimuli are part of typical maternal behaviors as she occasionally handles pups roughly or tramples them, such as when she enters or leaves the nest (Hofer & Sullivan, 2001; Roth & Sullivan, 2005). Indeed, assessment of pup behavior immediately following both of our animal models of abuse does not indicate abuse-related disruption of pup attachment learning. Abused pups show a paradoxical preference to their mother and cues associated with abuse (Raineki et al., 2010; Roth & Sullivan, 2005). Therefore, it seems that the neural pathway to support infant learning has been presumably designed through evolution to ensure caregiver attachment (and hence survival).
UNCOVERING THE EFFECTS OF EARLY LIFE ADVERSITY
While the effects of infant abuse can be identified, in general these effects are not always readily detectable (Pollak, 2008). However, the differences between abused and normal children become more apparent if the children are stressed (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996). One method of stressing a child is via the Strange Situation Test, in which a caregiver brings a child into a room and then leaves, after which a stranger comes in and attempts to engage the child. Only after repeated rounds of these stressful events, and after the final stage of this test when the child and caregiver are reunited, will an abused child finally begin to show signs of “disorganized attachment,” simultaneously showing a need for and avoidance of the caregiver (Ainsworth, 1969). Indeed, in both of our abusive mother and odor-shock models of abuse, stress (increased CORT) uncovers detrimental effects of early life abuse in infancy. Our abusive mother paradigm, which increases pups’ CORT levels through pup adrenal CORT and the mother’s milk, produces a decreased preference for maternal odor, decreased time nursing, and increased activation of the amygdala following abuse (relative to non-abused pups) (Raineki et al., 2010). The emergence of these deficits is related to increased CORT, for pairing our odor-shock abuse paradigm with systemic CORT injections similarly produces disrupted social behavior and recruitment of the amygdala in abused pups (Raineki et al., 2010). This suggests that normal and abusive attachments, while appearing to have similar supporting neural circuits in infancy, have divergent neural circuits that can be uncovered by CORT via recruitment of the amygdala (Raineki et al., 2010, 2012). As discussed below, as individuals mature, stress is no longer necessary to uncover the effects of early life adversity.
LONG-TERM COSTS: ENDURING EFFECTS OF EARLY LIFE ADVERSITY
With maturation the effects of early life abuse become more readily apparent, and include problems in self-regulation, excessive anxiety and fearfulness, aggression, substance abuse, mood disorders, and posttraumatic stress disorder (Righthand et al., 2003; Zeanah, Keyes, & Settles, 2003). Human and animal research have revealed that early life adversity produces long-term changes in brain areas that regulate stress, cognition, and emotion, suggesting that the altered development of these areas contributes to the emergence of psychiatric disorders (McEwen, 2003). However, the mechanisms by which adversity produces brain and behavioral changes remains unclear, and is hindered by our limited understanding of the age at which brain function emerges within specific behavioral systems.
Our abusive mother and odor-shock rodent models of early life abuse produce similar enduring negative effects, which emerge by adulthood, and provide insight into mechanisms by which early life abuse produces later life neurobehavioral deficits. Specifically, following both models of abuse, social behavior deficits emerge just before weaning (PN20) and persist into adulthood. Additionally, depressive-like behaviors (as assessed through sucrose consumption and Forced Swim Tests) emerge from weaning through peri-adolescence, persist into adulthood, and are associated with increased amygdala activation in central, lateral, and basal nuclei. In fact, a causal relationship between amygdala activity and depressive-like behavior was suggested through temporary deactivation of the amygdala (via muscimol infusions), which rescued depressive- like behavior seen in the Forced Swim Test (Raineki et al., 2012; Sevelinges et al., 2011).
