“…vitamin D and/or its nonhypercalcemic potent analogs, pending appropriate clincial trials evaluation, could be viable options for medical orally administered treatment of symptomatic uterine fibroids.”
Uterine fibroid represents a localized proliferation of smooth muscle cells surrounded by a pseudocapsule of compressed muscle fibers. It is the most common benign tumor in the female genital tract. The mainstay treatment of uterine fibroid is surgery, in the form of myomectomy or hysterectomy. More than 600,000 hysterectomy procedures are performed in USA alone each year. In addition to its cost burden on the USA healthcare system, these procedures cause considerable morbidity and possible mortality. Additionally, hysterectomy will preclude future fertility. Therefore, it is crucial to search for novel nonsurgical alternatives for the management of symptomatic uterine fibroids and develop strategies to prevent its occurrencein the first place. Vitamin D is known as the main regulator of calcium homeostasis. Vitamin D3 also functions as a strong antifibrotic factor. Additionally, recent studies have demonstrated that vitamin D3 is a potent antitumor agent that effectively inhibits human uterine fibroid cells in vitro and shrinks fibroid lesions in preclinical animal studies; however, no human trials have been conducted in this area thus far. Here, we will discuss the different mechanisms of action of vitamin D3 in inhibiting uterine fibroid growth and proliferation.
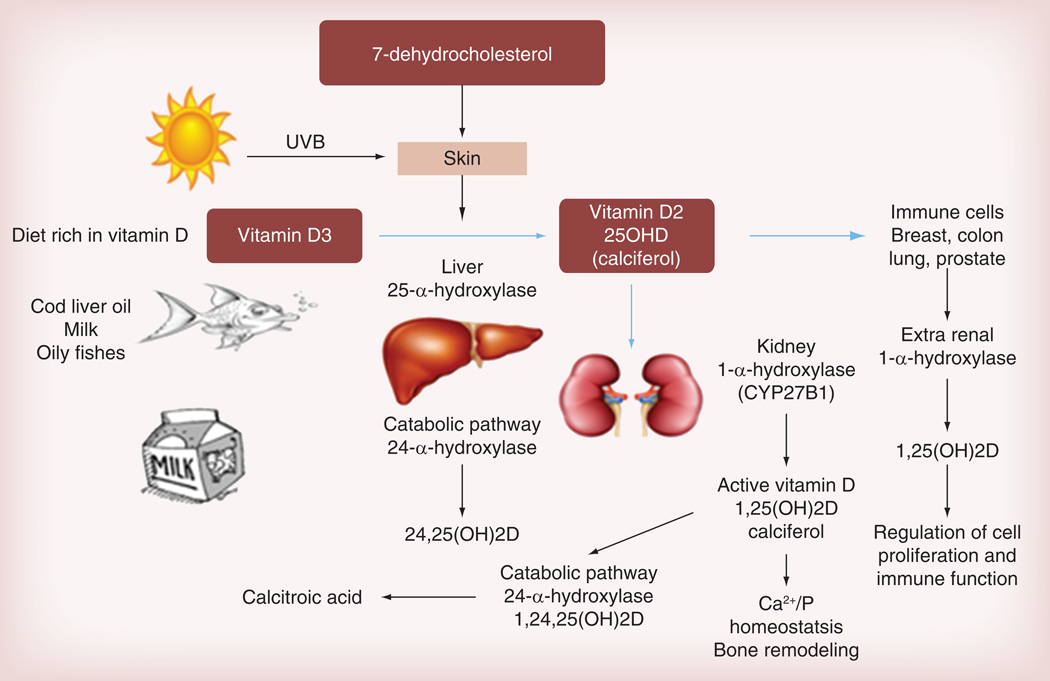
Vitamin D was first identified as a key regulator of calcium homeostasis as its deficiency was associated with rickets and osteomalacia [1]. Humans can find vitamin D within their diet, such as oily fish, cod liver oil and dairy products [2]. However, the main source is sun exposure. UVB light transforms 7-dehydrocholesterol to vitamin D through a nonenzymatic thermal isomerization found in the skin. Vitamin D is then metabolized in the liver to 25-hydroxyvitamin D (25OHD) by 25α-hydroxylase; 25OHD is converted to the active compound 1,25-dihydroxyvitamin D (1,25[OH]2D) by 1α-hydroxylase (or CYP27B1) [3], which is predominantly expressed in the kidney [4]. A catabolic pathway involving 24α-hydroxylase (CYP24A1) is responsible for 25OHD and 1,25(OH)2D hydroxylation to inactive metabolites, named 24,25(OH)2D and 1,24,25(OH)3D, respectively (Figure 1) [5]. In the kidney, 1α-hydroxylase activity is strictly controlled by calcium homeostatic signals, especially by parathyroid hormone, whose release by parathyroid glands is elicited by hypocalcemia. 1,25(OH)2D response to low serum calcium levels by stimulation of osteoclasts to release calcium from the bone, enhances intestinal calcium absorption and reduces renal calcium excretion [6]. 1,25(OH)2D3 is a biologically active vitamin D3 and, in cell systems, it functions through interacting with the vitamin D receptor (VDR) [7]. VDR is a nuclear transcription factor that plays a major role in the modulation of gene expression. The effects of VDR on cell signaling include growth arrest, differentiation and/or induction of apoptosis, thus, demonstrating the involvement of vitamin D signaling in the inhibition of cell growth.
