Abstract
Light-activatable drugs offer the promise of controlled release with exquisite temporal and spatial resolution. However, light sensitive pro-drugs are typically converted to their active forms using short wavelengths, which display poor tissue penetrance. We report herein erythrocyte-mediated assembly of long wavelength-sensitive phototherapeutics. The activating wavelength of the constructs is readily pre-assigned by using fluorophores with the desired λex. Drug release from the erythrocyte carrier was confirmed by standard analytical tools and by the expected biological consequences of the liberated drugs in cell culture: methotrexate, binding to intracellular dihydrofolate reductase; colchicine, inhibition of microtubule polymerization; dexamethasone, induced nuclear migration of the glucocorticoid receptor.
Keywords: Photochemistry, Drug Delivery, Cobalamins, Pro-drugs
The use of light to activate therapeutic agents at disease sites offers the advantage of aggressive treatment with exquisite spatial control, thereby reducing potential deleterious side effects at unintended sites. An excellent example of this concept is photodynamic therapy, which employs the delivery of a photosensitizer to the tissue of interest.[1] Upon excitation with the appropriate wavelength of light and, in the presence of oxygen, cytotoxic reactive oxygen species are generated, resulting in destruction of the target cells. This minimally invasive procedure furnishes control over where and when the reactive oxygen species are produced. However, a more general strategy that can control the delivery of any drug could profoundly influence the treatment of a variety of disorders, including cancer, diabetes, and autoimmune and vascular diseases. A major challenge in this regard is the so-called “optical window of tissue”, the wavelength of light that enjoys maximal tissue penetration, which lies in the range of 600 – 900 nm.[2] Wavelengths less than <600 nm are absorbed by hemoglobin in the circulatory system and melanin in the skin whereas water interferes with light penetrance >900 nm. Unfortunately, nearly all light-activatable pro-drugs described to date respond to short wavelengths <450 nm.[3] This limitation is responsible for the intense interest in two-photon[4] and up converting[5] technologies. However, as discussed in recent reviews,[4–5] both technologies must overcome daunting challenges before potential therapeutic applications are realized. We recently described the long wavelength (>600 nm) photolysis of alkylcobalamins (alkyl-Cbl).[6] We now report the cell-mediated assembly of lipid-Cbl-drug and lipid-fluorophore conjugates in which the latter serve as long wavelength-capturing antennas that promote drug release.
Erythrocytes have been called the “champions of drug delivery” due to their biocompatibility, their long life span (120 days), and their size, which allows large quantities of drug to be conveyed relative to other carriers.[7] However, “practically useful controlled release from carrier RBC (red blood cells) remains an elusive goal”.[7] Our strategy to address this issue is depicted in Figure 1. Based on a previously demonstrated energy transfer between fluorophores and Cbls in covalently appended Cbl-fluorophore conjugates,[6] we decided to explore the premise that the cell-mediated assembly of C18-Cbl-drug and C18-fluorophore conjugates could act in concert as a photo-responsive drug delivery system. Illumination of the fluorophore antenna at its λmax and subsequent energy transfer to the Cbl-drug moiety should result in cleavage of the weak Co-C bond,[6,8] thereby liberating the drug.
Figure 1.
