Abstract
Background
Previous attempts to support the single ventricle circulation mechanically have suggested that a custom-built assist device is needed to push rather than pull through the pulmonary circulation. We hypothesized that using a conventional ventricular assist device, with or without conversion of a total cavopulmonary connection to a bidirectional Glenn cavopulmonary connection would allow assistance by pulling blood through the circuit and improve cardiac index (CI).
Methods
Cavopulmonary connections were established in each of five Yorkshire pigs (25kg) using ePTFE conduits in a “Y” configuration with appropriate clamping of limbs of the Y to achieve: total cavopulmonary Fontan connection (TCPC), SVC cavopulmonary connection (SVC Glenn) and IVC cavopulmonary connection (IVC Glenn). A common atrium had been established previously by balloon septostomy. Mechanical circulatory assistance of the single systemic ventricle was achieved using a centrifugal pump with common atrial inflow and proximal ascending aortic outflow. CI was calculated using an ultrasonic flow meter placed on the distal ascending aorta and compared between assisted and non-assisted circulation for 3 conditions: TCPC, SVC Glenn and IVC Glenn. Mean pulmonary artery pressure (PAP), common atrial pressure (LAP), arterial oxygen saturation (SAT), partial pressure of arterial oxygen (PO2) and oxygen delivery (DO2) were calculated.
Results
Unassisted SVC Glenn CI tended to be higher than TCPC or IVC Glenn (Figure 1). Significant augmentation of total CI was achieved with mechanical assistance for SVC Glenn (109% ± 24%, P =.04) and also with TCPC (130% ± 109%, P = .01). Assisted CI achieved at least mean baseline biventricular CI for all 3-support modes. Oxygen delivery was highest for assisted SVC Glenn 1786 ± 1307 ml/l/min and lowest with TCPC 1146 ± 386 ml/l/min, with a trend toward lower common atrial pressure and lower pulmonary artery pressure for SVC Glenn.
Conclusions
SVC bidirectional Glenn circulation may allow optimal augmentation of cardiac index and oxygen delivery in a failing single ventricle using a conventional pediatric ventricular assist device. Our model also suggests that the Fontan circulation itself can be supported with systemic ventricular assistance of the single ventricle.
Introduction
Despite tremendous improvement in outcomes of adult patients with ventricular assist devices [1, 2], options for pediatric patients continue to be limited. A failing single ventricle circulation imposes particular challenges to providing mechanical circulatory support.
Previous attempts to support the single ventricle circulation mechanically have suggested that a custom-built assist device is needed to push [3–5] rather than pull through the pulmonary circulation. We hypothesized that using a conventional ventricular assist device, with or without conversion of a total cavopulmonary connection to a bidirectional Glenn cavopulmonary connection would allow assistance by pulling blood through the circuit and improve cardiac index (CI).
Methods
The Animal Care and Use Committee of Children’s National Medical Center approved the study and all animals received humane care in compliance with the "Guide for Care and Use of Laboratory Animals" published by the National Institutes of Health. Five 25 kgs naïve Yorkshire swine (Archer Farms, Darlington, MD) were used for the study. The animals underwent percutaneous atrial septostomy at the NIH followed by surgical construction of univentricular cavopulmonary connection at CNMC.
Percutaneous Balloon Atrial Septostomy
Animals were anesthetized with atropine, butorphanol, ketamine, and xylazine, and maintained on isoflurane and mechanical ventilation. Percutaneous arterial and venous access was obtained. If patent foramen ovale was not present, standard Mullins technique transseptal puncture was performed. Atrial septal communications were enlarged by inflation of large (18–20 mm diameter) balloon angioplasty catheters. Experiments were guided by X-ray fluoroscopy (Siemens Medical, Erlangen, Germany) and intracardiac echocardiography (AcuNav, Biosense-Webster, Diamond Bar, CA).
Surgical Creation of Univentricular Circulation
After premedication with intramuscular ketamine and xylazine, animals were maintained on intravenous continuous infusion of fentanyl, midazolam, pancuronium and mechanical ventilation.
