Abstract
Native arteriovenous fistula (AVF) is the vascular access of choice for hemodialysis patients. Compared with grafts and central venous catheters, AVFs last longer and are associated with fewer complications. The widespread use of the Doppler ultrasound (DUS) has increased the number of patients who are eligible for AVF by facilitating the identification of vessels that are suitable for fistula construction (preoperative vascular mapping). DUS can also extend native AVF survival by improving the early detection of complications (post-operative surveillance). It is the only imaging modality that furnishes both morphological and functional data on the native vascular access, and it is also the only imaging tool that can be used directly by the surgeon, an indisputable advantage. This review examines the numerous roles played by DUS in the construction and postoperative follow-up of AVFs, including preoperative vascular mapping, AVF maturation, and surveillance.
Keywords: Doppler ultrasound (DUS), Arteriovenous fistula (AVF), Preoperative vascular mapping, Access flow volume measurement, AVF monitoring and surveillance
Riassunto
La FAV confezionata con vasi nativi rappresenta l’accesso vascolare di scelta per il paziente emodializzato in quanto, a parità di flusso, presenta minore incidenza di complicanze e più lunga sopravvivenza rispetto alle protesi ed ai cateteri venosi centrali. L’avvento del DUS nell’armamentario di chi si occupa di chirurgia degli accessi vascolari ha, da un lato, aumentato il numero di pazienti in cui si riesce a confezionare una FAV con vasi nativi (grazie all’individuazione di vasi idonei all’intervento mediante il mapping pre-chirurgico), e, dall’altro, ha migliorato la sopravvivenza delle FAV grazie alla diagnosi precoce (monitoraggio post-operatorio) delle complicanze dell’accesso vascolare. L’eco-color-Doppler è l’unica tecnica in grado di dare informazioni sia morfologiche che di funzionalità (flusso) dell’accesso vascolare; inoltre, è l’unica tecnica (tra quelle di diagnostica per immagini) direttamente gestibile dal chirurgo e ciò rappresenta sicuramente un valore aggiunto. Questa review fornisce una panoramica sulle possibili applicazioni del DUS nell’ambito del confezionamento e del follow-up delle FAV, con particolare riferimento al mapping pre-chirurgico, alla maturazione della FAV e al monitoraggio/sorveglianza della FAV.
Introduction
Arteriovenous fistula (AVF) created with native vessels are the vascular access of choice for hemodialysis: at comparable flow rates, the AVF is associated with a lower incidence of complications and longer survival than prosthetic grafts or central venous catheters [1, 2]. However, because of the increasing prevalence of advanced age and co-morbidities such as diabetes mellitus and vascular disease among patients requiring dialysis, nephrologists and vascular surgeons are finding it increasingly difficult to locate native vessels suitable for creation of a well-functioning, persistently patent AVF [3]. The use of Doppler ultrasound (DUS) by physicians performing vascular access surgery has increased the number of cases in which AVFs can be created with native vessels by allowing preoperative mapping and identification of suitable vessels. It has also improved AVF survival by facilitating early diagnosis and rapid correction of complications that may arise (postoperative monitoring/surveillance) [4–8].
The aim of this article is to provide an overview of the possible applications of DUS during the creation and postoperative follow-up of AVFs, with particular emphasis on the following aspects:
Preoperative vascular mapping.
Maturation of the AVF.
Monitoring/surveillance of AVF (follow-up and early detection of complications).
Preoperative vascular mapping
For decades, the selection of vessels to be used for constructing an AVF was based exclusively on physical examination of the upper limbs, which is a low-cost, bedside procedure that requires no additional equipment. Although this approach provides acceptable information on the superficial venous circulation (vessel palpability, caliber, patency and course), it furnishes much more limited data on the arterial vessels (pulse palpability and patency of the arterial circulation of the hand based on the results of the Allen test) [9–11]. In addition, physical examination alone is insufficient in a considerable percentage of patients (~25–50 %) [10].
DUS is more time-consuming than physical examination, and it requires both an experienced examiner and special equipment. However, it also provides more information on the superficial and deep veins of the arm and a wealth of additional data on the arterial circulation. In addition, it is completely noninvasive, safe, and repeatable [9]. DUS is the only diagnostic imaging technique that allows simultaneous visualization of the anatomy of an area (B-mode imaging) and its blood supply (Color and Doppler imaging). It is also the only one that can be performed directly by the physician who will be creating the vascular access, and this is an indisputable advantage. Some authors maintain that DUS should not be part of the routine preoperative assessment but used only when anomalies emerge during the physical examination [4]. However, international guidelines recommend its use in all patients who are candidates for an AVF, as a natural complement to the physical examination [12].
Technical requirements and examination technique
The ultrasound scanner used to map the upper-extremity vasculature must be equipped with a linear probe with minimum frequencies of 7 MHz for the B-mode examination and 5 MHz for the Doppler study [13]. The patient should be examined in the supine position with the trunk moderately elevated to avoid flexion of the elbow. Alternatively, he/she may be seated in front of the operator with the forearm resting on a stand. Most examiners prefer the supine position because it simplifies the assessment of the vascular structures of the arm (subclavicular axillary region) and is more comfortable for the patient [13, 14]. The examination should be carried out in a comfortably warm room, and the gel should also be warmed to avoid triggering vasoconstriction of the structures being examined [13, 14]. Ideally, the arterial and venous districts should be evaluated consecutively, with transverse and/or longitudinal scans of the arteries (from the root of the arm towards the hand) and veins (from the periphery towards the thorax). The examination can begin with the arterial or the venous district, depending on operator preferences and the characteristics of the individual patient. A thorough examination of the circulation of the arm must include B-mode assessment of morphological aspects as well as Color and Doppler evaluation of arterial and venous blood flow.
Preoperative arterial mapping
Preoperative arterial DUS should include evaluation of subclavian, axillary, brachial, radial and ulnar arteries [15]. In clinical practice, however, most arterial evaluations begin with the distal subclavian artery or even the brachial artery [9, 16]: only when anomalies are found at these levels, is the investigation extended to the proximal portion of the subclavian artery?