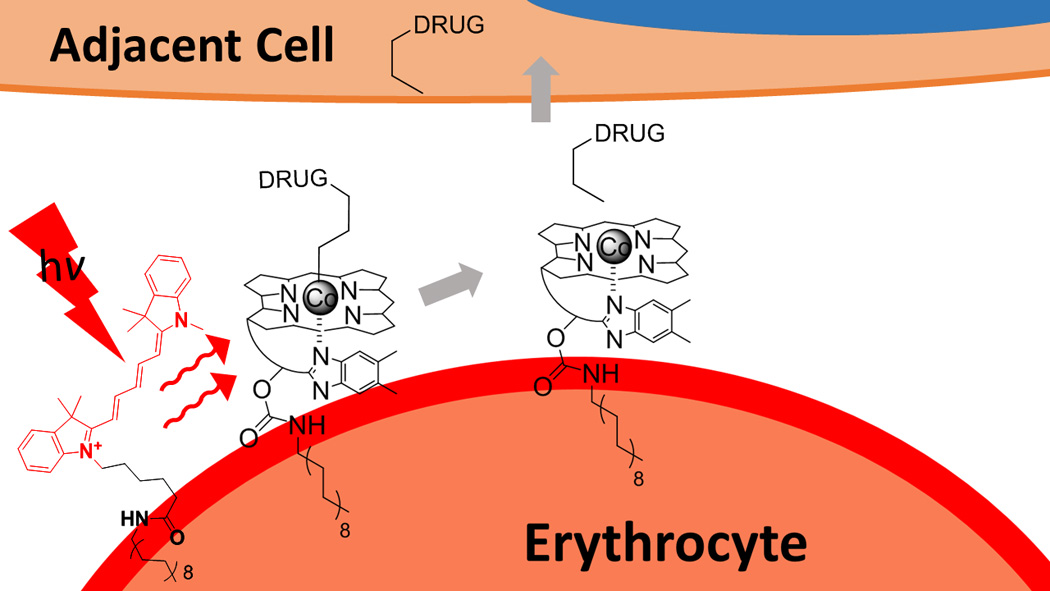
A wavelength-encoded drug release strategy. Anti-inflammatory drugs are covalently appended to Cbl via a photolabile Co-C bond. Lipidated-Cbl and -fluorophore constructs assemble on the plasma membrane of human erythrocytes. The fluorophore serves as an antenna, capturing long wavelength light and transmitting the energy to the Cbl-drug, resulting in drug release from the erythrocyte carrier.
A series of lipidated-Cbl (C18-Cbl) and C18–fluorophore derivatives were prepared (Figure 2, SI Figures S1–S4, Tables S3–S6 and Scheme S1). In the case of the Cbl derivatives, the C18 moiety was appended to the 5 ribose –OH of Cbl using octadecylamine and carbonyl-di-triazole. Subsequent alkylation of the Co furnished an amine or carboxylic acid handle, upon which drugs and fluorescent reporters were covalently attached (Figure 2, SI Figure S5 and Schemes S2 – S7). These species include the anti-inflammatories methotrexate (MTX), colchicine (COL), and dexamethasone (DEX), and the fluorescent reporters tetramethylrhodamine (TAM) and fluorescein (FAM). The C18–fluorophore derivatives were prepared via direct condensation of the activated carboxylate of the fluorophore with the amine of octadecylamine (SI Schemes S8 – S10).
Figure 2.
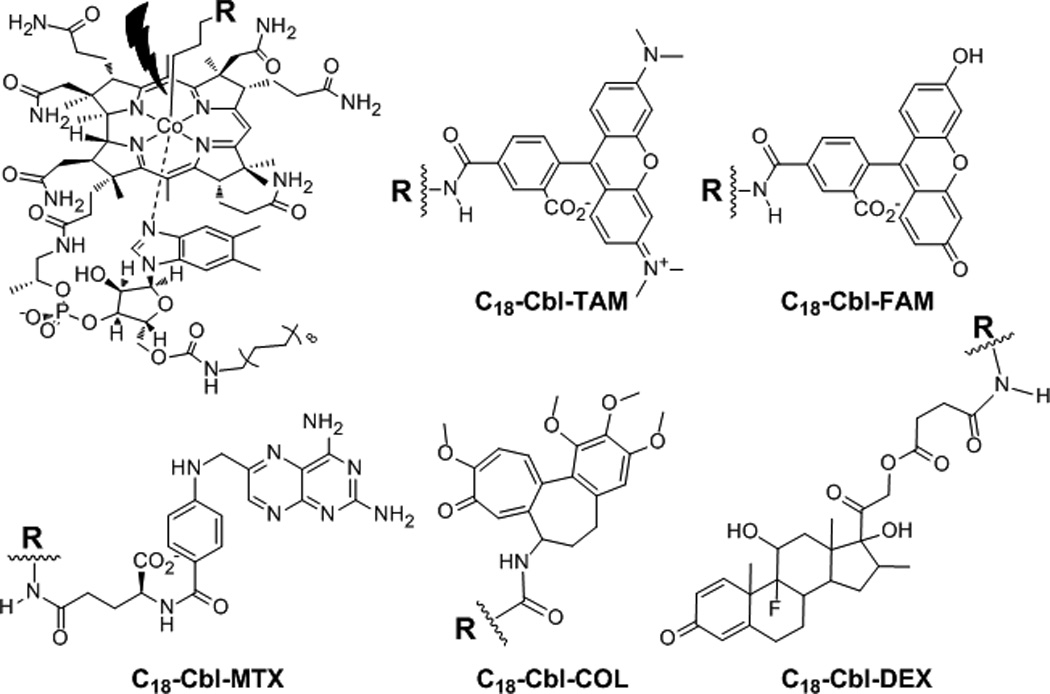
Anti-inflammatory drugs are Lipidated Cbl-reporters, TAM (C18-Cbl-TAM) and FAM (C18-Cbl-FAM) and lipidated Cbl-drug conjugates, MTX (C18-Cbl-MTX), COL (C18-Cbl-COL), and DEX (C18-Cbl-DEX).
Our initial studies sought to explore the premise of the Figure 1 strategy by establishing light-triggered movement of drugs and reporters from a hydrophobic environment to an aqueous one. For these preliminary experiments we directly photolyzed C18-Cbl-drug and C18-Cbl-reporter conjugates