Via a median sternotomy, after systemic heparinization and proximal ascending aortic and bicaval venous cannulation, normothermic cardiopulmonary bypass was established, using a continuous flow pump (Rotaflow, Maquet Inc., Wayne, NJ) and membrane oxygenator (Terumo FX-15, Ann Arbor, MI).
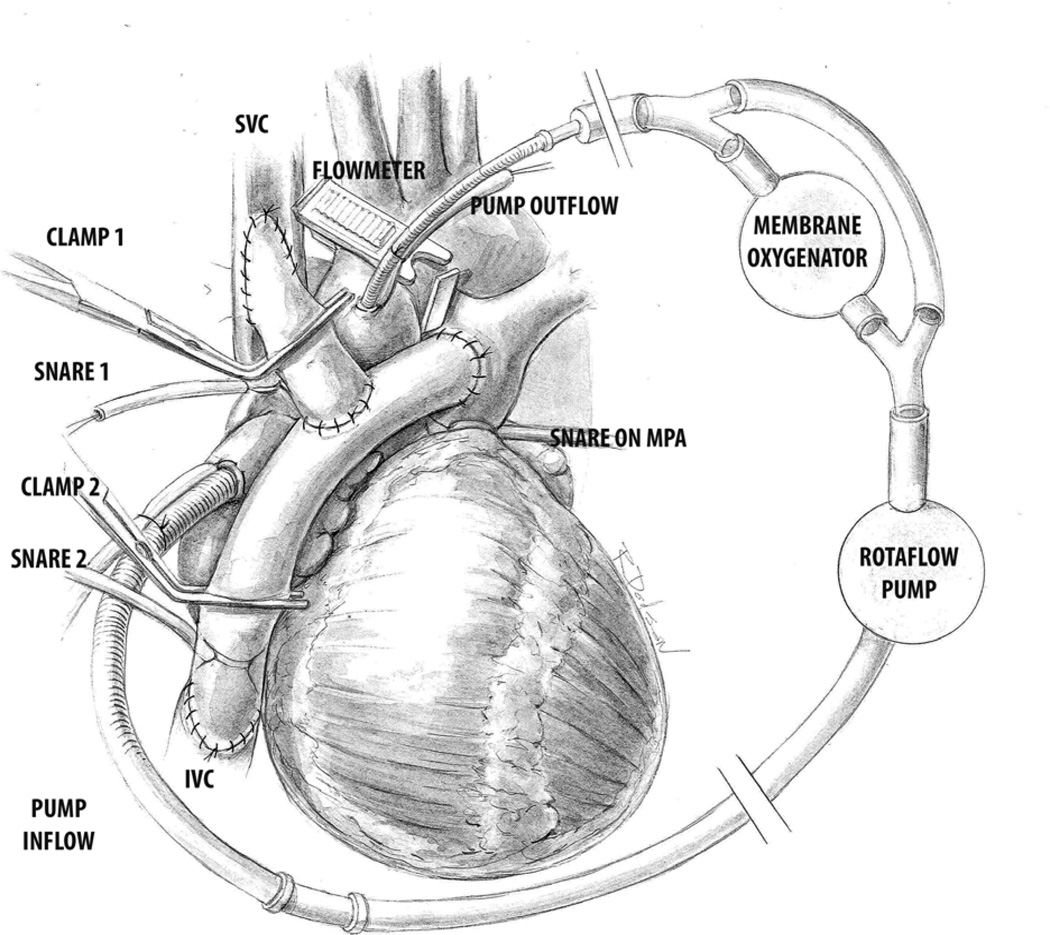
Using two limbs of 16mm ePTFE (W. L. Gore & Associates, Inc. Flagstaff, AZ) conduits in a “Y” configuration the anatomical substrate to establish cavopulmonary connection was created by construction of end to side anastomosis of the graft limbs to the superior vena cava (SVC), inferior vena cava (IVC) and the main pulmonary artery (MPA). With appropriate clamping of limbs of the Y, and snaring of the SVC, IVC or the MPA, total cavopulmonary Fontan connection (TCPC), SVC cavopulmonary connection (SVC Glenn) and IVC cavopulmonary connection (IVC Glenn) could be established. (Figure 1) An ultrasonic flow probe (Veri Q; MediStim USA Inc., MN) placed around the distal ascending aorta measured cardiac output/index. The superior caval venous cannula was removed and the inferior caval cannula moved to the right atrial appendage to drain the common atrium. By excluding the oxygenator & cardiotomy reservoir using a bypass line the cardiopulmonary bypass circuit was converted to function as a continuous flow ventricular assist device (VAD). Pressure was monitored in the common atrium (via a catheter placed via the left atrial appendage) and in the cavopulmonary circuit via a pulmonary arterial catheter.
