Abstract
Purpose
To evaluate the technical feasibility of real-time elastography (RTE) to assess the stiffness of the skin of the peri-oral region in patients affected by systemic sclerosis (SSc).
Methods
Six female patients affected by SSc (median age = 52 years) presenting with microstomia and six healthy controls matched for age and sex underwent RTE evaluation of the peri-oral region. Two operators with different experience evaluated the stiffness of the peri-oral region placing the probe in four different positions: parasagittal left (PL), parasagittal right (PR), upper axial (UA), lower axial (LA). Color map was converted into a semi-quantitative scale in which blue = 1, green = 2 and red = 3. Thus, each subject had a variable score ranging from 4 (four positions × value = 1) and 12 (four positions × value = 3). Mann–Whitney U and k statistics were used.
Results
RTE demonstrated that the skin of the peri-oral region of patients affected by SSc was stiffer than that of controls, both overall (6;4–6 [median; 25–75th percentile] vs. 11;9–11, p < 0.001) and for each probe position (PL = 1;1–2 vs. 2;2-3, PR = 1;1–2 vs. 2;2–3, UA = 1;1–2 vs. 2;2–3; LA = 1;1–1 vs. 3;3–3, p ≤ 0.011 for all). Interobserver reproducibility was excellent both overall and for each probe position (k = 1).
Conclusion
RTE is a feasible modality to assess peri-oral region skin stiffness with excellent interobserver reproducibility. Further studies on a larger cohort of patients including more clinical data and measures are warranted to confirm our initial results.
Keywords: Systemic sclerosis, Real-time elastography, Ultrasonography, Microstomia, Skin
Sommario
Obiettivo
Valutare la fattibilità dell’utilizzo dell’elastosonografia per studiare la rigidità della cute della regione periorale in pazienti affetti da sclerosi sistemica.
Metodi
Sei pazienti affette da sclerosi sistemica (età mediana = 52 anni) con microstomia e sei controlli sani appaiati per sesso ed età sono state sottoposte ad elastosonografia della regione periorale. Due operatori con differente esperienza hanno valutato l’elasticità della regione periorale con la sonda in quattro posizioni parasagittale sinistra (SX), parasagittale destra (DX), assiale superiore (AS), assiale inferiore (AI). La mappa colore è stata convertita in una scala semi-quantitativa in cui blu = 1, verde = 2 e rosso = 3. Pertanto, ogni soggetto ha avuto un punteggio totale variabile tra 4 (quattro posizioni × valore = 1) e 12 (quattro posizioni × valore = 3). Sono stati utilizzati i test U di Mann–Whitney e k di Cohen.
Risultati
L’elastosonografia ha dimostrato che la cute della regione periorale delle pazienti affette da sclerodermia è più rigida di quella dei controlli, sia globalmente (6;4–6 [mediana; 25–75th percentile] vs. 11;9–11, p < 0.001) che per ogni singola posizione (SX = 1;1–2 vs. 2;2–3, DX = 1;1–2 vs. 2;2–3, UP = 1;1–2 vs. 2;2–3; DN = 1;1–1 vs. 3;3–3, p ≤ 0.011 per tutti). La riproducibilità interosservatore è stata eccellente sia globamente che per singola posizione (k = 1).