DUS allows thorough assessment of the arterial circulation of the arm based on a series of morphological and functional parameters [10]. The morphological aspects include vessel diameter, wall thickness, wall alterations, vessel course, and any steno-obstructive lesions that may be present. The functional evaluation involves the assessment of blood flow and the artery’s ability to dilate.
The internal diameter of an artery can be measured on either longitudinal and transverse scans [14], but the former allows visualization of the intimal layers of the superficial and deep vessel walls, thereby facilitating more precise measurement of the intima–intima distance (i.e., the internal diameter of the vessel) [9]. To verify the precision of these measurements, sonographically measured vessel diameters have been compared with measurements obtained directly during surgery, and the correlation between the two proved to be good [17]. The relationship between arterial diameters and AVF outcomes has been studied in radial-cephalic fistulas. Immediate (on the day of surgery) and early (within the first 8–12 weeks after surgery) AVF failures were found to be quite frequent when small-caliber (<1.5–1.6 mm) arteries were used to create the fistula. Malovrh et al. reported immediate and early failure rates of 55 and 64 %, respectively, when the arteries used had diameters of ≤1.5 mm, whereas much lower rates (8 and 17 %, respectively) were observed when the arterial diameters were >1.5 mm [17]. Parmar et al. [18] reported an early failure rate of 46 % for arteries with diameters of <1.5 mm, while no failures were observed when vessel diameters were >1.5 mm. Wong et al. [11] encountered premature failures with all AVFs created with arteries whose diameters were ≤1.6 mm. In another study, patent fistulae had preoperative radial diameters of 2.7 mm as opposed to 1.9 mm for AVFs that failed [19]. Silva et al. proposed a minimal diameter of 2 mm, which in their experience was associated with an early failure rate of 8 % and a 1-year primary patency rate of 83 % [5]. However, AVF success rates of approximately 50 % have been reported even when the arterial diameter is <1.5 mm [18]. Therefore, indicating an ideal threshold for the diameter of the radial artery is inappropriate: the point to remember is that the likelihood of AVF patency and survival increases with the diameter of the artery used to create the fistula [5, 10, 11, 17, 19]. This also reflects the fact that the arterial diameter is only one of the factors that affect the probability of successful AVF creation. It has to be evaluated in conjunction with other clinical and ultrasound parameters which provide indications on the anatomic and functional status of the artery and the optimal site for AVF construction. In other words, the functional quality of the artery is an important determinant of AVF success, and it is not necessarily related to the internal diameter of the vessel (as shown, for example, by experience with AVFs created in pediatric patients).
There are no recommendations available regarding the diameter of the brachial artery, which is constitutionally larger than that of the radial artery. Consequently, its assessment is less crucial to the success of the surgical procedure [9].
The presence of arteriopathy that can jeopardize the success of the AVF can be easily detected with a high-resolution B-mode examination of the thickness and alterations of the vessel walls. Arterial wall changes are, in fact, common in patients with chronic renal insufficiency, diabetes, and atherosclerosis [10]. The intima-media thickness is estimated in a longitudinal scan of the distal wall of the artery. The ultrasonographic measurement shows good correlation with histologic measurement, and increased thickness seems to be closely correlated with fistula failure [20]. Calcifications are sonographically depicted as areas of hyperechogenicity (with or without posterior shadowing) within the arterial wall and irregularities of the intimal lamina. Although these alterations are easy to identify, they are difficult to quantify. In addition, they do not represent contraindications to the creation of a fistula although they can influence its outcome and/or render surgery more difficult [14].
DUS is also a very accurate method for identifying stenotic arterial lesions (sensitivity and specificity: 91 and 100 %, respectively, for the subclavian artery; 93 and 100 % for the arteries of the arm; 89 and 99 % for those of the forearm), obstructive arterial lesions (sensitivity and specificity 90 and 99 %) [21], and vascular abnormalities such as brachial artery bifurcation in the most proximal portion of the arm.
As noted earlier, the functional study involves the assessment of blood flow and the artery’s ability to dilate. Blood flow can be evaluated by measuring the vessel diameter and mean flow velocity (cm/s) on longitudinal scans (see below for calculation of flow volume). However, the value of these measurements in predicting AVF outcome has been assessed in relatively few studies on this subject [10, 22]. Malovrh et al. [10] found that successful radial-cephalic AVF construction was associated with radial artery flow exceeding 50 ml/min, and in the study by Sato et al. [22] a preoperative radial artery flow of <20 ml/min was associated with an increased risk of “primary AVF failure” within 8 months of surgery.
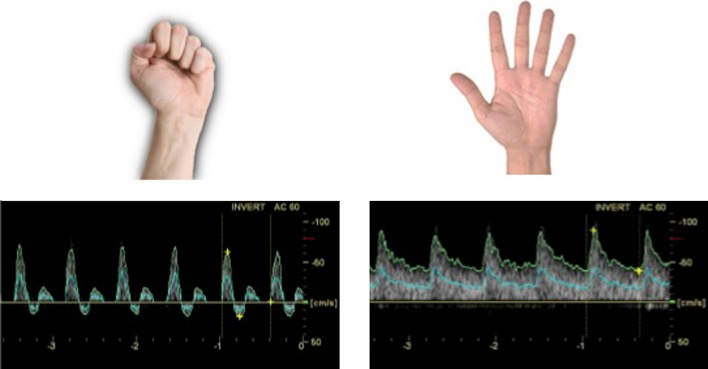
After surgery, adequate fistula maturation is associated with dilation of the artery that feeds the AVF. As a result, blood flow within the vascular access increases and the previously triphasic (high resistance) arterial spectrum becomes biphasic (low resistance). The artery’s ability to increase its caliber (distensibility) can be estimated preoperatively on the basis of variations in the radial artery Doppler spectrum during the reactive hyperemia test (Fig. 1) [10]. The term “reactive hyperemia” refers to the physiological increment in blood flow through an artery that occurs after a period of ischemia. In this test, ischemia is induced by having the patient make a fist for 2 min, and the increase in arterial flow (reactive hyperemia) is observed immediately after the hand is reopened [10]. During the phase of ischemia, the Doppler spectrum of the artery is normally triphasic, reflecting high resistance. If the vessel is capable of dilatation, the arterial spectrum becomes biphasic during the phase of reactive hyperemia (Fig. 1) [10]. This spectral variation can be quantified by calculating the resistance index (RI) [RI = (peak systolic velocity − end diastolic velocity)/peak systolic velocity]); in particular, the greater the intensity of the reactive hyperemia, the lower the RI will be [10]. Malovrh et al. [10] demonstrated that the absence of reactive hyperemia (reflected by an RI >0.7 after the fist is opened) indicates an insufficient increase in arterial flow during the test, which is predictive of immediate postoperative AVF failure. These findings show that the reactive hyperemia test provides an excellent index of the functional status of the artery, and it is particularly useful for selecting the artery and the surgical site (wrist, forearm, elbow) for AVF construction.