Figure 1.
Total or partial connections were established by application of clamps (Clamp 1 & 2 in figure) or snares (Snares 1 & 2 in figure) to achieve superior cavopulmonary connection (SVC Glenn), inferior cavopulmonary connection (IVC Glenn) or total cavopulmonary connection (TCPC-Fontan). Application of Snare 1 & Clamp 2 achieved SVC Glenn, Snare 2 and Clamp 1 achieved IVC Glenn and application of Snare 1 & 2 without any clamps led to TCPC. PA snare- Snare on main pulmonary artery was on for all the 3-cavopulmonary connection conditions.
All animals were weaned off cardiopulmonary bypass to achieve baseline biventricular data. Each of the 3 study conditions (TCPC, SVC Glenn & IVC Glenn) was created in random sequence in each animal. End points were measured after achievement of steady state in each of the 3 conditions in their native state (Unassisted) and when assisted by the continuous flow VAD (Assisted). While flow measurements were available in all animals, data required for calculation of tissue oxygen delivery was available in 3 animals only.
At the end of the study all animals were euthanized.
Statistical analysis
Continuous data are reported as means ± standard deviation. Paired t-tests were used to compare assisted vs. unassisted state for each of the 3 conditions. A probability value of less than 0.05 was taken to represent a statistically significant difference between groups. Analyses were performed using IBM SPSS Statistics, version 19 (SPSS Inc., Chicago, IL).
Results
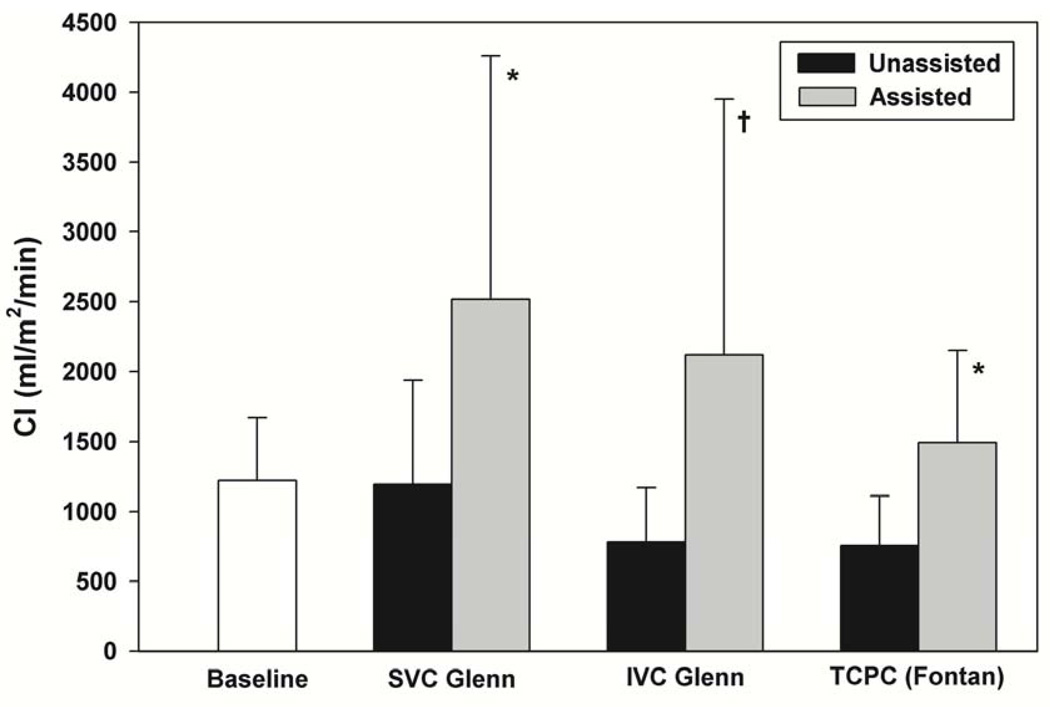
The baseline cardiac index in the animals after the surgical procedure with both limbs of the Y graft clamped was low (1221 ± 451 ml/m2/min) suggesting the marginal status of their circulation after the extensive procedure. The cardiac index was even lower in the unassisted state following appropriate clamp removal and placement for all three cavopulmonary connection conditions, but was higher in the SVC Glenn compared to the TCPC or IVC Glenn (Table 1)(Figure 2). (SVC Glenn-1191±747 ml/m2/min; IVC Glenn-781±389 ml/m2/min; TCPC- 754±357 ml/m2/min).