Conclusioni
L’elastosonografia può essere eseguita nella regione periorale per valutare il grado di rigidità della cute, con eccellente riproducibilità interosservatore. Ulteriori studi che includano un campione maggiore di pazienti con dati clinici sono necessari per confermare i nostri risultati preliminari.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by increased collagen deposition caused by an autoimmune activation of fibroblasts that may affect the skin, the deep organs, or both [1, 2]. Fibrosis can be considered as one of the most important aspects of SSc. Fibrosis occurrence is not restricted to the skin, but is also very prominent in the lung and heart, the gastrointestinal tract, in tendons and ligaments, as well as in endocrine glands and within the perivascular space [1–4]. An uncommon manifestation in SSc is microstomia, which is considered a direct consequence of subcutaneous matrix deposition around the mouth [1, 2]. Although not present in all patients, microstomia implies remarkable both functional and esthetical impairment with a significant overall reduction of life quality of affected patients. To date, assessment of skin involvement in patients affected with SSc has been traditionally performed clinically—using the modified Rodnan skin score, or mechanically —using a durometer, a standardized tool able to measure skin hardness [1, 2]. Ultrasonography (US) has been occasionally used to measure skin thickness [5]. Real-time elastography (RTE) is a recently developed, noninvasive US technique that allows for an in vivo assessment of the mechanical properties of tissues, enabling measurement of the changes in the native US signal before and after application of a mechanical stimulation [6]. Different US-based elastography technologies are available, with axial-strain elastography being the most widely available in commercial US systems. Originally used to diagnose malignancies throughout the body [7], RTE has been successfully used to evaluate the pathology of soft tissues and musculoskeletal system [6, 8–11]. Regarding SSc Iagnocco et al. [12] and Di Geso et al. [13] used RTE to evaluate skin involvement over the forearm, hand and fingers. However, no previous study has tested RTE in the evaluation of peri-oral soft tissues. Thus, the aim of our study was to test the technical feasibility of using RTE in the evaluation of peri-oral skin in patients affected by SSc with microstomia.
Materials and methods
Study population
We included in our study six women (median age; 25–75th percentile = 52; 45–64 years) that were diagnosed with SSc according to American College of Rheumatology criteria [2]. All patients presented with microstomia that was defined as a distance on the sagittal plane between the two right incisors less than 50 mm (with normal opening range between 50 and 60 mm) [2]. Patients who previously underwent surgery of the peri-oral area were a priori excluded from the present study.
Control group
Six healthy women, matched for age with patients with SSc, served as a control group. They were mainly recruited among hospital personnel. None of these subjects had known history of SSc or of other collagen pathologic condition, nor reported previous surgery around the mouth.
US and RTE evaluation
All patients and controls underwent B-mode US evaluation of the peri-oral region with a high-resolution linear probe 13–6 MHz (MyLab 70 XvG system; Esaote, Genoa, Italy). Examinations were performed by a radiologist and separately by a radiology resident with 10 and 2 years experience, respectively, in musculoskeletal US. The operators were not blinded to clinical condition of patients, as the disease is clearly clinically detectable. The operator placed the probe in different orientations around the mouth of subjects: parasagittal left (PL), parasagittal right (PR), upper axial (UA), and lower axial (LA). Immediately after B-mode evaluation, the same operator performed RTE evaluation with the probe placed in the same orientation as described for B-mode evaluation. The same US system equipped with axial-strain RTE software was used. To obtain a more reliable RTE evaluation, we used a 1 cm gel spacer (Parker Laboratories Inc., Fairfield, NJ, USA) that was interposed between the US probe and the skin [12]. A scheme for peri-oral evaluation is reported in Fig. 1. The force applied to the probe was adjusted appropriately, according to the visual indicator on the video screen, which showed optimal strain at the peri-oral region, as previously reported [11–13]. This indicator has a six-level scale: acceptable compression is achieved from level four to six. Each scan was repeated by compression and relaxation of the scan area several times (at least three compression–decompression cycles) until the findings were confirmed to be reproducible within the same observer.
Fig. 1.