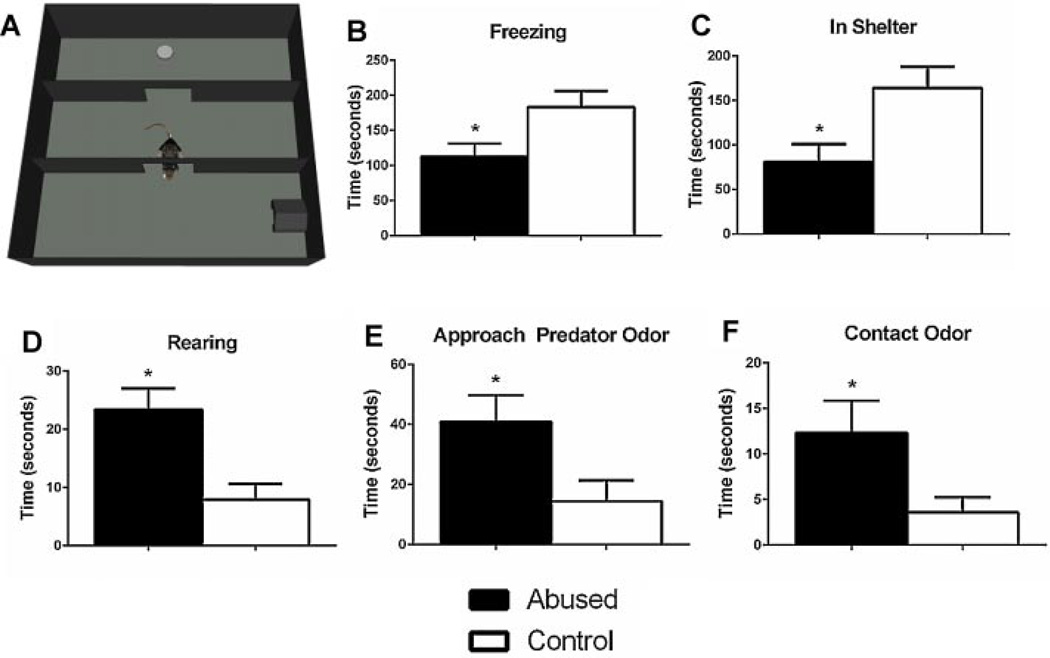
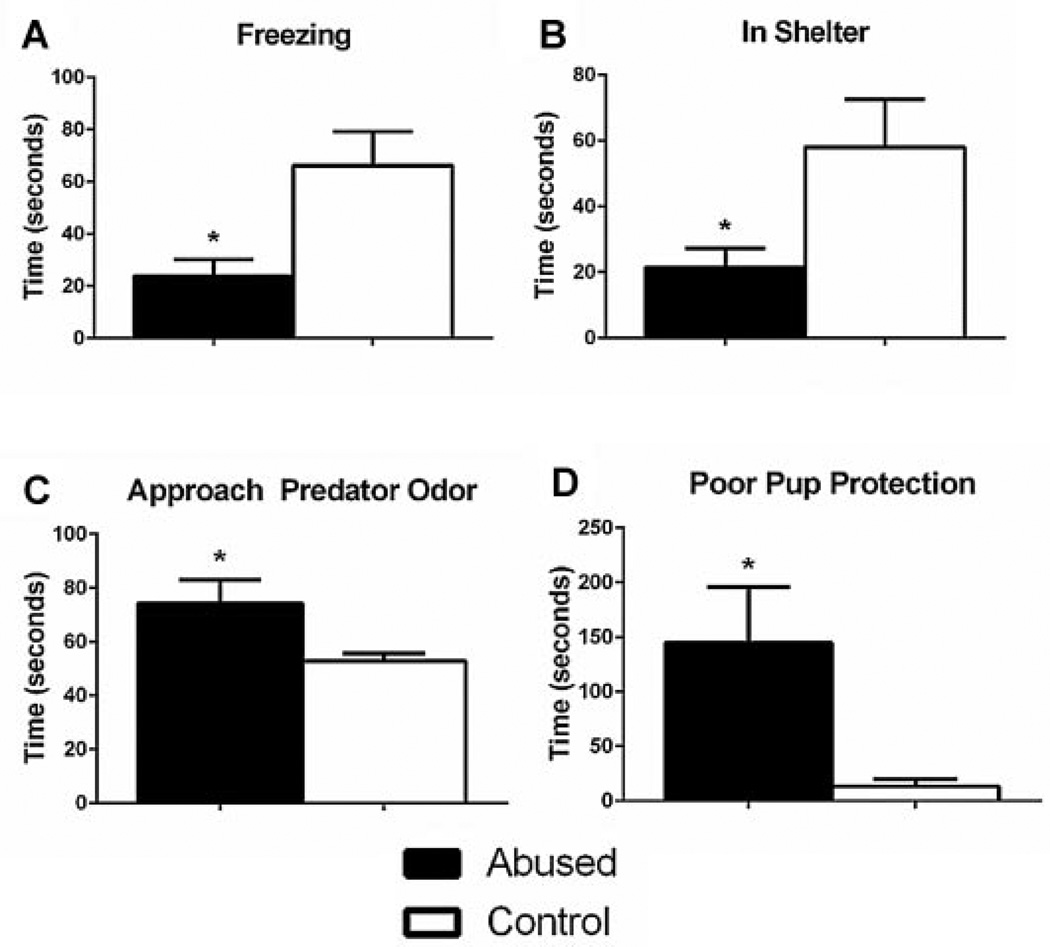
Interestingly, early life abuse rearing modifies other amygdala-dependent behaviors, such as defensive responses to threat. Abuse-experienced animals show maladaptive threat responding and poor maternal protection of pups. Specifically, in a Threat Response Selection Test (TRST), which permits defensive response options (i.e., freezing, hiding, approach), adults that were abused in infancy by their mothers show increased active responses to threat, including increased rearing, approach, and contact with the predator odor (Fig. 2D–F), which are behaviors typically associated with a heightened perception of predatory imminence (Blanchard & Blanchard, 1989; Fanselow, 1994). Furthermore, abuse-reared adults show decreased passive responses to threat relative to typically reared animals, as indicated by decreased time spent freezing and hiding in the provided shelter (Fig. 2B, C). Similarly, mothers that were abused in infancy exhibit less passive fear behaviors (as assessed by time spent freezing and hiding in the shelter), and increased approach to the predator odor, relative to typically reared control animals (Fig. 3A–C). Furthermore, early life abused mothers display poor protection of their pups in the TRST. Abused mothers spend increased time scattering their pups throughout the testing arena, transporting their pups, and placing pups close to the predator odor (Fig. 3D). Maternal observations confirmed that mothers that were abused in infancy show normal mother-infant interactions, unless they are stressed, which in turn produces poor maternal behavior. Thus, the results of this study reveal that infant abuse produces maladaptive threat responding and abolishes maternal protection of pups in a stressful situation, making it more likely for one to place itself and others in harm’s way. This altered response to threat is likely due to abuse-related changes in the amygdala. Indeed, a circuit has been identified in the amygdala that biases responses toward either passive or active coping strategies (Gozzi et al., 2010).
FIGURE 2.