Table 1.
Assisted vs. Unassisted circulation in three study conditions- SVC Glenn, IVC Glenn and TCPC
| SVC Glenn | IVC Glenn | TCPC | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Unassisted | Assisted | Unassisted | Assisted | Unassisted | Assisted | |
| CI,ml/m2/min | 1221±451 | 1191±747 | 2517±1741* | 781±389 | 2120±1832 | 754±357 | 1494±658* |
| PAPmmHg | 21.0±13.6 | 25.3±4.6 | 22.6±7.3 | 28.0±4.9 | 25.6±7.8 | 27.8±5.6 | 26.4±8.7 |
| LAPmmHg | 14.8±2.6 | 16.6±4.4 | 14.5±2.7 | 16.8±7.0 | 14.0±6.7 | 18.2±6.3 | 16.2±6.3 |
| SpO2 %$ | 89.8±19.1 | 63.7±11.9 | 50.5±16.6 | 84.7±6.4 | 73.8±13.9 | 78.7±15.5 | 73.8±15.1 |
| PaO2mmHg$ | 151.2±131.1 | 33.7±5.9 | 30.8±9.4 | 52.7±11.6 | 42.1±11.2 | 47±13.8 | 45.2±15.7 |
| DO2,ml/l/min$ | 1212±566 | 909±571 | 1786±1307 | 862±207 | 1715±1622 | 733±147 | 1146±386 |
CI-Cardiac Index, DO2- Oxygen Delivery, IVC Glenn- Inferior Cavopulmonary Connection, LAP-Left Atrial Pressure, PAP- Pulmonary Artery Pressure, PaO2- Partial Pressure of Oxygen in Arterial Blood, SVC Glenn- Superior Cavopulmonary Connection, TCPC- Total Cavopulmonary Connection
Data represented at Mean ± Standard Deviation
Significant Differences between Assisted vs. Unassisted Conditions
N=3 for these variables
Figure 2.
Mechanical Assistance of the Systemic Single Ventricle in Partial or Total Cavopulmonary Connection
* Paired t-tests revealed significantly greater CI for assisted vs. unassisted for SVC Glenn (P = .04) and TCPC (P = .01) but not during IVC Glenn (P = .13) †
Significant augmentation of total CI was achieved with mechanical assistance for each of the three conditions. A greater than twofold increase in total CI was seen in all the 3 cavopulmonary conditions and was statistically significant for SVC Glenn (109% ± 24%, P =.04) and TCPC (130% ± 109%, P = .01) (Table 1) (Figure 2). Assisted CI achieved at least mean baseline biventricular CI for all 3-support modes. The amount of flow contribution to the total cardiac output from the VAD was 60% in SVC Glenn, 64% in IVC Glenn and 76% in the TCPC group.