at the Cbl absorbance wavelength (525 nm). As expected, both C18-Cbl-TAM and C18-Cbl-MTX are soluble in octanol. Illumination at 525 nm cleaved the C18-Cbl-TAM Co-C bond and promoted migration of TAM from octanol to water as assessed by fluorescence (SI Figure S6). In an analogous fashion, 525 nm illumination of C18-Cbl-MTX triggered the release of MTX into the aqueous phase as demonstrated by LC-MS (SI Figure S7). We subsequently explored the light-triggered transfer of drug/reporter from the lipophilic plasma membrane of erythrocytes to the aqueous milieu. First, exposure of erythrocytes to C18-Cbl-TAM revealed even and extensive loading as assessed by widefield fluorescence microscopy. In addition, given the established photolytic sensitivity of the Co-C bond,[6, 8,9] we were not surprised to find that imaging C18-Cbl-TAM on erythrocytes results in the rapid migration (<1 s) of TAM fluorescence from erythrocytes into solution (SI Figure S8).
C18-Cbl-MTX, C18-Cbl-COL and C18-Cbl-DEX were loaded onto intact human erythrocytes with minimal lysis (~5% at 5 µM C18-Cbl-Drug, SI Figure S9). 0.3 – 1.0 fmol of C18-Cbl-Drug loaded per red blood cell. Subsequent photo-release of the drug was quantitative for C18-Cbl-COL and C18-Cbl-DEX and 35% for C18-Cbl-MTX (SI Figure S10):
C18-Cbl-MTX: LC-MS analysis revealed that photolysis of erythrocyte-anchored C18-Cbl-MTX primarily furnishes N-propylamide MTX (Figure 3, SI Scheme 11 and Tables S4 – S6). Consistent with the established structure activity relationship of MTX derivatives,[10] the photolyzed product of C18-Cbl-MTX serves as an effective inhibitor of dihydrofolate reductase (DHFR, SI Figure S11). In addition, photo-released MTX from erythrocyte-anchored C18-Cbl-MTX binds to DHFR in HeLa cells (SI Figure S12).
Figure 3.