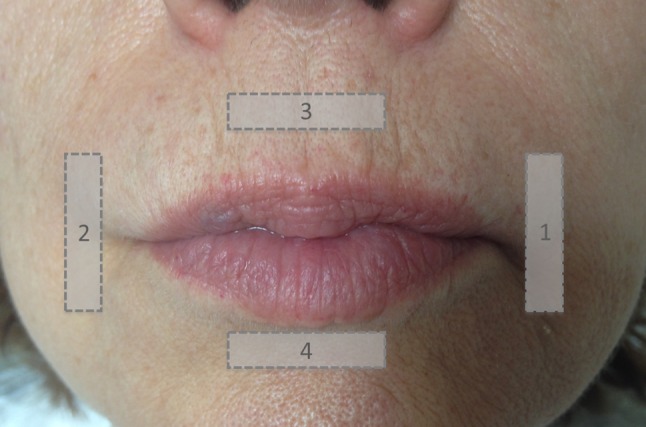
Probe positioning for real-time elastography evaluation of the peri-oral region. 1 parasagittal left scan; 2 parasagittal right scan; 3 upper axial scan; 4 lower axial scan
Image evaluation
The US system produces a colored map that is superimposed to B-mode images, similarly to what happens for power Doppler. The color scale ranges from blue—that corresponds to the hardest tissue—to red—that corresponds to the softest tissue, with green as the intermediate value. For each position, observers visually evaluated the predominant color, converting the color map into a semi-quantitative scale in which blue has a value of 1, green has a value of 2, and red has a value of 3. Thus, each individual could have a total score ranging from 4 (four positions × score = 1) to 12 (four position × score = 3) [11]. Due to the curvilinear shape of the dental arcade that underlies the mouth, the peripheral area of RTE images may be affected by artifacts. Thus, a peripheral section of 0.5 cm from each side of the image was excluded from our analysis.
Statistical analysis
RTE scores in patients affected by SSc were compared to those of controls using the Mann–Whitney U test. Interobserver reproducibility was assessed using the Cohen’s k. A P value less than 0.05 was considered to be statistically significant.
Results
Despite the curvilinear shape of the peri-oral region, we were able to perform RTE in all patients with no discomfort. This evaluation required approximately 1 min in addition to B-mode evaluation.
In SSc patients, RTE showed a predominantly blue pattern with green spots. In controls, the blue pattern was never detected and red color was predominantly seen. RTE scoring evaluation showed that that peri-oral skin region of patients is significantly less elastic than controls, both for each position and overall (p ≤ 0.011). Full data are reported in Table 1. RTE images of patient and control are reported in Fig. 2.
Table 1.
Real-time elastography scores of the peri-oral region in patients affected by systemic scleroderma and microstomia and in matched controls
| Evaluation Site | Patients | Controls | p value |
|---|---|---|---|
| Parasagittal left | 1 (1–2) | 2 (2–3) | 0.011 |
| Parasagittal right | 1 (1–2) | 2 (2–3) | 0.001 |
| Upper axial | 1 (1–2) | 2 (2–3) | 0.001 |
| Lower axial | 1 (1–1) | 3 (3–3) | 0.001 |
| Total | 6 (4–6) | 11 (9–11) | 0.001 |
Data are given as median and (25–75th percentiles)
Fig. 2.
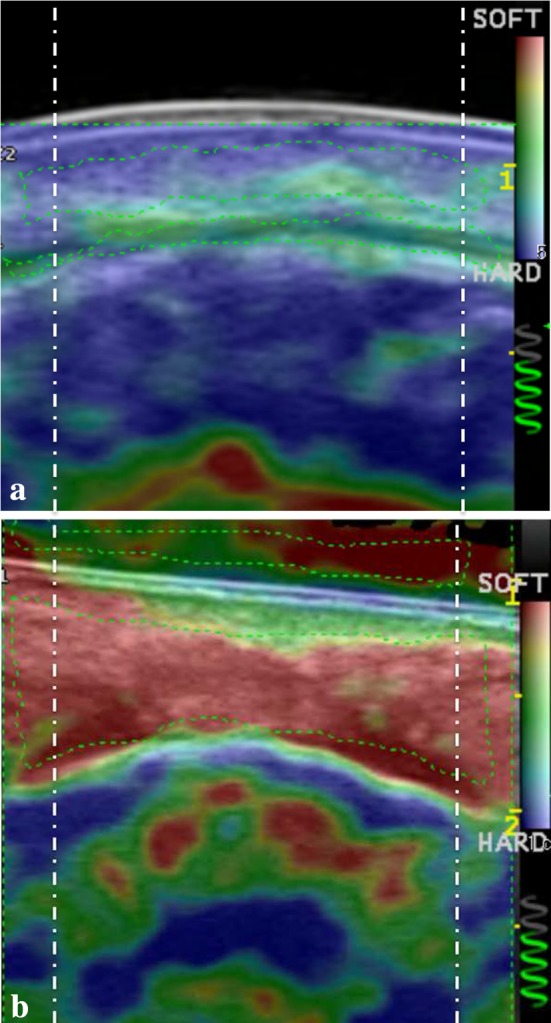
Real-time elastography evaluation of the peri-oral region in, a a patient affected by systemic scleroderma and b a control matched for age and sex. Note the patchy blue pattern of the scleroderma patient and the almost homogeneous red pattern of the healthy volunteer. Dashed-dotted vertical lines indicate the area excluded from the analysis
Interobserver correlation was excellent (k = 1), both for each position and overall.