Common atrial pressure (LAP) tended to be lower in the ‘Assisted’ state in all the 3 conditions compared to the ‘Unassisted’ state. (Table 1)
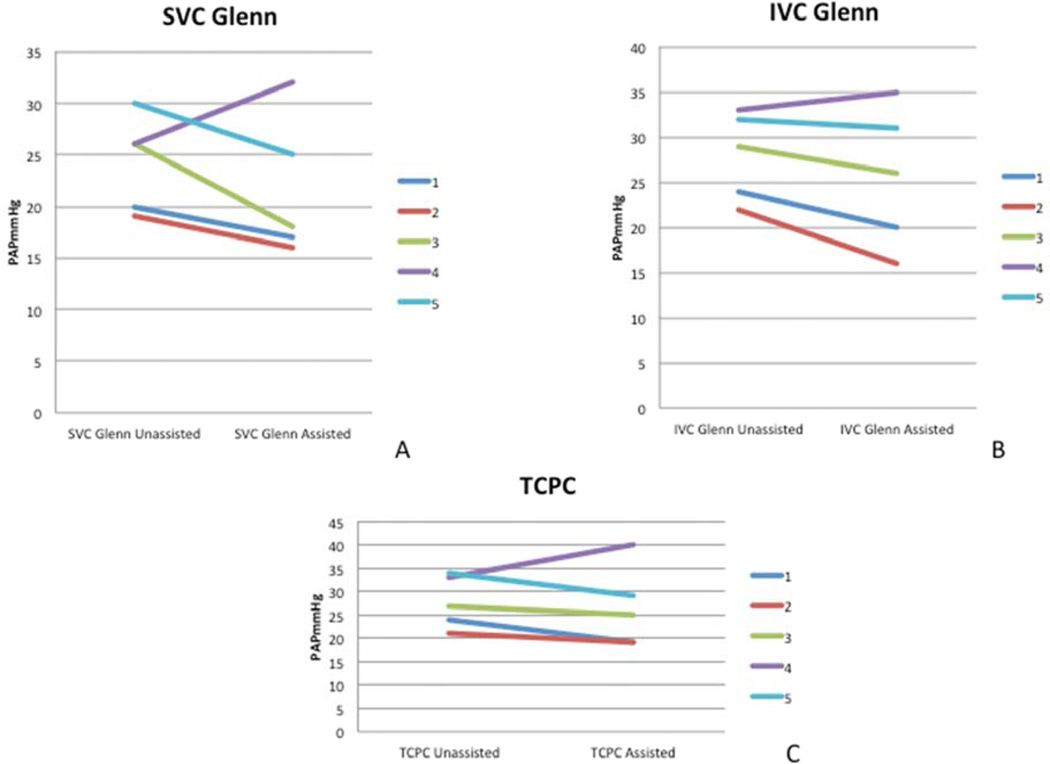
The mean pulmonary artery pressure was higher in all the 3-cavopulmonary connection conditions in the unassisted state compared to the baseline biventricular circulation. (Baseline-21.0±13.6 mm Hg; SVC Glenn-25.3±4.6 mm Hg; IVC Glenn-28.0±4.9 mm Hg; TCPC-27.8±5.6 mm Hg). The mean pulmonary artery pressure (PAP) was lower in the assisted state compared to the unassisted state in all the 3-cavopulmonary connection conditions for 4 out of 5 study animals (Figure 3) resulting in a lower group mean PAP in the assisted state in all 3 conditions (Table 1). A paradoxical rise in the PAP was seen in one study animal.
Figure 3.
A reduction in the mean pulmonary artery pressure (PAP) was seen in 4 out of 5 study animals with mechanical assistance in each of the 3-cavopulmonary connection conditions
A- SVC Glenn, B- IVC Glenn, C- TCPC
Oxygen saturation (SpO2) and arterial oxygen tension (PaO2) dropped in both the partial cavopulmonary connection conditions (SVC Glenn and IVC Glenn) in the assisted state compared to the unassisted state, but the overall augmentation of the cardiac output offset the desaturation and resulted in a higher oxygen delivery (DO2) in the assisted state for both these conditions. No drop in the SpO2 and the PaO2 was seen from unassisted to assisted state for the TCPC condition. Oxygen delivery (DO2) was higher in the assisted state for all 3 conditions, and was highest for assisted SVC Glenn 1786 ± 1307 ml/l/min and lowest with TCPC 1146 ± 386 ml/l/min. (Table 1)
Discussion
With improved outcomes of single ventricle palliation, there are an increasing number of Fontan survivors. Inherent inefficiencies of the Fontan circulation however lead to delayed Fontan failure in some patients and can lead to late mortality and morbidity [6–9].
