Abstract
Background and Purpose
Bone marrow derived mononuclear cells (MNCs) are an investigational autologous cell-based therapy for acute ischemic stroke. Both intravenous (IV) and intra-arterial (IA) administration routes have been used in clinical trials. However, the route of administration to optimize the effect of MNCs is unknown. In this study, we compared the effect of IV versus IA route of MNCs in the rat stroke model.
Methods
Long Evans rats were subjected to transient middle cerebral artery occlusion (MCAo). At 24 hrs after stroke, animals were randomly assigned to either receive autologous bone marrow derived MNCs using IV or IA delivery routes. IV saline served as control. 1 million cells/kg (low dose) and 30 million cells/kg (high dose) were assessed. Neurological testing, cavity size, serum cytokines, neuroregenerative endpoints, and MNC biodistribution were evaluated.
Results
High dose MNCs improved functional recovery, reduced lesion size and pro-inflammatory cytokines, and increased vessel density and neurogenesis markers compared with saline treatment (p<0.05). However, there were no significant differences between IV and IA MNC treated groups; though, IV MNCs reduced serum IL-1β levels compared with IA MNCs (p<0.05). IA MNCs at high dose led to a greater number of cells in the brain at 1 and 6 hrs after injection but not in the lungs and spleen. Low dose MNCs (by IV or IA) did not improve any functional or structural endpoint compared with saline.
Conclusion
At low and high doses of MNCs, we found that IV or IA achieves similar structural and functional outcomes after stroke.
Introduction
Bone marrow derived mononuclear cells (MNCs) are being tested as an autologous cell-based therapy in stroke patients in early phase clinical trials. We and others have reported that intravenous (IV) or intra-arterial (IA) delivery of MNCs enhance recovery after acute ischemic stroke in rodent models1, 2 and both routes of administration have been brought forward to clinical studies3-5. However, it is unclear which delivery route is better to enhance recovery after stroke.
IA administration carries the theoretical advantage of selective delivery to the injured brain but may carry risks of occlusion or embolization. IV administration is least invasive but given pulmonary sequestration may not deliver cells to the same extent to the brain2. Few studies have directly compared delivery routes and the results are conflicting6, 7. In this study, we performed a direct comparison of the effects of IV versus IA delivery of autologous MNCs in the rodent stroke model and assessed for potential differences in biodistribution, behavioral outcome, and selected mechanistic effects.
Methods
Animals
In this study,168 male Long Evans Rats (weight: 300-320 g) were employed. All animals were housed in pairs with free access to food and water and maintained on a standard 12:12 h light/dark cycle. All animal experiments and surgical procedures were approved by the University of Texas Health Science Center Animal Welfare Committee and followed NIH guidelines and regulations. Experimental Group information and flow chart is shown in Fig 1.
Fig 1.
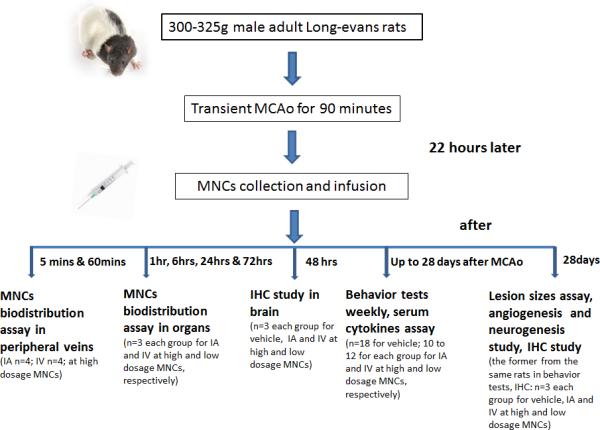
Schematic representation of the experimental groups, time line, and measured outcomes
Stroke Model
Transient focal brain ischemia was induced by a modified intraluminal middle cerebral artery occlusion (MCAO) suture method as previously described1, 8. Briefly, rats were anesthetized with 1-2% isoflurane in a mixture of 30% oxygen and 70% nitrous oxide by face mask. Through a midline neck incision, left common, internal and external carotid arteries were exposed and a 3-0 nylon filament with blunt tip is inserted through a stump of the external carotid artery. The common carotid artery was then clipped off and the filament was advanced into the internal carotid artery 19 to 21 mm beyond the carotid bifurcation. Mild resistance indicates proper filament placement in the middle cerebral artery confirmed by laser Doppler flow reduction of the MCA perfusion territory greater than 75% from baseline. Focal ischemic time was 90 minutes and reperfusion was accomplished by withdrawing of the suture and removing the CCA clip. The reperfusion was confirmed using laser Doppler. The body temperature was maintained at 36.5°C±0.5°C during surgery and animals were allowed to recovery at room temperature.