Discussion
The present paper is the first feasibility report that demonstrates RTE can be technically performed in a very small area such as the peri-oral region. We also found that RTE is able to demonstrate that the skin of the peri-oral region in patients with SSc was harder than in healthy controls.
Despite that RTE was used in several musculoskeletal conditions [8–11], to our knowledge only two previous papers reported the use of RTE to evaluate skin rigidity in patients affected by SSc. Iagnocco et al. [12] used RTE to evaluate the skin of the forearm and fingers in patients affected by SSc. Similarly to us, they found that the skin of forearms in patients with SSc was stiffer than that of healthy controls. However, they found that RTE evaluation in the skin of the finger was not really reliable, producing extremely variable patterns. They concluded that this variability may be due to the close vicinity of the underlying bone that is known to somewhat alter the results of RTE [11]. Di Geso et al. [13] further investigated whether RTE may increase the reliability of B-mode US in the measurement of dermal thickness at finger level in patients affected by SSc. They tested their hypothesis on the second finger of 22 patients and concluded that RTE allows for +29 % improvement in measuring dermal thickness, helping to better differentiate the interface between the dermis and hypodermis.
In our study, we found that RTE can be effectively used to assess skin elasticity in the peri-oral region. The adequacy of the scan was assessed looking at the semi-quantitative scale that is present on each RTE image. This result is peculiar, as it was always thought that RTE needed to be applied onto a perfectly flat surface. In this case, the peri-oral region is convex and irregular, due to the underlying dental arcades. Furthermore, our data differ from that found by Iagnocco et al., who reported that the close vicinity of the underlying bone interfered with the RTE signal.
The second important result is that RTE allowed for detecting the higher stiffness of the peri-oral skin of patients affected by SSc. This implies that RTE may be potentially used to routinely assess these patients in clinical practice, both when initially evaluated and to monitor the effectiveness of treatment. However, this is still a speculation, as this hypothesis cannot be derived directly from our data.
The main limitation of our report is the fact of being a feasibility study performed on a limited number of patients and controls, with no correlation with clinical data. However, our main goal was to demonstrate the technical application of this relatively new modality in such a particular area of the body. Future studies on this topic should certainly include a correlation between RTE and clinical data (e.g., Rodnan score) and other tools that are routinely used to evaluate SSc patients (e.g., durometer).
In conclusion, RTE is an imaging modality that can be effectively applied in the peri-oral region to assess skin stiffness in patients affected by SSc, who demonstrated harder skin compared to healthy controls. RTE seems to have high interobserver reproducibility. Further studies on a larger cohort of patients including more clinical data and measures are warranted to confirm our initial results.
Acknowledgments
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Institutional review board approval and informed patients’ consent were obtained for the present prospective study.