Fig. 1.
Reactive hyperemia test. Left ischemic phase with fist closed and corresponding Doppler spectrum, Right Doppler spectrum during the reactive hyperemia phase with the hand opened
Preoperative venous mapping
Preoperative venous DUS involves evaluation of the superficial and deep venous systems of the upper limb from the wrist up to the central veins. With ultrasound, the latter veins can be easily examined up to the distal segment of the subclavian vein, but direct visualization of the proximal portions of the subclavian vein and the innominate vein is not always possible [13]. The superior vena cava cannot be evaluated with DUS because it lies inside the rib cage.
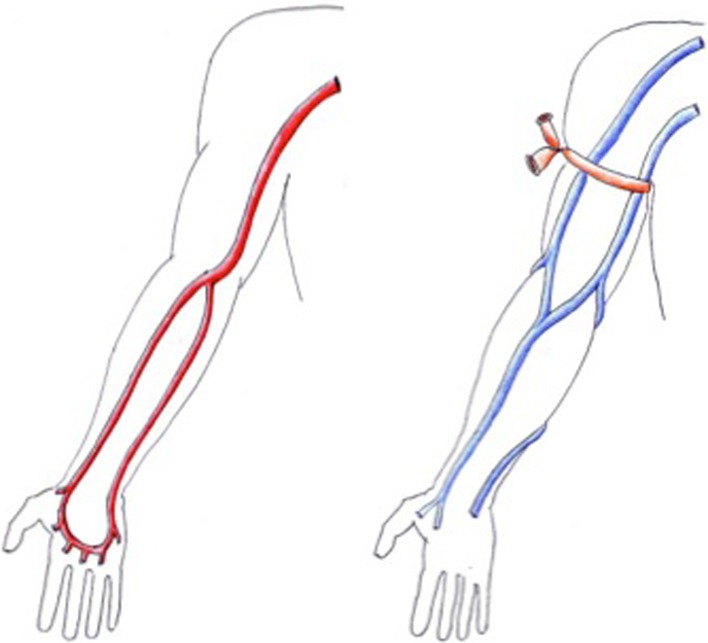
A tourniquet is placed around the root of the arm, and the superficial venous circulation is examined with transverse scans, beginning with the cephalic vein, from the wrist to the point where it drains into the deep venous system [9]. The full course of the basilic vein should also be examined, but this is often done only if the cephalic vein is not suitable for AVF creation [9]. With this assessment a map of the superficial venous circulation can be drawn (Fig. 2).
Fig. 2.
Examples of preoperative vascular mapping. Left arterial mapping, right venous mapping
Several ultrasound parameters can be helpful in deciding whether a superficial vein can be used to create an AVF. They include the appearance of the vein wall, the course of the vessel, its patency, caliber and distensibility, and the presence of collateral circuits [9, 13].
A normal vein is characterized by thin, regular walls and a completely anechoic lumen [23]. The course of the vein must be sufficiently linear (for a distance of at least 8–10 cm), and it should lie less than 6 mm below the skin surface to facilitate venipuncture [24]. Vein patency is assessed by exerting intermittent pressure with the transducer which causes complete collapse of the vessel walls [9]. Noncompressibility of the vein under the transducer’s pressure is sign of obstruction and is often associated with the presence of echogenic material in the lumen [23]. If doubts arise, the patency of the vein can be confirmed using the color Doppler module with a low pulse repetition frequency or by verifying the presence of the Doppler trace in a longitudinal scan [23]. A normal venous Doppler spectrum is characterized by continuous, low-velocity flow, which becomes increasingly phasic as the examination proceeds toward the central veins; the absence of such flow confirms the presence of an obstruction. Doppler spectral analysis can also provide an accurate indirect index of the patency of the innominate veins and the superior vena cava. In fact, the presence at the level of the subclavian and internal jugular veins of flow that varies in velocity with the respiratory and cardiac activity is an indirect index of the patency of the ipsilateral innominate vein and the superior vena cava, whereas a monophasic curve is indicative of steno-occlusion [10, 13, 14]. The suspicion of steno-thrombotic lesions involving a central vein should in any case be confirmed with phlebography [24].
The diameter of the vein should be measured at several points in the arm. This can be done on longitudinal or transverse scans [14]. To reduce the risk of underestimation, gel should be applied copiously to prevent the exertion of excessive pressure with the transducer. It is widely agreed that fistulas created with small-caliber veins (<1.6 mm) are at high risk for early failure [11], but there is no consensus on the minimum cephalic vein diameter that will ensure good maturation of a radial-cephalic AVF. Based on their findings, Silva et al. [5] suggest a minimum diameter of ≥2.5 mm when a tourniquet has been applied; in the absence of a tourniquet, Mendes et al. [25] propose a diameter of >2 mm. Well-documented indications on the minimum diameter for the veins of the arm are also lacking, but a value of at least 3 mm is recommended [14].
After the AVF has been created, the vein tends to dilate as a result of the increased blood flow. The vein’s ability to dilate (venous distensibility) can be evaluated during preoperative mapping. The diameter of the vessel is measured before and at least 2 min after placement of a tourniquet (or a sphygmomanometer cuff inflated to a pressure of 50–60 mmHg), and the percentage of increase is evaluated [14, 26]. The impact of venous distensibility on AVF outcomes has been evaluated in two studies: Malovrh et al. [10] concluded that venous distensibility is a predictor of outcome since the mean percentage of vein dilatation observed in veins used for successfully constructed AVFs was 48 versus 11 % in those used for fistulas that ended in immediate failure. Lockhart et al. [27, 28] reported that cephalic veins with a pretourniquet diameter of ≥2.5 mm and smaller veins with a post-tourniquet diameter ≥2.5 mm were equally useful for creating dialysis fistulas. They concluded that distensibility testing should be used mainly to identify the actual maximum diameter of apparently small-caliber arm veins.