Figure 1. Vitamin D sources, metabolism and biosynthesis in humans.
1,24,25(OH)2D: 1,24,25-dihydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; 24,25(OH)2D: 24,25-dihydroxyvitamin D; 25OHD: 25-hydroxyvitamin D; Ca2+: Calcium; P: Phosphate.
1,25-dihydroxyvitamin D3 (vitamin D3) is known as a strong growth inhibitor that induces apoptosis in human breast cancer cells [8]. Vitamin D3 suppresses proliferation of malignant cells, and it induces differentiation and apoptosis [9]. Vitamin D3 analogs have also been shown to potentiate antitumor activity in a murine squamous cell carcinoma model [10].
Uterine fibroid represents a localized proliferation of smooth muscle cells surrounded by a pseudocapsule of compressed muscle fibers. They are often detected incidentally in routine health examinations, through bimanual pelvic and/or ultrasound examination or other imaging modalities; uterine fibroids are rarely associated with symptoms. Sometimes, uterine fibroids may be complicated by a variety of symptoms, including menstrual disturbance (e.g., menorrhagia, dysmenorrhea and intermenstrual bleeding), pressure symptoms, bloated sensation, increased urinary frequency, bowel disturbance or pelvic pain; therefore, definite treatment is requested [11]. The pathophysiology behind the development of uterine fibroids is still unknown, but the most accepted theory supports the fact that both estrogen and progesterone play major roles in fibroid growth [12]. Hysterectomy is by far the mainstay option presented to women who have completed child birth; however, many women may prefer to keep the uterus if the uterine fibroids-related symptoms can be appropriately controlled by some other less invasive ways. Among these conservative therapies, myomectomy is mostly used for women who would like to preserve their future fertility [13].
Recent evidence from three independent research groups in populations in North Africa, east USA and central Europe demonstrate an association between serum vitamin D deficiency and increased risk of uterine fibroids. This is not a trivial finding but a significant one that can inform clinical practice and improve management of this common disease worldwide. The Al-Hendy group was first to report on the association between lower serum vitamin D level and increased susceptibility to uterine fibroids in 2012 in a cohort of black and white women in North Africa [12]. This was followed by two other major studies including; Baird et al. in a cohort of women from eastern USA [13], and Paffoni et al. in Italian women [14]. Table 1 summarizes the main finding in these studies.
Table 1.
A recently published study demonstrated the relationship between vitamin D and uterine fibroids.
| Sabry et al. (2013) | Baird et al. (2013) | Paffoni et al. (2013) | |
|---|---|---|---|
| Total number of participants | Total of 154 patients were included; 50 control group, 104 with fibroid. A total of 87 were black and 67 were white | 1036 (620 blacks and 416 whites) | 128 with fibroid; 256 control |
| OR/CI | Not reported | Adjusted protective OR: 0.68; 95% CI: 0.48–0.96 for 25OHD >20 ng/ml | 2.5 (95% CI: 1.2–4.9; p = 0.016) |
| Ethnicity | Different ethnicity (women; black and white) | African and Caucasian American women |
Italian women |
| Serum level 25OHD assay | UF: 19.7 ± 11.8 ng/ml Control: 22.3 ± 6.5 ng/ml Radio-immunoassay |
Only 10% of blacks and 50% of whites had levels of 25OHD regarded as sufficient (>20 ng/ml) Radio-immunoassay |
UF: 18.0 ± 7.7 ng/ml Control: 20.8 ± 11.1 ng/ml Chemiluminescence |
25OHD: 25-hydroxyvitamin D; OR: Odds ratio; UF: Uterine fibroid.