Bone Marrow Harvest
Bone marrow was harvest as previously described 1 from tibia at 22 hrs after ischemia. Briefly, the rats were anesthetized with isoflurane 1-2%. An incision was made through the skin to the medial aspect of the tibia. The periosteum was removed and a 1.25 × 1.25 mm burr hole was made extending into the medullary cavity. A 27×1/2 gauge hypodermic needle connected to a heparinized syringe was inserted into the medullary cavity and the bone marrow (1-1.5 mL) was aspirated while rotating and moving the needle back and forth. In the saline control group, a sham procedure was performed involving a burr hole and needle insertion of the tibia without bone marrow aspiration. The burr hole was sealed with bone wax and the skin closed with a nylon suture. This limited aspiration of the bone marrow did not cause impairment of the limbs and animals are able to fully participate in neurological testing1.
MNCs Isolation
MNCs were isolated from bone marrow using Ficoll density gradient centrifugation, as we published previously1. The cells from the bone marrow aspirate were triturated, centrifuged, and washed in PBS + 0.5% bovine serum albumin (BSA). Cells were then suspended in Media 199 and added on top of 20 mL Ficoll-Paque PLUS (GE Healthcare) in a 50mL conical tube and centrifuged. The MNCs layer were collected, washed with PBS+0.5% (BSA) and then counted. Cell viability was more than 98% by trypan blue detection. Cells were then washed and resuspended in 1ml sterile cold saline at the desired concentration for infusion.
MNCs administration
At 24 hrs after MCAo, animals were randomized to receive saline IV or autologous bone marrow derived MNCs by an IV route through the left jugular vein or IA route through the left internal carotid artery. MNC treated groups received either 1 million cells/kg or 30 million cells/kg. We chose these doses because we previously demonstrated a protective effect of MNCs at 30 million cells/kg (IV) but not at 1 million cells/kg (IV)2. We hypothesized that an IA delivery of the lower dose might lead to a therapeutic effect. In our experience (data not shown), we have found that IV or IA administration of saline results in indistinguishable behavioral outcomes in our rodent stroke model. We therefore chose IV saline as a common control group. Similar to our previous studies, cells were infused with an auto-injection pump at a rate of 0.2mL/min over 5 mins.
Behavior test
Animals underwent long term behavioral testing which was performed by an examiner blinded to treatment allocation. Animals were pre-tested before MCAo and then tested on days 1, 3, 7, 14, 21, 28 post-ischemia. We used the cylinder, circling, and adhesive removal tests as we have previously reported9 to evaluate dysfunction in our model. We determined that the other tests conducted for these behavioral analyses -- beam balance, placing, and flexion were not sensitive to detect long term deficits in our model. All animals were pre-trained for behavior tests for two weeks.
Lesion Size
After 28 days after stroke, animals were anesthetized and intracardially perfused with ice-cold PBS, followed by ice-cold 4% paraformaldehyde (PFA) in PBS and decapitated. Brains were harvested, post-fixed in 4% PFA in PBS for 24 hours, immersed in 20% Sucrose for 2 days and divided into 6 sections (2 mm). Coronal 20 μm frozen sections from each section were stained with cresyl violet. As we reported previously, tissue loss of the ipsilateral chronic infarct was measured using the indirect method and expressed as a percentage of the contralateral hemisphere by a researcher blinded to treatment groups 2.
Biodistribution
Bone marrow MNCs (1 million cells/kg and 30 million cells/kg) were labeled with Q-tracker655 (red) and then administrated via IV or IA to rats subjected to MCAo 24 hrs earlier. We used 5 rats per group. At 1, 6, 24 and 72 hrs after MNC injection, animals were anesthetized and intracardially perfused with ice-cold PBS followed by ice-cold 4% PFA. As we described previously 2, the brain, lungs and spleen were removed and post-fixed for 24 hrs in 4% PFA and then immersed in 20% sucrose and stored at 4°C for at least 48 hrs. 20 μm cryosections were then generated and counterstained with DAPI (blue) for microscopic analysis. Q-tracker positive MNCs were quantified with the assistance of Image J (NIH). Three randomly chosen sections per each organ of predefined regions of interest, involving 9 fields per section under 400x magnification were analyzed. The regions of interest were in the peri-ischemic tissue of the brain at the level of sections from +1 mm to −7 mm relative to the bregma, in the lower lobe of the lungs, and in the spleen. To assess the biodistribution of MNCs in the peripheral circulation, 1ml venous blood was sampled from the jugular and femoral vein at 5 and 60 minutes after Q-tracker labeled MNC infusion IV and IA at 30 million cells/kg. Then the blood was treated with Immunoprep Reagent System (Beckman Coulter, USA ) as per the manufacturer suggested and run using Gallios Flow Cytometer (Beckman Coulter, USA). Data was analyzed with Kaluza software (Beckman Coulter, USA).