Conflict of interest
The authors (Paola Maria Cannaò, Valeriano Vinci, Fabio Caviggioli, Marco Klinger, Davide Orlandi, Francesco Sardanelli, Giovanni Serafini and Luca Maria Sconfienza) have no conflict of interest to disclose.
References
- 1.Geyer M, Müller-Ladner U. The pathogenesis of systemic sclerosis revisited. Clin Rev Allergy Immunol. 2011;40(2):92–103. doi: 10.1007/s12016-009-8193-3. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA, Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savarino E, Ghio M, Marabotto E, Zentilin P, Sammito G, Cittadini G, Sconfienza L, Murolo C, Gemignani L, Indiveri F, Savarino V. Possible connection between gastroesophageal reflux and interstitial pulmonary fibrosis in patients with systemic sclerosis. Recenti Prog Med. 2009;100(11):512–516. [PubMed] [Google Scholar]
- 4.Savarino E, Mei F, Parodi A, Ghio M, Furnari M, Gentile A, Berdini M, Di Sario A, Bendia E, Bonazzi P, Scarpellini E, Laterza L, Savarino V, Gasbarrini A. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology. 2013;52(6):1095–1100. doi: 10.1093/rheumatology/kes429. [DOI] [PubMed] [Google Scholar]
- 5.Bendeck SE, Jacobe HT. Ultrasound as an outcome measure to assess disease activity in disorders of skin thickening: an example of the use of radiologic techniques to assess skin disease. Dermatol Ther. 2007;20(2):86–92. doi: 10.1111/j.1529-8019.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 6.Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28(3):373–378. [PubMed] [Google Scholar]
- 7.Guazzaroni M, Spinelli A, Coco I, Del Giudice C, Girardi V, Simonetti G. Value of strain-ratio on thyroid real-time sonoelastography. Radiol Med. 2014;119(3):149–155. doi: 10.1007/s11547-013-0320-9. [DOI] [PubMed] [Google Scholar]
- 8.Sconfienza LM, Silvestri E, Bartolini B, Garlaschi G, Cimmino MA. Sonoelastography may help in the differential diagnosis between rheumatoid nodules and tophi. Clin Exp Rheumatol. 2010;28(1):144–145. [PubMed] [Google Scholar]
- 9.Sconfienza LM, Orlandi D, Cimmino MA, Silvestri E. A few considerations on “sonoelastography of the plantar fascia”. Radiology. 2011;261(3):995–996. doi: 10.1148/radiol.11111255. [DOI] [PubMed] [Google Scholar]
- 10.Sconfienza LM, Orlandi D, Longo S, Silvestri E. RE: few comments on: “musculoskeletal applications of elastography: a pictorial essay of our initial experience”. Korean J Radiol. 2012;13(2):254–255. doi: 10.3348/kjr.2012.13.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sconfienza LM, Silvestri E, Orlandi D, Fabbro E, Ferrero G, Martini C, Sardanelli F, Cimmino MA. Real-time sonoelastography of the plantar fascia: comparison between patients with plantar fasciitis and healthy control subjects. Radiology. 2013;267(1):195–200. doi: 10.1148/radiol.12120969. [DOI] [PubMed] [Google Scholar]
- 12.Iagnocco A, Kaloudi O, Perella C, Bandinelli F, Riccieri V, Vasile M, Porta F, Valesini G, Matucci-Cerinic M. Ultrasound elastography assessment of skin involvement in systemic sclerosis: lights and shadows. J Rheumatol. 2010;37(8):1688–1691. doi: 10.3899/jrheum.090974. [DOI] [PubMed] [Google Scholar]
- 13.Di Geso L, Filippucci E, Girolimetti R, Tardella M, Gutierrez M, De Angelis R, Salaffi F, Grassi W. Reliability of ultrasound measurements of dermal thickness at digits in systemic sclerosis: role of elastosonography. Clin Exp Rheumatol. 2011;29(6):926–932. [PubMed] [Google Scholar]