Previous reports have focused on mechanical assistance of this failing circulation by use of subpulmonary blood pumps to drive blood forwards through the pulmonary circuit [4, 5, 10–14] or with a viscous impeller pump based on the Von Karman principle [3, 15]. Although there has been clinical success [10] with this approach of pushing blood through the pulmonary circuit, significant surgical modifications to the existing cavopulmonary circuit are required to implement this strategy.
Decompression of the systemic circulation in a patient with cavopulmonary circulation should ideally draw blood (pull) through the pulmonary circuit. This can be easily accomplished by the already tried and tested [1, 2] and readily available commercial continuous flow ventricular assist devices in a standard left ventricular assist device configuration. The strategy would also be effective irrespective of the type of the cavopulmonary circuit without the need for any modifications. While there are anecdotal case reports [16–22] and computer simulation studies [12], lack of an optimal single ventricle large animal model have prevented systematic preclinical studies so far. In particular it has been unclear if the bidirectional Glenn circulation might be preferable to the TCPC circulation for this method of single ventricle mechanical assistance. We developed our acute single ventricle porcine model to test the hypothesis that a standard method of ventricular assistance would be feasible with a single ventricle circulation and that the bidirectional Glenn would be optimal for mechanical assistance. The achievement of a univentricular circulation was achieved by a combination of percutaneous (balloon atrial septostomy) creation of common atrium, followed by surgical creation of cavopulmonary connection. The novel ‘Y’ graft configuration allowed establishment of total (Fontan) or partial (SVC-superior; or IVC- Inferior) cavopulmonary connection. The surgical procedure was done on cardiopulmonary bypass to improve safety. While this model is good for acute study, the animals tolerate the procedure poorly as suggested by low baseline cardiac index at the end of the procedure, and further refinements will be necessary for longer-term studies. For the same reason, all end points were measured soon after achievement of steady state hemodyanamics in each of the conditions (approximately 5–10 minutes from establishment of each condition).
We noticed a significant improvement in the total cardiac index in all 3-cavopulmonary conditions by assistance with a continuous flow pump. This was accompanied by a fall in the mean pulmonary artery pressure and the left atrial pressure, suggesting that decompression of the systemic circulation is transmitted to the cavopulmonary circuit, and is effective in pulling or drawing blood through the pulmonary circulation. The fall in the PAP was noted in 4 out of the 5 study animals, and was found to be paradoxically higher in one. Surgical dissection and ligation of the ductus arteriosus to exclude any effect of patency did not change the outcome. No other aortopulmonary collaterals could be found with intrapericardial dissection.
The common atrial pressure was lower in the assisted state compared to the unassisted state, however complete decompression of the systemic atrium was not achieved. This could be due to our choice of atrial inflow vs. the preferred ventricular inflow used for most left ventricular assist devices, which provided better systemic decompression. We however avoided direct ventricular apical cannulation to avoid any further ventricular dysfunction in an already marginal circulatory state. We do speculate that the better decompression with ventricular inflow cannulation would provide a large drop in the mean pulmonary artery pressure in the failing Fontan circulation and could offer more effective relief from the detrimental effects of high venous pressure in these patients. Return from the Thebesian veins directly to the right ventricle can be a problem over time in the setting of a competent tricuspid valve. In our model this was easily managed by pushing the tip of the atrial inflow cannula into the tricuspid valve to make it incompetent. Future studies with apical inflow cannulation may require direct procedures to induce tricuspid incompetence.
The augmentation in the cardiac output was maximum in the SVC Glenn, and lowest in the TCPC. This is possibly due to the added direct effect of the systemic circuit decompression on the IVC return in addition to additional flow through the cavopulmonary circuit. This is supported by the fall in the arterial oxygen saturation and PaO2 with use of mechanical assistance.