Western Blot Analysis
At 3 or 28 days after stroke rats were perfused intracardially with ice-cold PBS. Brains were harvested. The injured ipsilateral hemisphere was homogenized on ice in RIPA buffer (Invitrogen, USA). The protein concentration of each sample was determined using the Bicinchoninic Acid Assay (Sigma-Aldrich, USA). 50 μ g of protein was separated on a 4-12% gradient SDS-PAGE gel using a Novex Mini Cell (Invitrogen, USA). The proteins were transferred onto a PVDF membrane (Invitrogen, USA) using theNovex Mini Cell system. Membranes were blocked (5% non-fat milk, 0.1% Tween-20 in Tris-buffered saline, pH 7.6) at room temperature for 2 hrs and incubated with primary antibodies overnight at 4°C. We used 1:100 rabbit polyclonal anti-rat BDNF (Santa Cruz Bio, USA), 1:100 goat polyclonal anti-rat NGF (Santa Cruz Bio, USA), 1:100 goat polyclonal anti-rat NT-3 (Santa Cruz Bio, USA), 1:100 Rabbit polyclonal anti-rat iNOS (Abcam, USA), and 1:100 goat polyclonal anti-rat GAP-43 (Santa Cruz Bio, USA) as primary antibodies. Mouse monoclonal anti-rat β-actin (1:2,000, Sigma-Aldrich) was used as a normalizing control. HRP-conjugated mouse monoclonal antibodies (Ebioscience, USA) to rabbit and goat were used as secondary antibodies and membranes were incubated for one hour at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence (ECL) system (GE Healthcare, USA) according to the manufacturer's protocol. X-ray films were scanned and then analyzed with Image J for densitometrical analyses.
Immunofluorescence staining
For immunohistochemical analyses, coronal 10 μm frozen brain sections at the level of the infarction were blocked for 2 hrs in 5% goat-serum, 0.01% Triton-100 in 0.1M PBS at room temperature. Sections were then incubated with rabbit anti-rat CD31 antibody (1:100, Abcam, USA), or rabbit anti-rat Doublecortin (DCX) antibody (1:10000, Abcam, USA) overnight at 4°C and followed by secondary goat anti-rabbit antibody (1:500 Alex Fluor 594 for CD31, 1:1000 Alex Fluor 488, Invitrogen, USA) for 4 hrs at room temperature. For CD31 positive vessels analysis, six frozen sections were randomly selected from each of 2 mm sections for lesion size measurement. 9 views from each 10 μm section were collected in the ipsilateral hemisphere and the CD31 positive vessels were analyzed. For Doublecortin positive cells analysis, only sections at the level between −1mm to −4mm relative to the bregma were selected. All sections for analysis were mounted by Vectashield with DAPI (Vector Lab, USA). The immunofluorescence signal was captured using fluorescence microscope equipped with CCD camera and immunopositive vessels were counted by using software ImageJ (NIH).
Fluoro-J staining
To test whether IA MNCs cause micro-infarcts resulting in cell death, rats were perfused intracardially with ice-cold PBS at 24 hours after MNCs or saline infusion. 40 μm fresh frozen sections were generated and fixed with cold 2% PFA (for 20 minutes) following by immersion in 1% sodium hydroxide, in 80% ethanol (for 5 minutes), rinsing in 70% ethanol (for 2 minutes), rinsing in water (for 2 minutes) and incubation in 0.06% potassium permanganate solution (for 20 minutes). Slides were then transferred into a 0.0001% solution of Fluoro-Jade B (Histo-Chem Inc., USA) dissolved in 0.1% acetic acid. This was followed by three 1-minute rinses in distilled water. Dried slides were cleared in xylene for 1 minute and coverslipped with a xylene-based mounting media (Richard Allan Scientific, USA). Sections of the hemisphere ipsilateral to the carotid injection were visualized and analyzed for the abundance of fluorescent cells under a fluorescence microscope.
Serum cytokines measurements
Venous blood serum samples were collected at various time points. IL-1β, TNF-α, IL-10 (Thermo Scientific, USA), and IL-6 (Invitrogen, USA) levels were detected by ELISA according to the manufacture's protocols.
Statistical Analysis
Data are presented as Means±SD. Repeated measures, analysis of variance and Bonferroni posttest were used for comparison among groups at different days after stroke in the behavioral tests. For the lesion size, neurotrophins and regenerative responses, a one way ANOVA was performed with post-hoc Tukey-Kramer tests. For the cytokines and MNCs biodistribution measurement, a two-way repeated ANOVA and Bonferroni post-test were used. Behavior, cavity volume, histology, and serum markers were analyzed across all groups. Statistical significance was set at p<0.05 level.
Results
Mortality/Model Failure
Animals that did not have a reduction of cerebral blood flow greater than 80% (4 animals) upon MCA occlusion and animals that died within 22 hrs after MCAo (6 animals) were excluded from the experiment. The remaining animals were randomly allocated to saline or IV or IA MNCs [(high dosage at 30 million cells/kg, n=12) and low dosage at 1 million cells/kg, n=10 per group)]. Three animals died in the saline group that served as a control. There was no mortality after MNC administration in the IV and IA groups.
Behavior tests
We found a significant reduction in neurological deficits at 28 days after stroke (Fig 2A) in animals treated with 30 million MNCs/kg, irrespective of delivery route, compared with saline controls. Both IV and IA treated groups achieved similar degree of recovery compared with saline controls and no significant differences were found between the two groups by delivery route. In the animals treated with 1 million MNCs/kg, there was no difference in functional scores among IV, IA or saline treated groups (Fig 2A, B and C).