Additionally, we have reported [12] that vitamin D exhibited a significant (r = −0.31; p = 0.002) inverse dose–response relationship between its serum levels and the severity of fibroid disease in that cohort, which means the higher levels of serum vitamin D were associated with the least severe uterine fibroid burden (small fibroid size and low multiplicity) [12].
We have recently demonstrated that vitamin D3 inhibited the proliferation of human uterine fibroid cells. Blauer et al. confirmed the effect of vitamin D3 on the inhibition of human myometrial and fibroid cell growth [15]. Here, we will discuss the different mechanism of action of vitamin D3 in inhibiting uterine fibroid growth and proliferation. Although studies have shown this effect of vitamin D3 on fibroid growth in vitro and in animal studies, no human trials have yet been conducted to translate these important preclinical observations.
In a recent study, we have evaluated the efficacy and safety of vitamin D3 for the potential treatment of fibroid in the immune-competent authentic Eker rat model. The mutation of tuberous sclerosis suppressor gene makes the Eker rat more prone to fibroid development; its detailed phenotype has been described recently in a publication from our laboratory group [16]. We randomly assigned rats harboring visible fibroid tumors into two groups, with six in each group: the control group was administered with ethylene glycol (vehicle); and the treatment group was given vitamin D3 delivered by micro-osmotic pumps (Alzet Inc., CA, USA) implanted into the dorsal subcutaneous space at a rate of 0.5 µg/kg/day for 3 weeks. Methodological details of this experiment have been recently described by our group [16].
The physiological level of vitamin D is 30–80 ng/ml for 25-hydroxyvitamin D3 and can usually be accomplished by intake of 2000 IU/day. Chronic or acute administration of higher doses of vitamin D3 can lead to hypervitaminosis D, inducing hypercalcemia and functional hypoparathyroidism or adynamic bone disease in which the serum parathyroid hormone levels are suppressed below 100 pg/ml (100 ng/l), as well as the frequent fractures and bone pain usually seen in this condition. Hypercalcemia has been reported with daily doses of greater than 50,000 IU of vitamin D3. Studies have demonstrated that vitamin D toxicity is very unlikely in healthy people at intake levels lower than 10,000 IU/day [17]. In our study, we have used 0.5 µg/kg/day dose of vitamin D3, which is equivalent to 1400 IU for an adult individual with average bodyweight of 70 kg. We have calculated the dose of vitamin D3 using the conversion equation 1 µg of vitamin D3 is equivalent to 40 IU [18].
To understand the different antiproliferative mechanism of action of 1,25 dihydroxyvitamin D3, we are going to discuss each mechanism separately in the following discussion.
We detected that vitamin D3 reduced the expression levels of the cell proliferation marker PCNA on vitamin D3-treated Eker rats when compared with vehicle-treated controls. We used the western blot analyses using uterine fibroid tumor lysates obtained from both groups to determine the effects of vitamin D3 on protein expression associated with fibroid growth. Vitamin D3 also significantly reduced the expression levels of cell cycle regulatory proteins, such as CDK1, CDK2 and CDK4, in fibroid tumors [19].