Some authors have suggested that the likelihood of non-maturation is related to the presence and diameter of accessory veins. Wong et al. [11] found that the presence of accessory veins less than 5 cm from the site chosen for the anastomosis can alter the functionality of the fistula, while Beathard et al. stressed the importance of the dimensions of these veins and reported higher frequencies of non-maturation when the AVF was near large collateral veins [29].
AVF maturation and calculation of blood flow
What role does DUS play in the assessment of AVF maturation?
The term maturation refers to the development of those physical characteristics that render an AVF suitable for venipuncture with large-gage needles [24]. In many cases, non-maturation is the reason an AVF cannot be used for dialysis. In fact, despite the obvious long-term advantages in terms of morbidity and mortality that make AVFs the vascular access of choice for hemodialysis patients [24], arteriovenous anastomoses with native vessels has been associated with a high incidence of early occlusion and failure to mature (FTM) during the postoperative period. In different case series, the incidence of FTM for radiocephalic AVFs ranged from 30 to 60 % [15, 30].
But how does one assess AVF maturation? Generally, the physical examination conducted by an experienced dialysis nurse is sufficiently reliable for determining whether the fistula is mature and therefore ready for puncture [15]. The problem arises when the fistula does not appear clearly mature based on inspection alone, a situation that occurs with obese patients and with slow-maturing AVFs. In these cases, the ultrasound examination and assessment of hemodynamic parameters (AVF blood flow, RI) can help determine whether the AVF is suitable for cannulation or whether it has instead failed to mature and is therefore likely to undergo thrombosis or have a low flow volume.
In obese subjects, for example, even veins that are well developed can be difficult to visualize or palpate because of their depth; in these cases, DUS can reveal whether the fistula is mature, and US mapping of the out-flow veins can facilitate the first cannulation and simplify subsequent punctures [15]. In this regard, it is important to recall the proposal of Rayner et al. [31], which was incorporated in the K-DOQI Guidelines [24] as “the Rule of 6”. It identifies the ultrasound characteristics that confirm that a fistula is mature and, therefore, ready for use: a flow volume of >600 ml/min, an out-flow vein diameter of ≥6 mm, and an out-flow vein depth of ≤6 mm below the skin surface.
For slowly maturing AVFs, it is fundamental to determine whether the fistula is actually maturing—albeit slowly—or not (i.e., FTM). In these cases, the diagnosis can be made with DUS assessment and periodic calculation of the vascular access flow volume at the level of the brachial artery. Normally, the Doppler spectrum of brachial artery is classically triphasic (high resistance), with flow rates that vary from 80 to 150 ml/min [23, 32]. As soon as the arteriovenous anastomosis has been completed, a locus minoris resistentiae is created, and the velocity/time curve of the brachial artery becomes biphasic (low resistance). Blood flow increases dramatically in the first 24 h after surgery and more gradually thereafter, until after varying periods of time the vascular access reaches full maturity [32]. Lomonte and coworkers evaluated 17 radiocephalic AVFs, measuring brachial artery flow volumes preoperatively and 1, 7, 28, and 258 days after construction of the arteriovenous anastomosis. They confirmed that the most dramatic percentage increase in AVF flow occurs on postoperative day 1 and accounts for approximately 50 % of the flow volume measured on postoperative day 28. Subsequent increases were more gradual [33]. The authors concluded that serial measurement of AVF flow volumes during the first month after surgery can help distinguish fistulas that will mature correctly from those destined to fail. On the basis of the data they collected, it appears that maturation is likely if blood flow through the fistula is 250–500 ml/min on postoperative day 1 and 500–900 ml/min 1 month after construction of the anastomosis [33]. If lower flow rates are encountered or—worse yet—if the brachial artery flow volume tends to decline over time, proper maturation is unlikely, and the fistula will probably become unsuitable for use in dialysis owing to problems of thrombosis or low flow. Therefore, assessments of AVF maturation should always include a physical examination as well DUS measurements of the flow volume. The latter allows one to predict the probable outcome of the vascular access, and if FTM is observed, it can be helpful in identifying and in some cases correcting the cause.
In conclusion, in response to our initial question regarding the role played by DUS in assessing AVF maturation, the data discussed above clearly show that maturation should be sonographically monitored until the fistula is used, especially when maturation seems to be proceeding slowly and in patients whose veins cannot be easily assessed with physical examination alone (e.g., due to obesity). DUS measurement of AVF flow volumes is perhaps the only imaging tool that can be used to monitor the fistula even during its maturation. Even so, DUS should always be done before an AVF is first used. This examination provides baseline data on the vascular access, which can be useful in subsequent examinations performed to evaluate functional problems [15].
Calculation of AVF flow volume
When performed correctly, calculation of the AVF flow volume by DUS is a simple procedure that can be completed in a few minutes and is highly reproducible.