All animals, regardless of rearing conditions, display fear to predator odor. However, as illustrated by these data, infant abuse alters the adult response to threat by decreasing passive defensive behaviors and increasing active defensive behaviors in adults. (A) This figure illustrates the Threat Response Selection Test (TRST). This test permits the assay of both active and passive defensive responses. In this test, individuals are placed in a center “start” chamber and given the option to (i) freeze, (ii) approach a predator odor (fox urine), or (iii) hide in a provided shelter. The rat is allowed free roam of the apparatus for 5 min, and active and passive fear responses are scored. Each animal is habituated to the apparatus without predator odor on the day before testing, so they are familiar with the apparatus and the location of the shelter. In the TRST, early life abused animals (B) spend significantly less time freezing, relative to normally reared controls [unpaired t-test, t(12) = 2.383, p < .05, n = 7], (C) spend less time hiding in the shelter [unpaired t-test, t(12) = 2.660, p < .05, n = 7], (D) spend significantly more time rearing (standing on hindlimbs) [unpaired t-test, t(12) = 3.417, p < .01, n = 7], (E) spend more time approaching the section of the apparatus that contains predator odor [unpaired t-test, t(12) = 2.339, p < .05, n = 7], and (F) spend significantly more time in direct contact with the predator odor [unpaired t-test, t(12) = 2.225, p < .05, n = 7]. Thus, these data illustrate that early life abused animals spend significantly less time engaged in passive fear behaviors than typically reared control animals (as indexed by time spent freezing and hiding in the provided shelter), and significantly more time engaged in active fear behaviors than typically reared control animals (as indexed by time spent rearing, approaching the predator odor, and contacting the predator odor). *Significantly different from all other groups, p < .05, unpaired t-test. Error bars represent SEM.
FIGURE 3.
All mothers, regardless of rearing condition, display fear to predator odor. However, as illustrated in the TRST, mothers abused in infancy show a heightened threat response and display poor protection of their pups in a threatening situation relative to controls. Mothers that were abused in infancy: (A) exhibit significantly less time freezing [unpaired t-test, t(16) = 2.959, p < .01, n = 9], (B) spend less time hiding in the shelter [unpaired t-test, t(16) = 2.333, p < .05, n = 9], (C) spend significantly more time in the section of the arena that contains predator odor [unpaired t-test, t(16) = 2.342, p < .05, n = 9], and (D) fail to protect their pups in response to predator odor [unpaired t-test, t(16) = 2.546, p <.05, n = 9]. Poor pup protection is determined by total time spent scattering their pups, transporting their pups, and placing their pups close to the predator odor. *Significantly different from all other groups, p < .05. Error bars represent SEM.
The usefulness of the TRST is that it may provide insight into human research that has identified abused children as having a heightened sensitivity to threat (Pollak et al., 2000; Pollak & Tolley-Schell, 2003), and also a behavioral repertoire that is more likely to include active responses to threat, including aggression (Malinosky-Rummell & Hansen, 1993). Specifically, abused children are able to accurately identify threatening facial expressions using limited perceptual information (Pollak & Sinha, 2002), devote more attention to the processing of angry faces (Pollak, Cicchetti, Klorman, & Brumaghim, 2006; Pollak, Klorman, Thatcher, & Cicchetti, 2001), and have greater difficulty disengaging their attention from angry faces (Pollak & Tolley-Schell, 2003). This increased sensitivity to threat following early life abuse is paired with an increased prevalence of aggressive and violent behaviors emerging by adolescence (Malinosky-Rummell & Hansen, 1993).
The use of our novel TRST has demonstrated for the first time in an animal model that infant abuse seems to heighten the perception of danger, by producing increased active defensive responses that are indicative of high levels of predatory imminence (Blanchard & Blanchard, 1989; Fanselow, 1994). These data, combined with the human literature, suggest that abused infants show amplified attention to threat, perhaps as a form of adaptation to an environment where threat signals may predict the occurrence of abuse.
The use of the TRST following models of early life adversity allows for the controlled study of the response selection process in response to threat. Importantly, the use of the TRST to assess the behavioral response to threat not only compliments the assessment of freezing and avoidance behaviors (Choi, Cain, & LeDoux, 2010; Phillips & LeDoux, 1992), but also compliments the use of other expanded apparatuses that permit the assay of a more inclusive behavioral repertoire, going beyond the assessment of just freezing and avoidance in response to threat (Blanchard, Yang, Li, Gervacio, & Blanchard, 2001; Do Monte, Canteras, Fernandes, Assreuy, & Carobrez, 2008).