In addition, we have found the expression of the proapoptotic Bad protein to be increased in fibroid tumors in Eker rats treated with vitamin D3 when compared with vehicle-treated controls. The effects of vitamin D3 treatment on cellular apoptosis-related protein expression were verified using western blot analyses using the same fibroid tumor lysates. Vitamin D3 treatment significantly reduced the antiapoptotic BCL2 and BCL2L1 protein expressions in fibroid tumors. The cell proliferation marker cyclin D1 and the protooncogene MYC has been reported to be overexpressed in uterine fibroid compared with normal myometrium [20]. We have shown vitamin D3 treatment significantly reduced both cyclin D1 and MYC protein expression in Eker rat fibroid tumors by using western blot analyses. Additionally, vitamin D3 treatment induced the caspase-3-cleaved product in fibroid tumors in the treatment group, suggesting the activation of the caspase signaling cascade. These last two mechanisms of actions demonstrate the strong antiproliferation function of 1,25-dihydroxyvitamin D3 in controlling fibroid growth [19].
As mentioned above, both estrogen and progesterone play major roles in fibroid growth and function through their nuclear receptors [11]. We used western blot analysis to verify the regulatory effect of vitamin D3 treatment on uterine fibroid steroid receptor expression. These results demonstrated that vitamin D3 reduced fibroid tumor size by decreasing the protein expressions of estrogen receptor ESR1, as well as progesterone receptor PGR-A and PGR-B and increasing the VDR expression in Eker rats [19].
We have found that the expression of cell proliferating markers PCNA and MKI67 was significantly lower (p < 0.05) in fibroid tumors obtained from vitamin D3-treated Eker rats when compared with vehicle-treated controls. In addition, the hematoxylin and eosin staining revealed a reduced number of tumor cells in vitamin D3-treated Eker rats when compared with vehicle-treated controls. Caspase-3 immunoreactive cells denote the high apoptotic activity in a given tissue; we have found a significantly higher levels of caspase-3 immunoreactive cells in fibroid tumors derived from vitamin D3-treated Eker rats when compared with vehicle-treated controls. Taken all together, we can suggest that vitamin D3 shrinks uterine fibroid tumor size in the Eker rat preclinical model by reducing cell proliferation and by activating the intrinsic apoptosis pathway [19].
In addition, there were a remarkable reduction of the mRNA levels of MMP-1, MMP-3, MMP-13 and MMP-14 in human fibroid cells after treatment with vitamin D. The reduction of MMP-2 and MMP-9 protein were in a vitamin D concentration-dependent manner in uterine fibroid cells (p > 0.05 to p > 0.001). Uterine fibroids grow slowly by the deposition of a wide array of extracellular matrix (ECM) components. This ECM is under continuous physiological degradation process important for development, tissue repair and remodeling. Although the major proteinases enzymes called matrix metalloproteinases (MMPs) are involved in ECM degradation [21], the MMP family of proteases can digest fibrillar collagen and collagen type I via collagenases such as MMP-1, MMP-8, MMP-13, MMP-14, gelatinase A (MMP-2) and gelatinase B (MMP-9). In addition, the dysfunction and dysorganization of ECM homeostasis causes a paradoxical increase in MMPs in uterine fibroids. Vitamin D treatment led to a decrease in the expression of these MMPs, down to the level noticed in the normal myometrium [19].
TGF-βs, which are multifunctional peptides, are the key regulators of cell growth, differentiation, inflammation, apoptosis and tissue remodeling, and these processes are important contributors to tissue fibrosis [22]. The mRNAs and proteins for TGF-β1, TGF-β2 and TGF-β3 and their receptors have been detected in both human myometrium and fibroids [23]. TGF-βs also upregulate the synthesis of many of the ECM proteins that are involved in fibrosis [24]. TGF-β3 is elevated three- to five-fold in fibroid compared with adjacent myometrium tissues [25]. TGF-β3 plays an essential role in ECM overproduction in uterine fibroids by inducing the expression of collagen type 1, fibronectin, laminin and proteoglycans [26]. These ECM-related genes such as collagen type 1 and fibronectin are overexpressed in uterine fibroids. Vitamin D3 also functions as a strong antifibrotic factor by inducing a remarkable reduction in the expression of collagen and other TGF-β3-dependent key profibrotic factors in human fibroid cells in a dose-dependent fashion [27].