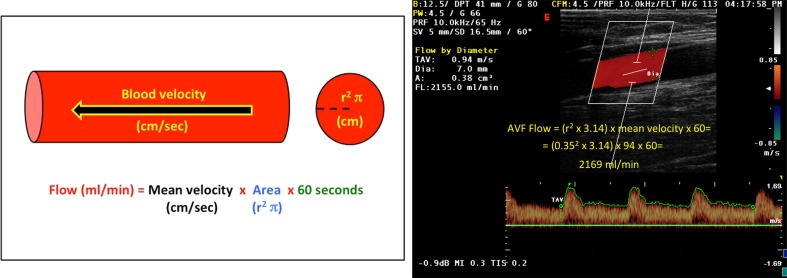
The formula used to calculate flow volumes is area × mean velocity × 60, where area is the cross-sectional area of the vessel in square centimeters (since the vessel is cylindrical, its section is a circle whose area is calculated as the square of the radius × 3.14) (Fig. 3), mean velocity (in cm/s) is that of the red blood cells measured from the Doppler trace recorded at the site used to measure area, and 60 is the number of seconds in a minute (since flow volumes are expressed in milliliters per minute) [13, 32]. The vessel diameter and mean flow velocity necessary for calculating flow volume according to this formula can be measured on a single longitudinal scan of the vessel. First, the vessel diameter is measured on the appropriately enlarged B-mode image. The pulsed Doppler module is then activated and the PRF adjusted to eliminate artifacts, and the mean flow velocity is calculated from the time/velocity curve (using the time-averaged velocity option available on most scanners) (Fig. 3). Measuring both variables on the same scan ensures that both have been made at the same site in the vessel. In fact, once the two measurements have been made, further calculations are really not necessary: modern ultrasound scanners are equipped with computing algorithms for automatic calculation of the AVF flow volume (Fig. 3).
Fig. 3.
Calculation of the AVF flow volume. Left theoretical basis of the formula used to calculate blood flow volume of a blood vessel. Right example of AVF flow volume calculated at the level of the brachial artery, manually with the proposed formula (AVF flow volume) and with scanner software (“Flow by diameter”)
As for the sampling site, the arterialized vein is punctured during hemodialysis, so the outflow vein of the AVF should be an ideal site for measuring the vascular access flow volume. However, measurements made at this level are actually fairly imprecise because this vein can be easily compressed with the probe. In addition, its diameter varies widely due to its tortuous course and the presence of collateral circuits, and these variations make it difficult to calculate the cross-sectional area of the vessel with any degree of precision. Moreover, calculation of the mean velocity is complicated by the turbulent flow that characterizes the venous side of the AVF. For these reasons, measuring the flow volume at the level of the inflow artery improves accuracy and reproducibility. However, measuring the flow volume of a distal AVF at the level of the radial artery can lead to underestimation because a variable portion of the fistula flow (approximately 25–30 %) may come from the ulnar artery, via the palmar arch. This “reverse flow” occurs if the diameter of the anastomotic chamber is larger than that of the artery that supplies arterial blood to the fistula. Therefore, in clinical practice, the brachial artery is the preferred site for measuring the flow volume of distal and proximal/proximalized AVFs [13, 24, 32, 33], for several reasons. It is easy to sample and does not collapse under normal transducer pressures. In addition, just above the elbow crease, there is an oblique segment of the brachial artery, where the sample volume can be easily positioned at an appropriate insonation angle. And finally, its laminar flow allows one to record suitable tracings for precise calculation of the mean velocity. In patients with proximal or prosthetic fistulas, the brachial artery flow downstream from the AV anastomosis should be subtracted from the value obtained to improve the accuracy of the flow volume measurement. Alternatively, in prosthetic grafts the flow volume can be measured directly in the prosthetic conduit, which is more regular in caliber than a native outflow vein and more resistant to pressure exerted with the transducer.
To reduce the risk of over- or underestimating the AVF flow volume, several things should be kept in mind. In the first place, use of the zoom function: measurement of the vessel diameter must be as precise as possible because minimal variations in diameter translate into major variations in flow volume (indeed, the formula used to calculate flow volume entails squaring the vessel radius, which thus becomes an important determinant of flow.) Second, when data for the Doppler curve are being acquired, the sample volume should be oriented parallel to the direction of blood flow and the angle of insonation maintained at <60°. The sample volume must always be positioned at the center of the vessel, but the amplitude should be adjusted to allow sampling of 50–70 % of the vessel lumen. This prevents measurement restricted to the red blood cells that pass through the central part of the vessel, which move faster than those flowing close to the vessel walls. Acquisition of velocity data must be as precise as possible; this can be achieved by careful regulation of the PRF to eliminate all types of artifacts.
Monitoring/surveillance of the AVF (follow-up and early detection of complications)
For a well-functioning AVF, the nephrologist’s job is to maximize its survival by prevention, early detection and prompt treatment of complications. To this end, the international guidelines recommend a specific protocol for vascular access monitoring (physical examination of the AVF before each dialysis session) and surveillance (assessment of recirculation, venous/arterial pressures, calculation of flow volume and other parameters, which provide information on AVF function and should be evaluated on a monthly basis) [24]. Measurement of blood flow is now considered the best means of surveillance for a vascular access [24]: reduced flow volumes or values that decrease over time are predictive of thrombosis for both native and prosthetic AVFs [32, 34, 35]. There are several methods for calculating AVF flow (DUS, MR angiography, the ultrasound dilution technique, the Crit-line monitor, glucose infusion, differential conductivity, ionic dialysance), and none is considered unequivocally superior to the others in the main international guidelines [12, 24]. One of the drawbacks of DUS with respect to the other methods is that it cannot be used during hemodialysis, and it also offers a major advantage, i.e., it can be used to document the presence of a low AVF flow volumes and simultaneously explore possible causes (e.g., by providing direct visualization of an area of stenosis, with precise information on its location and severity) [13, 15, 32]. To minimize the risk of underestimates caused by hemodynamic factors (e.g., hypotension), DUS should not be used to calculate AVF flow volumes during the immediate post-dialysis period: measurements made between one session and the next or immediately before a dialysis session are preferable.
Data in the literature on DUS flow volume assessment indicate that a well-functioning AVF will be characterized by a flow rate of 700–1,300 ml/min [32, 33]. Values of <500 ml/min [13, 24] and <300 ml/min [13, 32] are considered predictive of access dysfunction and imminent thrombosis, respectively. Aside from these absolute values, subsequent studies have shown that, in a vascular access that has previously been stable with flow volumes of >1,000 ml/min, further investigation is warranted when consecutive monthly measurements reveal a decrease in flow volume of >25 % over a relatively short period of time (1–4 months) since these findings are predictive of stenosis and vascular access thrombosis [15, 24, 34, 35].
DUS calculation of AVF flow volume can also be useful for assessing the effectiveness of a therapeutic intervention carried out to resolve a complication. The absence of an increase in flow of at least 20 % after such an intervention (e.g., percutaneous transluminal angioplasty to eliminate stenosis) indicates that the treatment has failed and an alternative solution is needed [24].