Fig 2. Autologous MNCs have the same impact on recovery and lesion size by IV or IA routes.
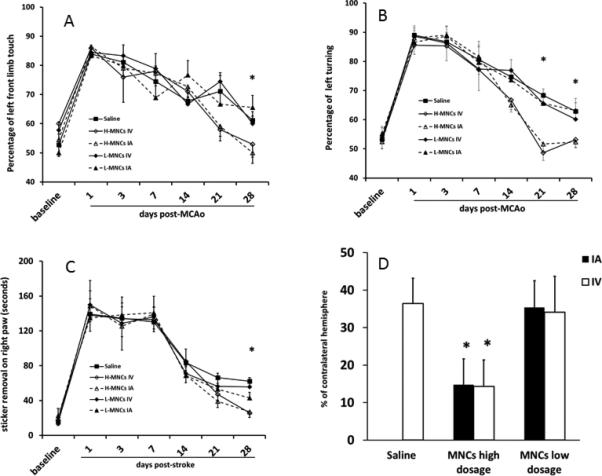
Fig 2A, B and C: Graphical representation of performance on the cylinder test (A), circling test (B) and adhesive removal test (C). Rats treated with autologous MNCs at 30 million cells/kg (high dose) achieved better neurological outcomes by IV or IA routes compared with saline controls at 28 days after stroke. However, there was no difference in outcome between the two routes. Rats treated with 1 million cells/kg showed no differences in neurological outcomes with saline controls. Higher numbers indicate more severe deficits.
Fig 2D: At 28 days after stroke, cavity size was reduced in animals treated with 30 million cells/kg of MNCs but not 1 million cells/kg. There were no differences between IV and IA routes.
Data are Means±SD. *: p< 0.05, comparing high dose IA or IV groups with saline controls. Saline Group n=18; High dosage (H-MNC) groups: H-MNCs IV n=10, H-MNCs IA n=11; low dosage (L-MNC) groups: L-MNCs IV n=9, L-MNCs IA n=9.
Lesion Size
We found a significant reduction in brain lesion size in animals treated with 30 million MNCs/kg of in both IV and IA groups compared with the saline treated controls. The robustness of lesion size reduction was similar for the IV and IA treated groups. In the animals treated with 1 million MNCs/kg, there was no difference among the three groups (Fig 2D).
Biodistribution
Given that IV and IA delivery of MNCs led to equal functional benefit, we assessed for similarity in the biodistribution of MNCs between these delivery routes. In a separate experiment, rats were subjected to MCAo and randomly divided into four groups, where the same high dosage of MNCs (30 million cells/kg) and low dosage (1 million MNCs/kg), as described previously, were given IV or IA (n=5 per time point per group). At a dose of 30 million cells/kg, there were significantly more Q-tracker labeled cells observed in the peri-infarct area at 1 and 6 hours after cell infusion in animals that received IA MNCs, compared with animals that received IV MNCs (Figure 3A). However, there were no significant differences in the number of labeled cells between IV and IA groups at 24 hours and 3 days (Fig 3A) after injection. There were also no significant differences in the number of labeled MNCs in the spleen and lungs at all four time points examined between IV and IA groups (Fig 3A). At a dose of 1 million MNCs/kg, we found no significant differences in the number of labeled cells at any time points after IV or IA injection in the brain, spleen, or lungs (Figure 3A). Representative photomicrographs of labeled cells in the brain are shown for high and low dose (Figure 3B). We then quantified the presence of labeled cells in the venous circulation in the jugular and femoral vein after IV or IA injection of 30 million MNCs/kg. We found a significant reduction in the number of labeled MNCs in both jugular and femoral vein at 1hour, compared to 5 minutes after cell infusion, irrespective of the route. However, we found no differences in the number of labeled cells between the two delivery routes at either time points (Figure 3C).
Fig 3. MNC Biodistribution based on IV and IA Delivery Routes.
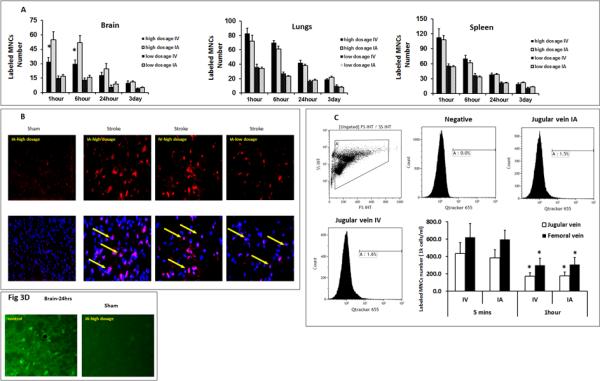
Fig 3A: Comparison of IA vs IV delivery of MNC biodistribution at two different doses in brain, lungs, and spleen. At the high dosage of 30 million cells/kg, there were more labeled cells detected in the brain at 1 and 6 hours in the IA group; however, there was no significant difference in labeled cells at later time points. There was no significant difference in labeled cells in the lungs or spleen. At the lower dosage of 1 million cells/kg, there was no difference in labeled cells in any of the organs. Data are Means±SD. *: p< 0.05, compared to IA. N=5 animals per time point each group. Time points reflect time in hours after MNC administration.