ATTACHMENT CUES AS SAFETY SIGNALS
Compelling evidence from a variety of studies converge in suggesting that early life abuse produces heightened or proactive responses to threatening stimuli, and moreover produces an increased prevalence and severity of fear-disorders and aggression throughout life (Andersen, 2003; Famularo, Kinscherff, & Fenton, 1992; Heim & Nemeroff, 2001). As outlined in this review, both our abusive mother and odor-shock paradigms of infant abuse converge with the findings of many abuse related-attachment models, by producing long-term negative consequences that emerge with maturation, including decreased social behavior, increased depressive-like behavior, and an amplified response to threat (Pryce et al., 2005; Sanchez et al., 2001; Teicher et al., 2003). These behaviors are central to many fear and anxiety-related disorders, and thus our models of abuse have begun to provide mechanistic insight into how infant abuse leads to the increased prevalence of these disorders, including depression (Raineki et al., 2012), and anxiety (Sarro, Sullivan, & Barr, 2014; Tyler, Moriceau, Sullivan, & Greenwood-van Meerveld, 2007).
Importantly, through the use of our models of abuse, we have begun to identify potential ways to rescue the negative long-term effects of infant abuse, by using early life attachment odors. Indeed, an odor cue paired with stroking in infant pups enhances later life sexual behavior (Fillion & Blass, 1986). Amazingly, even when an odor is paired with shock (as in our odor-shock paradigm of abuse), we see similar results. We have found through the use of our infant odor-shock paradigm that pairing a neutral peppermint odor with aversive shock in infancy produces a seemingly paradoxical odor preference, which endures into adulthood. Specifically, infant odor-shock conditioning produces an odor preference that enhances sexual and social behavior, and combats depressive-like behavior when the early life peppermint cue is presented (Raineki et al., unpublished observations; Sevelinges et al., 2011). Furthermore, the presentation of this odor prevents deficits in paired-pulse inhibition in the amygdala and piriform cortex, which are seen in animals following infant abuse (Moriceau, Raineki, Holman, Holman, & Sullivan, 2009; Sevelinges et al., 2007, 2011). Similarly, animals reared by an abusive mother develop a strong preference for her maternal odor, or other odors that have been associated with infant abusive caregiving. These odors acquire the ability to reduce fear and amygdala neural activity (Sevelinges et al., 2007; Sevelinges, Levy, Mouly, & Ferreira, 2009). Thus, it seems that early life odors associated with our paradigms of infant abuse acquire the properties of a “safety signal.”
Indeed, it has long been documented that cues associated with maltreatment are believed to sometimes elicit safety, comfort, and strong attraction (Freud, 1997; Haynes-Seman, 1987). Furthermore, learned safety cues, which are produced by negatively correlating a signal with aversive events, reduce the expression of learned and instinctive fear, and are associated with positive affective responses in rodents, as well as decreased amygdala activity (Rogan, Leon, Perez, & Kandel, 2005). The ability of cues associated with our early life abuse paradigms to normalize behavior and amygdala activity suggests that these cues provide adaptive value in adulthood, and could provide use for clinical intervention.
CONCLUSION
Infants form attachments to their caregivers regardless of the quality of the parental care (Hofer & Sullivan, 2001). This is true for a wide range of species, suggesting that our lab’s animal models of early life abuse are useful in assessing principles with wide phylogenetic importance (Helfer, 1997). However, trauma within the attachment system leaves the infant particularly vulnerable to the development of psychiatric disorders, behavioral changes in fear and anxiety, and alteration of neural circuits, particularly those regulating stress and emotion (Gunnar, 2003; Teicher et al., 2003). Overall, our animal models of abuse demonstrate that early life adversity affects the developing brain and offer potential sites (limbic system, stress axis) to understand the damaging effects of early adversity on subsequent behavioral development (Bremner, 2003; Sanchez et al., 2001; Teicher et al., 2003). Furthermore, our studies suggest that the effect of early life experiences differ during development and are specific to the age of assessment. Importantly, stimuli associated with early life abuse (which serve as a sort of paradoxical safety signal), may provide potential use for novel treatments of developmental effects of trauma.