Similar effects were seen with the IVC Glenn, probably attributed to the direct effect on the SVC flow, but did not achieve significance probably due to the proportional flow differences between SVC and IVC territories compounded by the small number of study animals. Remarkably though the augmentation in the flow offset this drop in the oxygen content in the blood to achieve oxygen delivery similar or higher than baseline biventricular levels in all the 3 assisted conditions. While significant augmentation of cardiac index and oxygen delivery can be achieved in the Fontan circulation with this approach, more resistant cases may need a combination of takedown of the total cavopulmonary circulation to a partial cavopulmonary circulation and a systemic ventricular assist device to effectively palliate a failing circulation. As this approach relies on augmenting blood flow through the low resistance cavopulmonary circuit by systemic decompression the efficacy of this therapy may vary upon the etiology of Fontan failure e.g. systemic side failure vs. high pulmonary vascular resistance or anatomic issues with the Fontan circuit.
While our study was underpowered to detect anything other than differences in Cardiac Index, it is the first large animal model of a univentricular heart with a systemic mechanical assist device that allows comparison of hemodynamics of the bidirectional Glenn versus TCPC circulation.
Conclusions
SVC bidirectional Glenn circulation may allow optimal augmentation of cardiac index and oxygen delivery in a failing single ventricle using a conventional pediatric ventricular assist device. Our model also suggests that the Fontan circulation itself can be supported with systemic ventricular assistance of the single ventricle.
Acknowledgments
The authors would like to thank
1. The Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health, for the percutaneous procedure on the study animals.
2. Rebekah Dodson, for the illustration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, Miller M, Young JB. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? The Journal of thoracic and cardiovascular surgery. 2012;144(3):584–603. doi: 10.1016/j.jtcvs.2012.05.044. discussion 597-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SJ, Milano CA, Tatooles AJ, Rogers JG, Adamson RM, Steidley DE, Ewald GA, Sundareswaran KS, Farrar DJ, Slaughter MS, et al. Outcomes in Advanced Heart Failure Patients With Left Ventricular Assist Devices for Destination Therapy / Clinical Perspective. Circulation: Heart Failure. 2012;5(2):241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 3.Rodefeld MD, Coats B, Fisher T, Giridharan GA, Chen J, Brown JW, Frankel SH. Cavopulmonary assist for the univentricular Fontan circulation: von Karman viscous impeller pump. The Journal of thoracic and cardiovascular surgery. 2010;140(3):529–536. doi: 10.1016/j.jtcvs.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Throckmorton AL, Kapadia JY, Chopski SG, Bhavsar SS, Moskowitz WB, Gullquist SD, Gangemi JJ, Haggerty CM, Yoganathan AP. Numerical, hydraulic, and hemolytic evaluation of an intravascular axial flow blood pump to mechanically support Fontan patients. Annals of biomedical engineering. 2011;39(1):324–336. doi: 10.1007/s10439-010-0159-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Plunkett M, Lynch J, Zhou X, Ballard-Croft C, Zwischenberger JB. Wang-Zwische Double-Lumen Cannula Leads to Total Cavopulmonary Support in a Failing Fontan Sheep Model. Ann Thorac Surg. 2011;91(6):1956–1960. doi: 10.1016/j.athoracsur.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PAW, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, et al. Contemporary Outcomes After the Fontan Procedure A Pediatric Heart Network Multicenter Study. Journal of the American College of Cardiology. 2008;52(2):85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 8.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A Cross-Sectional Study of Exercise Performance During the First 2 Decades of Life After the Fontan Operation. Journal of the American College of Cardiology. 