Fig 3B: Representative fluorescence images of Q-tracker labeled MNCs in the brain at 24 hours after IA or IV delivery. No labeled MNCs were found in the brains in the sham MCAo model. Red: Q-tracker 655. Blue: DAPI. Co-labeled MNCs (arrow). Maginification: 200X
Fig 3C: Comparison of IA vs IV delivery of high dosage MNCs biodistribution in the peripheral venous circulation. Fig 3C shows flow cytometric analysis and a bar graph representing the percentage of labeled MNCs in the sampled blood. There are no significant differences in labeled MNCs between IV and IA routes in either jugular or femoral vein at 5 minutes and 1 hour after cell infusion, respectively. Compared to 5 minutes after cell infusion, there were significantly less labeled MNCs in the venous circulation at 1 hour after cell infusion (N=4 animals, p<0.05). Data are Means±SD. *: p<0.05, compared to 5 minutes group.
Fig 3D: High dosage MNCs infusion via IA did not cause microinfarcts. Representative fluorescence images of Fluro-J B staining show dead neurons in the infarct area after MCAo. No stained neurons were detected in the sham animals given 3×107 MNCs /kg IA.
Microstroke formation
Given that IV and IA lead to equivalent benefit at the higher dose of 30 million MNCs/kg, yet IA leads to higher deposition of labeled cells in the brain, we explored the question whether intra-arterial delivery could cause diffuse damage in the form of microembolic strokes (vascular plugging) explaining the limited benefits despite the larger number of MNCs in the affected brain. We therefore injected MNCs IA at 30 million MNCs/kg to healthy rats at two rates: 0.2mL/min and 1.5mL/min (n=3, per group). The former rate has been used in previous studies, and the latter rate was reported to cause microstrokes by others using neural stem cells (NSCs)10. As a positive control for Fluro-J staining, we used a brain from a rat at 24 hours after MCAo. At 24 hours after IA injection, we did not detect Fluro-J positive cells in the middle cerebral artery territory of healthy rats at the injection rate of 0.2mL/min nor at the rate of 1.5mL/min (figure 3D).
Inflammation
Given similar cell biodistributions between IV and IA delivery routes, we turned to other mechanisms involving the systemic inflammatory response. Recent studies indicate that immune modulation may be an important mechanism underlying how MNCs enhance recovery after stroke. Here, we measured serum cytokine levels before stroke (pre-stroke), before treatment (pre-treatment), 3 days and 28 days after stroke. Compared with saline, MNCs at 30 million/kg IV reduced serum IL-1β, IL-6 and TNF- α levels, while increasing IL-10 at 3 days after stroke. MNCs IA showed a generally similar profile regarding TNF-α and IL-6, although MNCs IA did not decrease IL-1β or increase IL-10 to the same extent compared with IV MNCs. At 28 days, all cytokines returned to pre-stroke levels (Fig 4A). At the 1 million MNC//kg, there were no differences in cytokine levels among saline, IV and IA treated MNC groups (data not shown). In a parallel experiment to more directly explore the effects of the treatment on inflammation in the brain, we found that high dose MNCs significantly reduced iNOS expression as assessed at 3 days post MCAo in the ipsilateral hemisphere compared to saline controls (Fig 4B). However, we found no differences between IV and IA MNC treated groups regarding this effect. At the lower dosage, MNCs were not effective in reducing iNOS expression.
Fig 4. High dosage MNCs altered the profile of inflammatory cytokines and Neurotrophin expression after stroke.
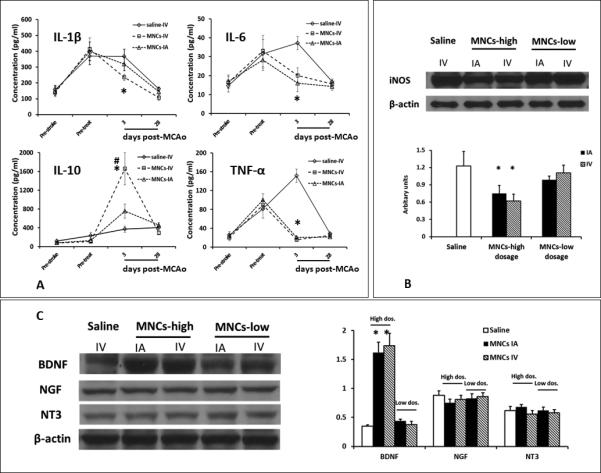
4A: Line diagrams indicating the alterations of serum pro-inflammatory and anti-inflammatory cytokines in animals treated with 3×107 cells/kg MNCs via IA or IV delivery. Data are Means±SD. Animals treated with low dose MNCs (1 × 106 cells/kg) are not shown to preserve clarity of the graph as the cytokine profile was not significantly different from saline treated animals. Statistical tests were performed across all groups including high and low dose treated animals. *p< 0.05, compared to saline group. #: p<0.05, compared to IA delivery route. Saline n=18, MNCs IV n=10, MNCs IA n=11.