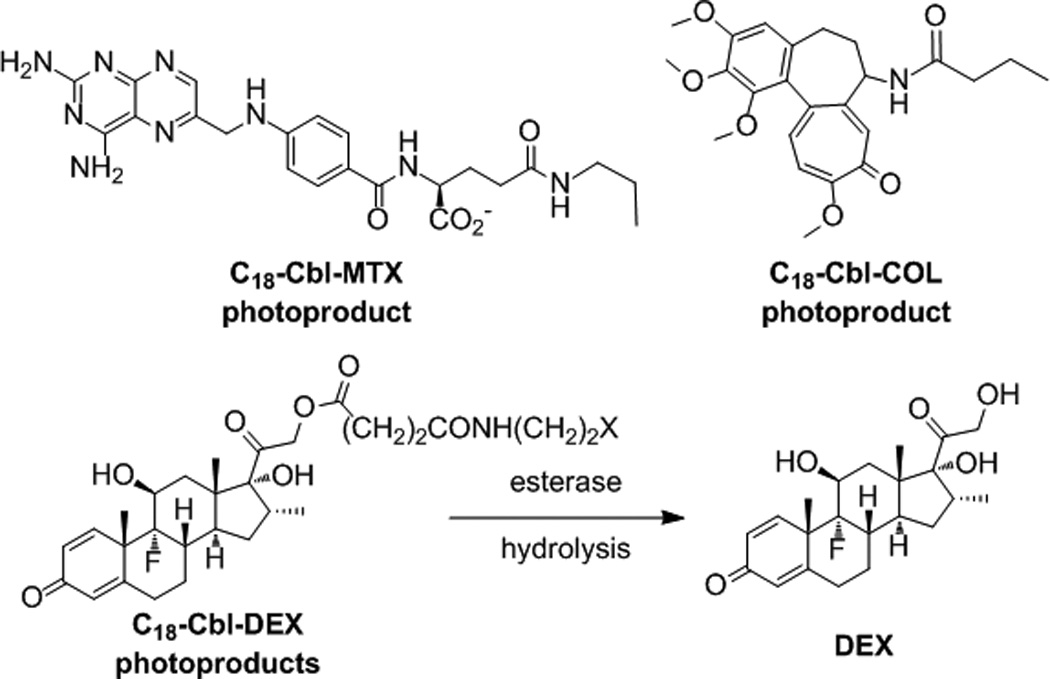
Erythrocyte-released photoproducts of C18-Cbl-MTX, C18-Cbl-COL and C18-Cbl-DEX. In the case of C18-Cbl-DEX, DEX is observed, instead of the possible photoproducts (X = H, OH, or =O).
C18-Cbl-COL: N-butanoyl COL is the primary photoproduct of C18-Cbl-COL-loaded erythrocytes (Figure 3 and SI Tables S4 – S6). Photolyzed C18-Cbl-COL disrupts microtubules in HeLa cells as effectively as COL itself (SI Figures S13 – S14).
C18-Cbl-DEX: DEX is the observed product from photolysis of C18-Cbl-DEX-loaded erythrocytes, instead of one or more of the expected photoproducts (Figure 3 and SI Tables S4 – S6). The 21-esters of DEX are pro-drugs that are hydrolyzed to DEX in vivo.[11] The possible presence of blood esterases[12] may explain the formation of DEX once freed from the RBC surface. Photo-release of DEX from C18-Cbl-DEX-loaded erythrocytes co-cultured with HeLa cells results in the migration of glucocorticoid receptor α (GRα) from the cytosol to the nucleus in HeLa cells [SI Figures S15 – S16]. We note that, in the absence of illumination, C18-Cbl-MTX-, C18-Cbl-COL-, and C18-Cbl-DEX-loaded erythrocytes do not release their therapeutic contents (SI Figures S12, S14, S16).
We also examined whether C18-Cbl-drugs transfer from erythrocytes to HeLa cells in the absence of light. HeLa cells were incubated with C18-Cbl-DEX-loaded erythrocytes and, subsequently, the adherent HeLa cells were washed to remove the red blood cells. If C18-Cbl-DEX had migrated to HeLa membranes during the incubation process, illumination at 525 nm should result in DEX release, uptake, and GRα migration. However, we did not observe receptor relocation to the nucleus, suggesting that C18-Cbl-DEX is retained by erythrocytes in the dark (SI Figure S17).