2008;52(2):99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 9.Diller G-P, Giardini A, Dimopoulos K, Gargiulo G, Müller J, Derrick G, Giannakoulas G, Khambadkone S, Lammers AE, Picchio FM, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. European Heart Journal. 2010;31(24):3073–3083. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 10.Pretre R, Haussler A, Bettex D, Genoni M. Right-Sided Univentricular Cardiac Assistance in a Failing Fontan Circulation. Ann Thorac Surg. 2008;86(3):1018–1020. doi: 10.1016/j.athoracsur.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Lacour-Gayet FG, Lanning CJ, Stoica S, Wang R, Rech BA, Goldberg S, Shandas R. An Artificial Right Ventricle for Failing Fontan: In Vitro and Computational Study. Ann Thorac Surg. 2009;88(1):170–176. doi: 10.1016/j.athoracsur.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 12.Durham LA, Dearani JA, Burkhart HM, Joyce LD, Cetta F, Cabalka AK, Phillips SD, Sundareswaran K, Farrar D, Park SJ. Application of Computer Modeling in Systemic VAD Support of Failing Fontan Physiology. World Journal for Pediatric and Congenital Heart Surgery. 2011;2(2):243–248. doi: 10.1177/2150135110397386. [DOI] [PubMed] [Google Scholar]
- 13.Myers CD, Boyd JH, Presson RG, Jr, Vijay P, Coats AC, Brown JW, Rodefeld MD. Neonatal cavopulmonary assist: pulsatile versus steady-flow pulmonary perfusion. Ann Thorac Surg. 2006;81(1):257–263. doi: 10.1016/j.athoracsur.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Myers CD, Mattix K, Presson RG, Jr, Vijay P, Maynes D, Litwak KN, Brown JW, Rodefeld MD. Twenty-four hour cardiopulmonary stability in a model of assisted newborn Fontan circulation. Ann Thorac Surg. 2006;81(1):264–270. doi: 10.1016/j.athoracsur.2005.06.063. discussion 270-261. [DOI] [PubMed] [Google Scholar]
- 15.Rodefeld MD, Frankel SH, Giridharan GA. Cavopulmonary assist: (em)powering the univentricular fontan circulation. Seminars in thoracic and cardiovascular surgery Pediatric cardiac surgery annual. 2011;14(1):45–54. doi: 10.1053/j.pcsu.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardarelli MG, Salim M, Love J, Simone S, Tumulty J, Conway D, Griffith B. Berlin Heart as a Bridge to Recovery for a Failing Fontan. Ann Thorac Surg. 2009;87(3):943–946. doi: 10.1016/j.athoracsur.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 17.Chu MWA, Sharma K, Tchervenkov CI, Jutras LF, Lavoie J, Shemie SD, Laliberte E, Calaritis C, Cecere R. Berlin Heart Ventricular Assist Device in a Child With Hypoplastic Left Heart Syndrome. Ann Thorac Surg. 2007;83(3):1179–1181. doi: 10.1016/j.athoracsur.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Frazier OH, Gregoric ID, Messner GN. Total circulatory support with an LVAD in an adolescent with a previous Fontan procedure. Tex Heart Inst J. 2005;32(3):402–404. [PMC free article] [PubMed] [Google Scholar]
- 19.Morales DLS, Adachi I, Heinle JS, Fraser CD., Jr A new era: Use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulation. The Journal of thoracic and cardiovascular surgery. 2011;142(3):e138–e140. doi: 10.1016/j.jtcvs.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Nathan M, Baird C, Fynn-Thompson F, Almond C, Thiagarajan R, Laussen P, Blume E, Pigula F. Successful implantation of a Berlin heart biventricular assist device in a failing single ventricle. The Journal of thoracic and cardiovascular surgery. 2006;131(6):1407–1408. doi: 10.1016/j.jtcvs.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Pearce FB, Kirklin JK, Holman WL, Barrett CS, Romp RL, Lau YR. Successful cardiac transplant after Berlin Heart bridge in a single ventricle heart: Use of aortopulmonary shunt as a supplementary source of pulmonary blood flow. The Journal of thoracic and cardiovascular surgery. 2009;137(1):e40–e42. doi: 10.1016/j.jtcvs.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 22.VanderPluym CJ, Rebeyka IM, Ross DB, Buchholz H. The use of ventricular assist devices in pediatric patients with univentricular hearts. The Journal of thoracic and cardiovascular surgery. 2011;141(2):588–590. doi: 10.1016/j.jtcvs.2010.06.038. [DOI] [PubMed] [Google Scholar]