4B: The Western blots indicate that iNOS was reduced in the brain from animals treated with high dosage MNCs IA or IV at day 3 after stroke but not with low dosage MNCs IA or IV, compared to saline control. Data are Means±SD. N=3 animals. *: p<0.05, compared to saline group.
4C: Representative immunoblots of neurotrophins and a quantitative bar graph. High dosage MNCs by IA or IV routes increased BDNF expression compared with saline controls. However, there was no significant difference between IV and IA delivery. Low dosage MNCs did not change the levels of any of the neurotrophins. Data were analyzed across all groups and represented as Means±SD. N=3 animals. *: p<0.05, compared to saline group.
Neurotrophins
We also explored differences in neurotrophin levels between the MNC delivery routes. At the higher MNCs dosage, we detected a robust increase in BDNF protein levels but not in NGF or NT-3 between MNC and saline treated groups in the injured hemisphere at 3 days after stroke. However, there was no difference between IA or IV MNCs groups in BDNF levels (Fig 4C). Lower dose of MNCs at 1 million MNCs/kg did not affect BDNF levels.
Neuroregenerative response
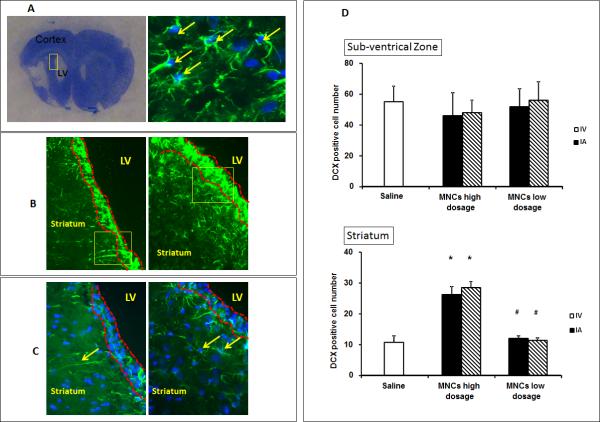
To address one further mechanism reported to be important in the positive effects of MNCs, we examined changes in various aspects of regeneration and repair. To assess neurogenesis associated with the delivery routes of MNCs, we measured changes of Doublecortin(DCX), a marker of neuroblasts, in the subventricle zone (SVZ) and the striatum near the SVZ at day 28 after stroke. In animals treated with high dosage MNCs IV or IA, there were more DCX positive cells in the striatum compared to low dosage MNC treatment or saline controls (Fig 5A, B & C). There was no difference between the IV and IA MNCs group. There were no changes in DCX positive cells between saline and low dosage MNC groups. Interestingly, we also found that GAP-43, an important regulator of synaptic plasticity, was expressed at much higher levels in the ipsilateral cortex of animals treated with high dose MNCs, compared to saline controls, at 28 days after stroke (Fig 6A). Again, we did not find any differences between IV and IA MNC treated groups. We then measured changes in the density of CD-31-positive vessels in the peri-infarct at 28 days after stroke. High dose MNC treatment led to an increase in vessel density compared with saline controls (Fig 6B). There was no difference between IV and IA MNC groups at the higher dosage. Lower dosage of MNCs had no effect on GAP-43 or vessel density.
Fig 5. High dosage of MNCs increases the number of neuroblasts in the striatum at 28 days after stroke.
5A: The frame to the left depicting the region of interest containing the striatum and the sub-ventricular zone, and the image on the right showing a representative neuroblast with characteristic double staining with DCX(green) and nuclear marker DAPI. Only cells with double staining were enumerated as DCX positive cells (arrows).
5B-C: Images showing DCX double positive cells in the region of interest from the MNC low dosage (left panels) and high dosage groups (right panels). 5B–200x; 5C–400x magnification of insert in 5B. Subventricular zone is shown as the area between two broken red lines. LV indicates lateral ventricle. Arrows in the left and right panel of Fig 5C represent neural dendrites and neuroblasts, respectively.
5D: Bar graphs showing the number of DCX positive cells in SVZ and striatum, respectively.
*: compared to saline control group, p<0.05; #: compared to high dosage MNCs treatment group, p<0.05. Saline n=18; MNC High dosage group: IV n=10, IA n=11; MNC Low dosage group: IV n=9, IA n=9.
Fig 6. High dosage MNCs improves other aspects of repair and regeneration after stroke.