With the biological efficacy of the light triggered release of drugs from erythrocytes established, we turned our attention to the assembly of photo-responsive constructs that (1) operate within the optical window of tissue and (2) are encoded to respond to specific wavelengths. Our choice of anti-inflammatory drugs is based upon their role in the treatment of rheumatoid arthritis (RA). The long-term administration of anti-inflammatory agents produce moderate to severe side effects, such as weight gain, osteoporosis, diabetes mellitus, hypertension, skin fragility and infections arising from being systemically immunocompromised.[13–14] Not surprisingly, there is significant interest in the development of therapeutics that can be selectively delivered to RA joints.[15] With this in mind, we prepared C18 derivatives of Cy5 (C18-Cy5; λex 646 nm), Alexa Fluor 700 (C18-AF700; λex 700 nm), Cy7 (C18-Cy7; λex 747 nm), and a DyLight 800 analog (C18-DY800; λex 784 nm) and examined whether they are capable of serving as long wavelength antennas for drug release. Photolysis experiments employed in-house constructed LED circuit boards centered at 660 nm (Cy5), 725 nm (AF700 and Cy7), and 780 nm (DY800) (SI Figure S18 and Table S7).
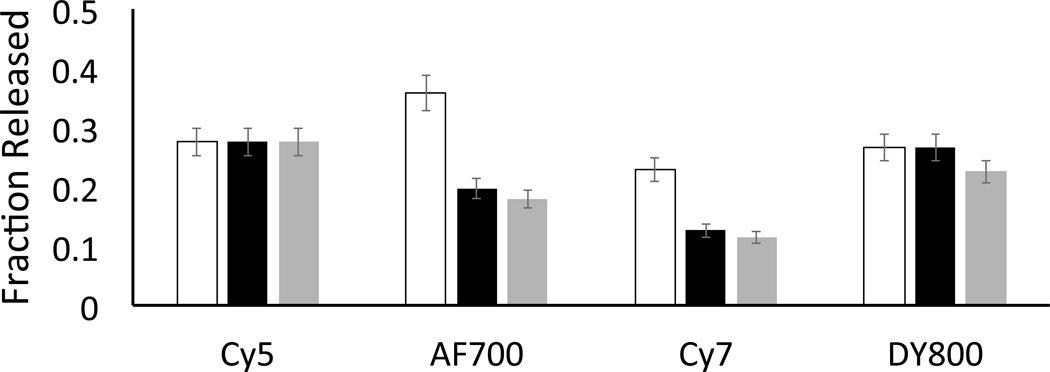
Our initial studies employed C18-Cbl-TAM in the presence of the C18-fluorophores, since the photo-release of TAM from C18-Cbl-TAM-labelled erythrocytes is readily monitored by fluorescence. In order to identify optimal energy transfer conditions, we first exposed erythrocytes to various relative concentrations of C18-Cbl-TAM and C18-Cy5. After washing the erythrocytes to remove any unbound C18-Cbl-TAM and C18-Cy5, the loaded RBCs were illuminated at 660 nm. The carrier erythrocytes were subsequently pelleted via centrifugation and TAM fluorescence in the supernatant measured. Although the most efficient TAM release was observed with erythrocytes exposed to a 1 µM:10 µM of C18-Cbl-TAM:C18-Cy5 (SI Figure S19), we employed a 1 µM:5 µM ratio to keep disruption of the erythrocyte’s structural integrity at an absolute minimum. In addition to the C18-Cy5 @ 660 nm antenna, the photo-release of TAM from C18-Cbl-TAM-loaded erythrocytes can be triggered at longer wavelengths in the presence of other antennas [C18-Cy7 @ 725 nm, C18-Cy7 @ 725 nm, C18-DY800 @780 nm]. Figure 4 compares the fraction of TAM released as a function of (a) antenna wavelength, (b) power of the different LEDs, and (c) photon density. An analogous set of experiments with similar results was performed with C18-Cbl-FAM/C18-fluorophores (SI Figure S20). Erythrocytes in the dark do not release TAM or FAM and, erythrocytes lacking a fluorophore antenna do not release FAM or TAM at wavelengths longer than those absorbed by Cbl (SI Figures S21 – S23). Finally, confocal microscopy revealed that the structural integrity of erythrocytes is not altered by the addition of the C18-fluorophores (SI Figure S24).