Acknowledgments
Contract grant sponsor: NIH
Contract grant numbers: MH091451, DC009910, T32MH096331
REFERENCES
- Ainsworth MD. Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child Development. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Research Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S. Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yang M, Li CI, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neuroscience and Biobehavioral Reviews. 2001;25(7–8):587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. New York, NY: Basic Books; 1965. [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child and Adolescent Psychiatric Clinics of North America. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learning & Memory. 2010;17(3):139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(5):541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Do Monte FH, Canteras NS, Fernandes D, Assreuy J, Carobrez AP. New perspectives on beta-adrenergic mediation of innate and learned fear responses to predator odor. The Journal of Neuroscience. 2008;28(49):13296–13302. doi: 10.1523/JNEUROSCI.2843-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: Preliminary findings. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(5):863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1(4):429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231(4739):729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Freud SS. Collected writings compiled by the International Psychoanalytic Association. Vol. 5. Yale University Press; 1997. A child is being beaten. [Google Scholar]
- Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ. A neural switch for active and passive fear. Neuron. 2010;67(4):656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Annals of the New York Academy of Sciences. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Haynes-Seman C. Developmental origins of moral masochism: a failure-to-thrive toddler’s interactions with mother. Child Abuse & Neglect. 1987;11:319–330. doi: 10.1016/0145-2134(87)90005-6. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Helfer ME. The battered child. Chicago: University of Chicago Press; 1997. [Google Scholar]
- Hofer MA, Sullivan RM. Towards a neurobiology of attachment. Cumberland, RI: MIT Press; 2001. [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Child abuse and neglect: Usefulness of the animal data. Psychological Bulletin. 1998;123(3):211–223. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- Malinosky-Rummell R, Hansen DJ. Long-term consequences of childhood physical abuse. Psychological Bulletin. 1993;114(1):68–79. doi: 10.1037/0033-2909.114.1.68. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: A review of neurobiological and genetic research. Journal of the Royal Society of Medicine. 2012;105(4):151–156. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Frontiers in Behavioral Neuroscience. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. The Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17(6):370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Klorman R, Brumaghim JT. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Development. 2006;68(5):773–787. doi: 10.1111/j.1467-8624.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38(2):267–274. [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38(5):784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112(3):323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29(4–5):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro EC, Rincon Cortes M, Perry R, Boggs J, Holman CJ. Abusive attachment odor cues learned during infancy rescue adult depressive-like behaviors and enhance sexual motivation. (unpublished observations). [Google Scholar]
- Righthand S, Kerr B, Drach K. Child maltreatment risk assessments: An evaluation guide. New York: The Haworth Press, Inc; 2003. [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46(2):309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Levy F, Mouly AM, Ferreira G. Rearing with artificially scented mothers attenuates conditioned odor aversion in adulthood but not its amygdala dependency. Behavioural Brain Research. 2009;198(2):313–320. doi: 10.1016/j.bbr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biological Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Developmental Cognitive Neuroscience. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach EP, Toth SL, Rogosch F, Oshri A, Manly JT, Cicchetti D. Child maltreatment, attachment security, and internal representations of mother and mother-child relationships. Child Maltreatment. 2011;16(2):137–145. doi: 10.1177/1077559511398294. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19(6):625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psychobiology (Austin, Tex) 1991;19(4):308–312. doi: 10.3758/bf03332084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. The Journal of Neuroscience. 1989;9(11):3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Research Developmental Brain Research. 1992;70(2):279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Tyler K, Moriceau S, Sullivan RM, Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterology and Motility. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Keyes A, Settles L. Attachment relationship experiences and childhood psychopathology. Annals of the New York Academy of Sciences. 2003;1008:22–30. doi: 10.1196/annals.1301.003. [DOI] [PubMed] [Google Scholar]