Conclusion
Vitamin D is known as the main regulator of calcium homeostasis. Recent studies have demonstrated that vitamin D3 is a potent antitumor agent that shrinks uterine fibroids in vitro and in appropriate preclinical animal studies; however, human trials are yet to be conducted in this important area of women’s health, which should be considered a high clinical research priority to verify these important preclincial observations. 1,25(OH)2D3 is the biologically active form of vitamin D3 and, in cell systems, functions through interacting with the VDR. VDR is a cell membrane as well as a nuclear transcription factor that includes growth arrest, differentiation and/or induction of apoptosis demonstrating the involvement of vitamin D signaling in the inhibition of cell growth. Vitamin D3 reduces the expression levels of cell proliferation marker PCNA; MKI67 protein expressions; antiapoptotic BCL2, BCL2L1, ESR1, PGR-A, PGR-B protein expressions; and the expression levels of cell cycle regulatory proteins (CDK1, CDK2 and CDK4) in fibroid tumors, but increases the expression of the proapoptotic Bad protein and the VDR itself. Vitamin D3 also functions as a strong antifibrotic factor as it dramatically inhibit the expression of collagen and other TGF-β3-dependent key profibrotic factors in human fibroid cells in a dose-dependent fashion. Finally, there is a paradoxical increase in MMPs in uterine fibroids. The MMP system is responsible for ECM homeostasis and a decrease in the expression of MMPs caused by 1,25 dihydroxyvitamin D3 is associate with fibroid shrinkage. We believe vitamin D and/or its nonhypercalcemic potent analogs, pending appropriate clincial trials evaluation, could be viable options for medical orally adminstered treatment of symptomatic uterine fibroids.
Supplementary Material
Biographies

Ayman Al-Hendy

Marwa Badr
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Ayman Al-Hendy, Email: aalhendy@gru.edu, Georgia Regents University, Division of Translational Research, Department of Obstetrics & Gynecology, Medical College of Georgia, 1120 15th Street, BA-7300, Augusta, GA 30912, USA.
Marwa Badr, University of South Alabama, Department of Obstetrics & Gynecology, Subdivision of Reproductive Endocrinology, 251 Cox Street, Suite 100 A, AL, USA.
References
- 1.Mozolowski W. Jedrzej Sniadecki (1768–1838) on the cure of rickets. Nature. 1939;143:121. [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Stoffels K, Overbregh L, Bouillon R, Mathieu C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: unravelling the IFN-gamma pathway. J. Steroid Biochem. Mol. Biol. 2007;103:567–571. doi: 10.1016/j.jsbmb.2006.12.091. [DOI] [PubMed] [Google Scholar]
- 5.Bikle D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: a millennium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 7.Adams JS, Hewison M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bc-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 9.Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil. Steril. 2001;75:405–410. doi: 10.1016/s0015-0282(00)01714-3. [DOI] [PubMed] [Google Scholar]
- 10.Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–3764. [PubMed] [Google Scholar]
- 11.Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am. J. Obstet. Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int. J. Womens Health. 2013;5:93–100. doi: 10.2147/IJWH.S38800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–453. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paffoni A, Somigliana E, Viganó P, et al. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013;98(8):E1374–E1378. doi: 10.1210/jc.2013-1777. [DOI] [PubMed] [Google Scholar]
- 15.Blauer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009;91:1919–1925. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 16.Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the eker rat model. Biol. Reprod. 2012;86(4):116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001;73:288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 18.Jeffers MD, Richmond JA, Macaulay EM. Overexpression of the c-myc proto-oncogene occurs frequently in uterine sarcomas. Mod. Pathol. 1995;8:701–704. [PubMed] [Google Scholar]
- 19.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 20.Othman E, Al-Hendy A. Molecular genetics and racial disparities of uterine leiomyomas. Best Prac. Res. Clin. Obstet. Gynecol. 2008;22:589–601. doi: 10.1016/j.bpobgyn.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factors and TGF-beta receptor mRNA and protein and the effect of TGF-betas on human myometrial smooth muscle cells in vitro. Mol. Hum. Reprod. 1997;3:233–240. doi: 10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- 23.Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil. Steril. 2007;87:1166–1172. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- 25.Norian JM, Malik M, Parker CY, et al. Transforming growth factor 3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator in-hibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 2004;89:5549–5557. doi: 10.1210/jc.2004-0161. [DOI] [PubMed] [Google Scholar]
- 27.Halder SK, Goodwin JS, Al-Hendy A. 1,25-dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011;96(4):E754–E762. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.