Figure 4.
Photo-release of TAM from C18-Cbl-TAM/C18-fluorophore-loaded erythrocytes. LED arrays centered at 660 nm (Cy5), 725 nm (AF700 and Cy7), and 780 nm (DY800) were employed as the light sources. TAM release is displayed as the fraction liberated from erythrocytes. Total erythrocyte bound C18-Cbl-TAM was determined via a time study where 3 h of illumination generated maximal TAM release. Unfilled bars: fraction of TAM release; Black bars: fraction of TAM release normalized by the power of the different light sources; Grey bars: fraction of TAM release normalized by photon density produced by the light sources; n = 3 for all experiments. See the legend to SI Figure 20 for an explanation of the normalizations.
We subsequently examined drug photo-release from the erythrocyte-assembled drug/antenna array. C18-Cbl-MTX, C18-Cbl-COL, and C18-Cbl-DEX were each paired with C18-fluorophores and illuminated. LC-MS confirmed the release of MTX, COL, and DEX at the wavelength absorbed by the partner C18-fluorophore (SI Figures S25 – S26 and Table S8):
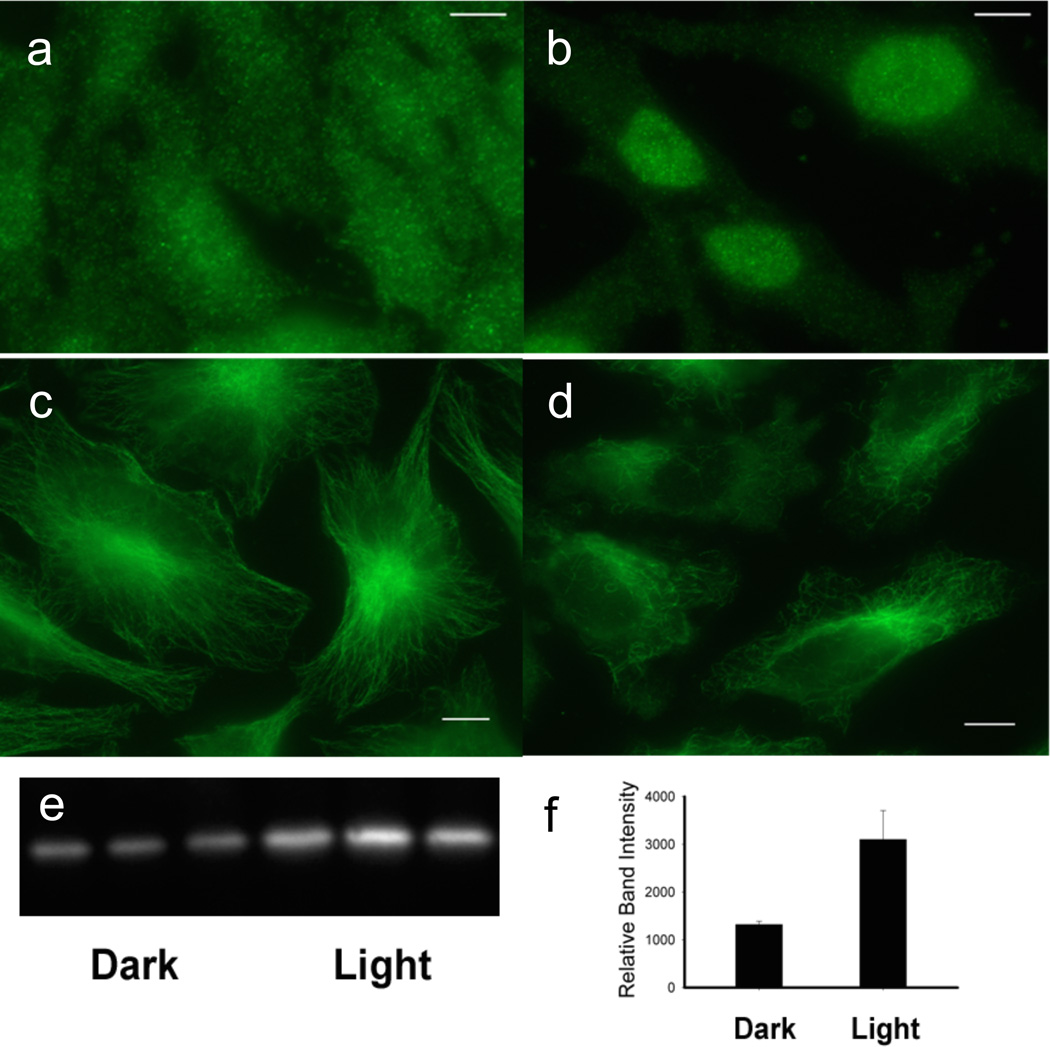