As mentioned, in AVF surveillance DUS can also be used to explore the possible causes of vascular access malfunction. However, while flow volume calculation is fairly simple and takes very little time, systematic assessment of an AVF with DUS is a challenging and more time-consuming procedure that should be done only by experienced operators. Therefore, this examination should be used only when monitoring/surveillance methods have revealed anomalies or when problems arise that prevent regular dialysis (difficult venipuncture, insufficient blood flow, high venous pressure, prolonged bleeding after removal of fistula needles).
The minimal technical requirements and the position of the patient are those used for preoperative mapping. Given the relatively superficial position of the vessels used to create an AVF, high-frequency (7.5–13 MHz) linear probes are fundamental for obtaining maximum anatomical details on the vessel walls and for accurate assessment of the superficial wall of the outflow vein, where puncture damage is more likely (approximately 300 punctures/year with 15–16 gage needle) [23]. The wall of the outflow vein is only a few millimeters below the skin surface. If a lower frequency transducer is used, this area will inevitably be out of focus, and it will be very difficult to identify wall lesions [23]. The vessels of an AVF almost always run parallel to the skin surface. Therefore, the Doppler examination should always be performed with a beam-steering transducer that will allow the operator to maintain the correct angle of incidence (30°–60°) relative to the direction of flow [23].
The examination should include the following steps:
Study of arterial inflow side of the fistula (including AVF flow volume).
Study of anastomotic chamber.
Study of venous outflow side of the fistula.
A thorough evaluation of the AVF includes exploration of each of these three areas with both transverse and longitudinal scans and assessment of morphological (B-mode) as well as hemodynamic aspects (with color Doppler and Doppler analysis).
B-mode, color Doppler, and Doppler analysis findings that are typical of a well-functioning AVF are described separately below (for purely educational purposes, as in clinical settings the three studies are generally carried out concomitantly). Descriptions of the ultrasound findings associated with the main complications of AVFs would render this review excessively long, and the reader is therefore referred to specialist publications on this subject [23, 32].
Morphological assessment (B-mode)
The study typically begins proximally, with an examination of the brachial artery. On a longitudinal view, a normal artery appears as a completely anechoic band delimitated by two three-layered walls. On real-time images, the arteries can be easily distinguished from the veins by their walls, which move in synchrony with cardiac systole, and by their noncompressibility under probe pressure.
The brachial artery is followed down to the antecubital crease, where it divides into radial and ulnar arteries, which run along the lateral and medial side of the forearm, respectively. The AVF inflow artery, generally the radial artery, is characterized by a constant, regular increase in caliber and modest tortuosity, which are more marked in high-flow AVFs. Another common finding is pulsatility, which is much stronger than that of the same vessel in the contralateral arm (especially in the area near the anastomosis) [23, 32].
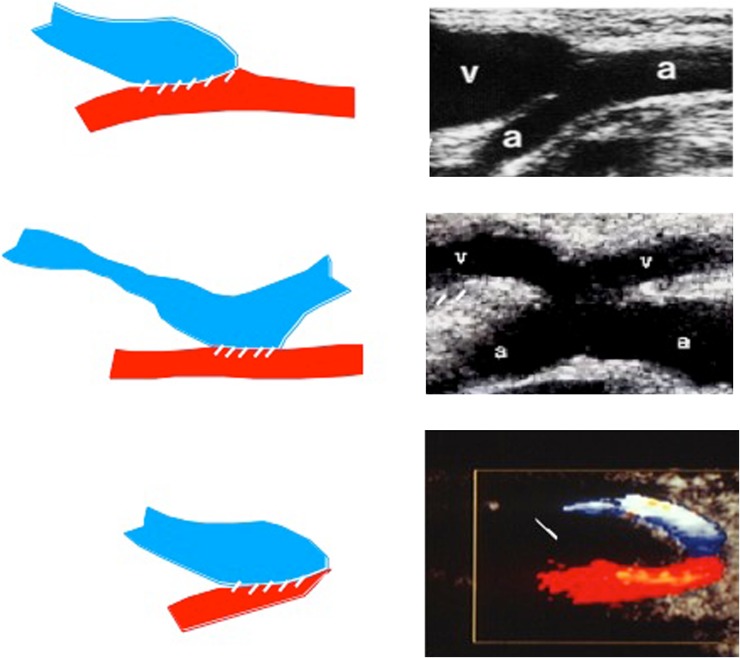
Exploration of the arterial side of the fistula proceeds distally to the surgical anastomosis, which frequently has a winding course. Vessel pulsatility at the anastomotic region is so marked that it produces a “thrill” caused by the turbulence of the flow. It is characterized by fine, rapid, palpable and sonographically documented vibrations involving the tissues surrounding the vessel [23, 32]. The sonographic image of the anastomotic region can help us to define the AVF type: end-to-end (E-E), side-to-end (S-E), or side-to-side (S-S) (Fig. 4).
Fig. 4.
Longitudinal ultrasound scans of the anastomotic region of various AVF types. Top side-to-end AVF (S-E), Center side-to-side AVF (S-S), Bottom end-to-end AVF (E-E)
If the non-linearity of the anastomotic chamber precludes acquisition of such images, the presence or absence of the arterial and distal venous segment with respect to the site of the anastomosis can be used to identify the type of anastomosis.
The outflow vein is characterized by tortuosity, ectasia, and segmental variations in caliber that are generally due to wall damage caused by repeated venipuncture. The vessel walls generally appear to be mildly thickened as a result of intimal hyperplasia, a phenomenon that renders the vessel capable of withstanding repeated venipuncture with large-caliber needles [23, 32].
Color Doppler examination
The appearance of color confirms the patency of the vessels being examined. As a general rule, the scanner is usually set to “map” arterial flow in red (flow moving towards the transducer) and venous flow in blue (moving away from the transducer).