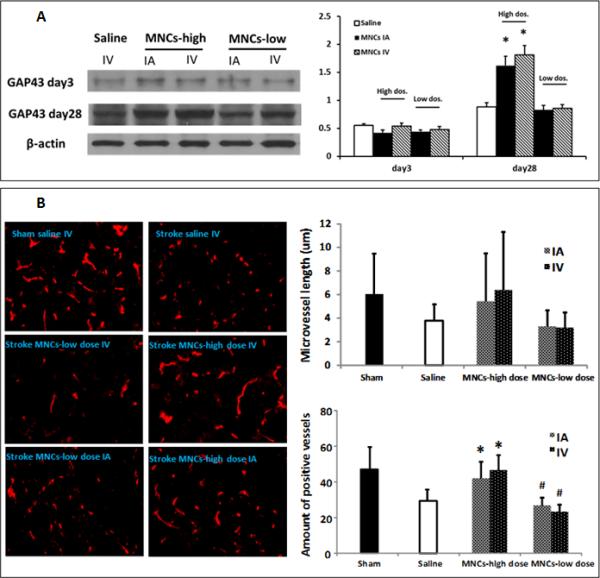
6A: Representative immunoblots of GAP-43 at day 3 and day 28 after stroke. Bar graphs illustrate that high dosage MNCs but not low dosage MNCs increased GAP-43 expression at day 28 but not at day 3 after stroke. N=3 animals. *: p<0.05, compared to saline control group.
6B: Representative fluorescence images of CD31 positive blood vessels at 28 days after stroke. Bar graphs on the right indicate that high dosage MNC administration increased the vessel numbers but not the vessel length at 28 days after stroke, compared with the saline group. Low dosage MNCs had no effect on the vessels. There were no significant differences in vessel numbers or length between the delivery routes at either dose. Data are Means ±SD. *: p< 0.05, compared to saline group. #: p<0.05, compared to high dosage. Sham n=3; Saline n=18, MNC high dose: MNCs IV n=10, MNCs IA n=11; MNC low dose: MNCs IV n=9, MNCs IA n=9.
Discussion
Autologous bone marrow derived mononuclear cells are a promising potential therapy to promote stroke recovery but the optimal delivery route for these cells remains unknown and is an important translational issue. Intravenous and intra-arterial routes of delivery are being pursued in clinical trials. We therefore investigated differences between them to provide important information that could help in designing future clinical trials. We first used a high dose of MNCs that was previously shown by intravenous administration to exert a therapeutic effect2. Considering that more cells in the peri-infarct area might lead to better recovery, we expected that the IA route would allow for more MNCs to reach the affected brain and be associated with a better outcome. We also used a low dosage of MNCs which in our hands did not enhance stroke recovery using an IV delivery route2, with the expectation that an IA administration of the same dose might, unlike IV, lead to a better outcome. Irrespective of the delivery route, MNCs at the higher dose improved functional recovery but we found no added benefit of IA over IV. The high dose of MNCs improved functional recovery to the same extent by intravenous or intra-arterial delivery routes and the lower dose had no effect on stroke outcome whether by IV or IA.
There are a number of possible factors to account for the similar outcomes between the delivery routes at the low and high cell doses. We first explored the biodistribution of MNCs. As expected, infusion via IA compared with IV of 30 million MNCs/kg delivered more cells into the peri-infarct area acutely at 1 and 6 hours after injection. Unexpectedly, the presence of a higher number of cells in the brain did not correlate with improvement in functional outcome or tissue repair as previously suggested by others5. On the other hand, infusion of 1 million MNCs/kg deposited a significantly lower number of cells in the brain, and was not associated with a therapeutic effect. These results suggest the possibility that the number of cells delivered to the brain may not be critical to influence functional outcomes. However, another possibility is that a minimum threshold of cells delivered to the brain is needed and that a higher cell presence at least acutely does not lead to a greater effect on recovery in our rodent stroke model. The data also suggest that a lower dose of MNCs, that is not efficacious IV2, does not lead to better outcomes when more directly administered to the brain with an IA route.
Since MNCs are overall smaller in size than purified stem cells and have a 30 fold increased pulmonary passage than mesenchymal stem cells (MSCs)11, IV delivery of MNCs may thus lead to the same effects within the brain as IA because a sufficient number of cells may penetrate the CNS. Higher numbers of MNCs thus does not lead to a greater effect (“ceiling effect”) but lower doses such as 1 million cells/kg do not lead to enough cells entering the brain. This notion is supported by our previous experiment in which pre-treatment with a nitric oxide antagonist inhibited vasodilation, significantly reduced the entrance of MNCs into the peri-infarct area and prevented MNCs from reducing neurological deficits9.
In further support of the concept that IV and IA MNCs lead to the same effects within the CNS, we investigated various mechanisms that may be therapeutic targets of MNCs. We found that IA and IV MNCs at 30 million cells/kg led to a similar degree of infarct cavity reduction. We1 and others12 have reported that some types of cell therapies may decrease infarct cavity by possibly reducing infarct maturation and delayed cell loss in the peri-infarct areas in the days to weeks after stroke onset. A high dose of MNCs by IV or IA delivery also led to upregulation of various aspects of the regenerative response such as DCX positive cells in the striatum (reflective of neurogenesis), vessel density within the peri-infarct area, GAP-43 (reflective of synaptic plasticity), and the neurotrophin, BDNF. These results suggest that the intracerebral responses were similar after IV and IA delivery of MNCs. Unfortunately, since 1 million cells/kg had no effect on any of these endpoints (compared with saline), we could not dissect specific targets in the CNS that were differentially affected by the mode of delivery.