C18-Cbl-DEX/C18-Cy5: DEX is released from C18-Cbl-DEX/C18-Cy5-loaded erythrocytes in culture with HeLa cells upon 660 nm exposure. As a consequence, GRα in HeLa cells migrates from the cytoplasm to the nucleus (Figure 5a–b). By contrast, C18-Cbl-DEX loaded erythrocytes lacking a long wavelength absorbing antenna fail to trigger GRα migration when exposed to long wavelengths (e.g. 780 nm; SI Figures S27 – S28).
Figure 5.
Release of anti-inflammatory agents from erythrocytes and their affect on HeLa cells. (a) – (b) DEX release from C18-Cbl-DEX/C18-Cy5-loaded erythrocytes at 660 nm triggers HeLa cell GRα nuclear localization, where (a) is dark and (b) 660 nm. HeLa GRα visualized with Alexa488 antiRabbit/anti-GRα. (c) – (d) COL release from Cbl-COL/C18-DY800 erythrocytes at 780 nm initiates HeLa microtubule depolymerization, where (c) is dark and (d) 780 nm. HeLa microtubules visualized with Alexa488 antiMouse/anti-tubulin. Bar = 5 µm. (e) – (f) MTX release from C18-Cbl-MTX/C18-Cy7-loaded erythrocytes at 725 nm shifts the thermal stability of DHFR where left 3 lanes (dark) and right 3 lanes (725 nm). GAPDH used as loading control (SI Figure S30).
C18-Cbl-COL/C18-DY800: 780 nm elicits COL release from erythrocyte-anchored C18-Cbl-COL/C18-DY800, which induces microtubule depolymerization in the plated HeLa cells (Figure 5c–d, SI Figure S29).