The pulse repetition frequency (PRF) is one of the most important settings that need to be adjusted for a proper flow metric analysis. The PRF selected for AVFs is usually higher (1,000–6,000 Hz) than those commonly used to study the upper limbs, because of the higher flow velocities in AVFs [23, 32]. It is important to recall that this is a “dynamic” setting, which has to be re-adjusted several times during the examination to eliminate aliasing (especially near the anastomosis, where the flow is faster) and to avoid non-coding of the venous flow, which can occur, for example, during exploration of the outflow vein after assessment of the AVF with a high PRF [23, 32]. Lower PRFs (<1,000 Hz) can be used when a vessel seems to be patent on the basis of morphological findings and responses to compression maneuvers, but intraluminal flow signals are lacking. This can occur, for example, at the level of large venous aneurysms, where flow slows considerably as a result of the large caliber of the lumen. Low PRFs are also recommended—although not absolutely necessary—during exploration of collateral vessels near a thrombosed or complex AVF [23, 32].
On color Doppler imaging, the inflow artery of the AVF is characterized by relatively homogeneous, laminar flow (with maximum velocity at the center of the lumen and the lowest values near the vessel walls). Near the anastomosis, there is an increase in flow velocity (reflected by lighter colors, even white) and turbulence (reflected by a disorderly alternation of reds and blues within the same luminal segment). Because of the high flow volumes and caliber irregularities that characterize the outflow vein, areas of vortex flow are often observed at this level, especially near the anastomosis. They are reflected by alternating intraluminal color signals with a typical spiroidal configuration [23, 32]. Moving away from the AVF, the caliber of the vessel tends to decrease, vortexing diminishes, and the venous flow gradually becomes more homogeneous and regular [23, 32].
Doppler ultrasound assessment
On Doppler ultrasound imaging, the inflow side of a normal AVF is characterized by an appreciable reduction in peripheral resistance relative to the contralateral limb, with copious anterograde flow during the entire diastolic phase [23]. As the transducer moves closer to the anastomosis, flow through the afferent artery undergoes a progressive and constant increase in velocity that involves both the systolic and diastolic phases, and spectral broadening is observed, which is maximal in vicinity of the anastomosis, where it reaches the baseline with the disappearance of the acoustic window [23]. At the level of the anastomosis, purely turbulent flow profiles are observed, with loss of arterial phasicity, a broad spectrum extending above and below the baseline, and high peak systolic velocities that are extremely variable in subsequent moments [23]. On the venous side, near the anastomosis, flow is “arterialized” with obvious systolic–diastolic phasicity and a particularly broad spectrum. As the distance from the AVF increases, arterial phasicity is progressively lost, the mean flow velocity diminishes, and the spectrum takes on the characteristics of regular venous flow [23].
Conclusions
The native AVF is the vascular access of choice for patients who require hemodialysis: it lasts longer and is associated with fewer complications than other types of vascular access; for hemodialysis patients, these benefits translate into better quality of life and longer survival.
Doctors involved in the construction and maintenance of vascular accesses for hemodialysis know that DUS is fundamental for identifying vessels that are suitable for creating an AVF (preoperative mapping) and for early detection of complications (surveillance). Indeed, DUS is the only surveillance method that allows one to monitor AVF blood flow and simultaneously explore possible causes of vascular access malfunction. This facilitates timely, targeted salvage interventions that can prolong the survival of the vascular access and consequently that of the patient as well.
Conflict of interest
Pasquale Zamboli, Fulvio Fiorini, Alessandro D’Amelio, Pasquale Fatuzzo, Antonio Granata declare that they have no conflict of interest.
Human and animal studies
The study described in this article did not include any procedures involving humans or animals.
References
- 1.NKF-K/DOQI III (2001) NKF-K/DOQI Clinical practice guidelines for vascular access: update 2000. Am J Kidney Dis 37(Suppl 1):S137–S181 [DOI] [PubMed]
- 2.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO. Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg. 2001;34:694–700. doi: 10.1067/mva.2001.117890. [DOI] [PubMed] [Google Scholar]
- 3.Konner K, Hulbert-Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney Int. 2002;62:329–338. doi: 10.1046/j.1523-1755.2002.00436.x. [DOI] [PubMed] [Google Scholar]
- 4.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–2020. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 5.Silva MB, Jr, Hobson RW, 2nd, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of pre-operative non invasive evaluation. J Vasc Surg. 1998;27:302–307. doi: 10.1016/S0741-5214(98)70360-X. [DOI] [PubMed] [Google Scholar]
- 6.Shenoy S, Darcy M. Ultrasound as a tool for preoperative planning, monitoring and interventions in dialysis arteriovenous access. AJR. 2013;201(4):W539–W543. doi: 10.2214/AJR.13.11277. [DOI] [PubMed] [Google Scholar]
- 7.Wong CS, McNicholas N, Healy D, Clarke-Moloney M, Coffey JC, Grace PA, et al. A systematic review of preoperative duplex ultrasonography and arteriovenous fistula formation. J Vasc Surg. 2013;57:1129–1133. doi: 10.1016/j.jvs.2012.11.094. [DOI] [PubMed] [Google Scholar]
- 8.Ilhan G, Esi E, Bozok S, Yürekli I, Özpak B, Özelçi A, et al. The clinical utility of vascular mapping with Doppler ultrasound prior to arteriovenous fistula construction for hemodialysis access. J Vasc Access. 2013;14(1):83–88. doi: 10.5301/jva.5000097. [DOI] [PubMed] [Google Scholar]