Given the lack of differences in brain responses to MNC therapy, we turned to other mechanisms that may account for their positive effects in the stroke model. Some cell therapies may exert their beneficial effects by targeting systemic immune responses emanating from peripheral organs such as the spleen13 rather than within the brain or in addition to their effects in the brain. In this study, we found similar numbers of MNCs in the lungs and spleen from 1 hour to 3 days after stroke at either dosage. It is therefore possible that IV and IA led to similar effects on functional outcome because both delivery routes deposited similar numbers of MNCs to these organs. In support of this notion, IV and IA MNCs led to similar reductions of serum pro-inflammatory cytokines such as IL-6 and TNF-α. However, IV MNCs reduced IL-1β and increased IL-10 more effectively than IA MNCs, suggesting the high level of complexity involving immune regulation.
The similar biodistrubtion profiles of MNCs by either IV or IA may be due to the possibility that IA delivery flushes MNCs through the microcirculation rather than causing retention in the brain, leading to the deposition of the cells into the venous system and then to the heart. We tested this hypothesis by assessing the presence of labeled cells in the circulation and found no differences in the number of cells in the jugular or femoral vein at 5 minutes or 1 hour after IV or IA injection. Therefore, after the initial injection, MNCs appear to circulate through the body to the same extent by IA and IV routes. IA delivery therefore does not bypass the filters of the lungs or other organs such as the spleen and may not confer selective advantages over IV in preferentially directing cells to the brain, at least for MNCs. In contrast, MSCs are cultured and adhesive and may therefore differ in their retention within the cerebral vasculature.
A final interpretation to account for the lack of differences between the two groups could be that the IA route does lead to a greater impact on CNS targets such as vessel density or infarct size but also causes brain injury that may offset the benefits. In reports testing MSCs, IA injection causes microembolization, a reduction in cerebral perfusion, and death14, 15. Thus, in the present study, we first tested the safety of IA injection with a higher dosage from our previous study2. We found that there was no mortality after IA or IV injection, which suggests that a high dosage of MNCs was safe even when given intra-arterially. We then infused the same dosage IA and found no evidence for microinfarcts using two injection rates: a low rate over 5 minutes and a high rate over 40 seconds; the latter infusion rate IA had been reported to cause neuronal death in animal models using NSCs10.
Other research groups have investigated the differences between IV and IA delivery of MNCs in the rodent stroke brain. Kamiya, et al also used autologous MNCs harvested prior to stroke and administered the cells immediately after the stroke. The authors also found that IA delivery compared with IV led to more homing of cells in the brain at 2 hours but not at 24 hrs after injection but they did find that IA led to better tissue protection6. In a prior report, we found no differences in the biodistribuition of MNCs that were derived from bone marrow before versus after stroke when the MNCs were examined at 24 hrs after injection but we did not examine earlier time points16. In another relevant study, Vasconcelos-dos-Santos et al labeled donor-derived bone marrow MNCs with 99mTc7 and found no difference in labeling in the brain or other organs from early time points to 24 hrs after delivery and similarly found that both IV and IA led to similar effects on stroke recovery7.
In conclusion, we found no evidence that IA delivery is superior to IV as a mode of administration for bone marrow derived MNCs in our rodent stroke model. Despite a higher number of cells in the brain initially after IA delivery, there was no difference in functional or structural outcomes between the two routes. The IA route of delivery does not prevent cell trapping in peripheral organs. Our findings suggest that IA does not confer additional benefits beyond IV administration for MNCs in our animal stroke model.
Acknowledgments
FUNDING SOURCES
This research work was supported in part by grants from NIH R21 NS064316, and R-01 NS071127.
Footnotes
DISCLOSURES
No competing financial interests exist.
References
- 1.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr., Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr., Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(Suppl 1):S13–21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 5.Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos-dos-Santos A, Rosado-de-Castro PH, Lopes de Souza SA, da Costa Silva J, Ramos AB, Rodriguez de Freitas G, et al. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9:1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Kasam M, Yang B, Strong R, Schaar K, Misra V, Xi X, et al. Nitric oxide facilitates delivery and mediates improved outcome of autologous bone marrow mononuclear cells in a rodent stroke model. PLoS One. 2012;7:e32793. doi: 10.1371/journal.pone.0032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua JY, Pendharkar AV, Wang N, Choi R, Andres RH, Gaeta X, et al. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J Cereb Blood Flow Metab. 2011;31:1263–1271. doi: 10.1038/jcbfm.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, et al. Infusion of Human Umbilical Cord Blood Cells in a Rat Model of Stroke Dose-Dependently Rescues Behavioral Deficits and Reduces Infarct Volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 13.Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an mri study. J Cereb Blood Flow Metab. 2010;30:653–662. doi: 10.1038/jcbfm.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bing Y, Xi X, Aronowski J, Savitz SI. Ischemic Stroke May Activate Bone Marrow Mononuclear Cells to Enhance Recovery After Stroke. Stem Cells and Development. 2012;21:3332–3340. doi: 10.1089/scd.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]