C18-Cbl-MTX/C18-Cy7: MTX is released from C18-Cbl-MTX/C18-Cy7-loaded erythrocytes upon 725 nm exposure. The MTX photo-product associates with endogenous DHFR in the co-cultured HeLa cells as assessed by the cellular thermal shift assay (Figure 5e).[16]
In summary, we’ve described the cell-mediated assembly of photo-responsive drug release constructs. Release is triggered upon exposure to far-red and near-IR light that falls well within the optical window of tissue. Furthermore, drug release is wavelength-encodable; enabling the action wavelength to be chosen in advance based upon the known excitation properties of a given fluorophore. Finally, although this study focused on erythrocytes as drug carriers, the strategy described herein should prove applicable to nanoparticle drug carriers as well.[17]
Supplementary Material
Acknowledgements
We thank the NIH for financial support (R01 CA79954)
Footnotes
Supportinginformationforthisarticleisgiven via a link at the end of thedocument.((Pleasedeletethistextif not appropriate))
References
- 1.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Chem. Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Lee HM, Larson DR, Lawrence DS. ACS Chem. Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Chem. Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bort G, Gallavardin T, Ogden D, Dalko PI. Angew. Chem. Int. Ed. Engl. 2013;52:4526–4537. doi: 10.1002/anie.201204203. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Qiu H, Prasad PN, Chen, X X. Chem. Rev. 2014;114:1236–1250. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shell TA, Shell JR, Rodgers ZL, Lawrence DS. Angew. Chem. Int. Ed. 2014;53:875–878. doi: 10.1002/anie.201308816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzykantov VR. Expert Opin. Drug Deliv. 2010;7:403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Dolphin D, Johnson AW, Rodrigo, R R. Ann. N Y Acad. Sci. 1964;112:590–600. doi: 10.1111/j.1749-6632.1964.tb45035.x. [DOI] [PubMed] [Google Scholar]; (b) Taylor RT, Smucker L, Hanna ML, Gill J. Arch Biochem Biophys. 1973;156:521–533. doi: 10.1016/0003-9861(73)90301-9. [DOI] [PubMed] [Google Scholar]; (c) Halpern J, Kim S-H, Leung TW. J. Am. Chem. Soc. 1984;106:8317–8319. [Google Scholar]; (d) Kozlowski PM, Kumar M, Piecuch P, Li W, Bauman NP, Hansen JA, Lodowski P, Jaworska M. J. Chem. Theory Comp. 2012;8:1870–1894. doi: 10.1021/ct300170y. [DOI] [PubMed] [Google Scholar]
- 9.Priestman MA, Shell TA, Sun L, Lee HM, Lawrence DS. Angew. Chem. Int. Ed. 2012;51:7684–7687. doi: 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) McGuire JJ. Cur. Pharm. Design. 2003;9:2593–2613. doi: 10.2174/1381612033453712. [DOI] [PubMed] [Google Scholar]; (b) Antonjuk DJ, Boadle DK, Cheung HTA, Friedlander ML, Gregory PM, Tattersall MHN. Arzneimittel-Forschung. 1989;39:12–15. [PubMed] [Google Scholar]
- 11.(a) Markovic BD, Vladimirov SM, Cudina OA, Odovic JV, Karljikovic-Rajic KD. Molecules. 2012;17:480–491. doi: 10.3390/molecules17010480. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Civiale C, Bucaria F, Piazza S, Peri O, Miano F, Enea V. J. Ocul. Pharmacol. Ther. 2004;20:75–84. doi: 10.1089/108076804772745482. [DOI] [PubMed] [Google Scholar]
- 12.Richter D, Croft PG. Biochem. J. 1942;36:746–757. doi: 10.1042/bj0360746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. Ann. Rheum. Dis. 2009;68:1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 14.Baschant U, Lane NE, Tuckermann J. Nat. Rev. Rheumatol. 2012;8:645–655. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 15.(a) Mitragotri S, Yoo JW. Arch. Pharm. Res. 2011;34:1887–1897. doi: 10.1007/s12272-011-1109-9. [DOI] [PubMed] [Google Scholar]; (b) Fiehn C. Clin. Exp. Rheumatol. 2010;28:S40–S45. [PubMed] [Google Scholar]; (c) Ulbrich W, Lamprecht A. J. R. Soc. Interface. 2010;7(Suppl 1):S55–S66. doi: 10.1098/rsif.2009.0285.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina DM, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, Sreekumar L, Cao Y, Nordlund P. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 17.(a) Puri A. Pharmaceutics. 2013;6:1–25. doi: 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tong R, Kohane DS. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4:638–662. doi: 10.1002/wnan.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Alvarez-Lorenzo C, Bromberg L, Concheiro A. Photochem. Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.