- 9.Ferring M, Henderson J, Wilmink A, Smith S. Vascular ultrasound for the pre-operative evaluation prior to arteriovenous fistula formation for haemodialysis: review of the evidence. Nephrol Dial Transpl. 2008;23:1809–1815. doi: 10.1093/ndt/gfn001. [DOI] [PubMed] [Google Scholar]
- 10.Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002;39:1218–1225. doi: 10.1053/ajkd.2002.33394. [DOI] [PubMed] [Google Scholar]
- 11.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis accesses. Eur J Vasc Endovasc Surg. 1996;12:207–213. doi: 10.1016/S1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 12.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D et al (2007) EBPG on vascular access. Nephrol Dial Transpl 22(Suppl 2):ii88–ii117 [DOI] [PubMed]
- 13.Wiese P, Nonnast-Daniel B. Colour Doppler ultrasound in dialysis access. Nephrol Dial Transpl. 2004;19:1956–1963. doi: 10.1093/ndt/gfh244. [DOI] [PubMed] [Google Scholar]
- 14.Malovrh M. The role of sonography in the planning of arteriovenous fistulas for hemodialysis. Semin Dial. 2003;16:299–303. doi: 10.1046/j.1525-139X.2003.16069.x. [DOI] [PubMed] [Google Scholar]
- 15.Davidson I, Chan D, Dolmatch B, Hasan M, Nichols D, Saxena R, et al. Duplex ultrasound evaluation for dialysis access selection and maintenance: a practical guide. J Vasc Access. 2008;9:1–9. [PubMed] [Google Scholar]
- 16.Ferring M, Claridge M, Smith SA, Wilmink T. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis: a randomized trial. Clin J Am Soc Nephrol. 2010;5(12):2236–2244. doi: 10.2215/CJN.02820310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malovrh M. Non-invasive evaluation of vessels by duplex sonography prior to construction of arteriovenous fistulas for haemodialysis. Nephrol Dial Transpl. 1998;13:125–129. doi: 10.1093/ndt/13.1.125. [DOI] [PubMed] [Google Scholar]
- 18.Parmar J, Aslam M, Standfield N. Pre-operative radial arterial diameter predicts early failure of arteriovenous fistula (AVF) for haemodialysis. Eur J Vasc Endovasc Surg. 2007;33:113–115. doi: 10.1016/j.ejvs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Lemson MS, Leunissen KM, Tordoir JH. Does pre-operative duplex examination improve patency rates of Brescia-Cimino fistulas? Nephrol Dial Transpl. 1998;13:1360–1361. doi: 10.1093/oxfordjournals.ndt.a027893. [DOI] [PubMed] [Google Scholar]
- 20.Ku YM, Kim YO, Kim JI, Choi YJ, Yoon SA, Kim YS, et al. Ultrasonographic measurement of intima-media thickness of radial artery in pre-dialysis uremic patients: comparison with histological examination. Nephrol Dial Transpl. 2006;21:715–720. doi: 10.1093/ndt/gfi214. [DOI] [PubMed] [Google Scholar]
- 21.Wittenberg G, Schindler T, Tschammler A, Kenn W, Hahn D. Value of color coded duplex ultrasound in evaluating arm blood vessels-arteries and haemodialysis shunts. Ultraschall Med. 1998;19:22–27. doi: 10.1055/s-2007-1000454. [DOI] [PubMed] [Google Scholar]
- 22.Sato M, Io H, Tanimoto M, Shimizu Y, Fukui M, Hamada C, et al. Relationship between preoperative radial artery and postoperative arteriovenous fistula blood flow in hemodialysis patients. J Nephrol. 2012;25(5):726–731. doi: 10.5301/jn.5000050. [DOI] [PubMed] [Google Scholar]
- 23.Rabbia C, Matricardi L (2006) Eco-Color-Doppler Vascolare. Minerva Medica, III Edizione
- 24.NKF-K/DOQI (2006) Clinical practice guidelines for vascular access update 2006. Am J Kidney Dis 48(Suppl 1):s176–s322 [DOI] [PubMed]
- 25.Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg. 2002;36:460–463. doi: 10.1067/mva.2002.126544. [DOI] [PubMed] [Google Scholar]
- 26.Planken RN, Keuter XH, Hoeks AP, Kooman JP, van der Sande FM, Kessels AG, et al. Diameter measurement of the forearm cephalic vein prior to vascular access creation in end-stage renal disease patients: graduated pressure cuff versus tourniquet vessel dilatation. Nephrol Dial Transpl. 2006;21:802–806. doi: 10.1093/ndt/gfi340. [DOI] [PubMed] [Google Scholar]
- 27.Lockhart ME, Robbin ML, Fineberg NS, Wells CG, Allon M. Cephalic vein measurement before forearm fistula creation: does use of a tourniquet to meet the venous diameter threshold increase the number of usable fistula? J Ultrasound Med. 2006;25:1541–1545. doi: 10.7863/jum.2006.25.12.1541. [DOI] [PubMed] [Google Scholar]
- 28.Planken RN, Tordoir JH, Duijm LE, de Haan MW, Leiner T. Current techniques for assessment of upper extremity vasculature prior to hemodialysis vascular access creation. Eur Radiol. 2007;17:3001–3011. doi: 10.1007/s00330-007-0662-6. [DOI] [PubMed] [Google Scholar]
- 29.Beathard GA, Arnold P, Jackson J, Litchfield T, Physician Operators Forum of RMS Lifeline Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 30.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidrogel on early failure of arteriovenous fistula for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayner HC, Pisoni RL, Gillespie BW, Goodkin DA, Akiba T, Akizawa T, et al. Creation, cannulation and survival of arteriovenous fistulae: data from the dialysis outcomes and practice patterns study. Kidney Int. 2003;63:323–330. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Zamboli P, Calabria M, Camocardi A, Fiorini F, D’Amelio A, Lo Dico C, et al. Color-Doppler imaging and arteriovenous fistula: preoperative evaluation and surveillance. G Ital Nefrol. 2012;29(Suppl 57):S36–S46. [PubMed] [Google Scholar]
- 33.Lomonte C, Casucci F, Antonelli M, Giammaria B, Losurdo N, Marchio G, et al. Is there a place for duplex screening of the brachial artery in the maturation of arteriovenous fistulas? Semin Dial. 2005;18(3):243–246. doi: 10.1111/j.1525-139X.2005.18320.x. [DOI] [PubMed] [Google Scholar]
- 34.Smits JH, van der Linden J, Hagen EC, Modderkolk-Cammeraat EC, Feith GW, Koomans HA, et al. Graft surveillance: venous pressure, access flow or the combination? Kidney Int. 2001;59:1551–1558. doi: 10.1046/j.1523-1755.2001.0590041551.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim YO, Yang CW, Yoon SA, Chun KA, Kim NI, Park JS, et al. Access blood flow as a predictor of early failures of native arteriovenous fistulas in hemodialysis patients. Am J Nephrol. 2001;21:221–225. doi: 10.1159/000046251. [DOI] [PubMed] [Google Scholar]