Abstract
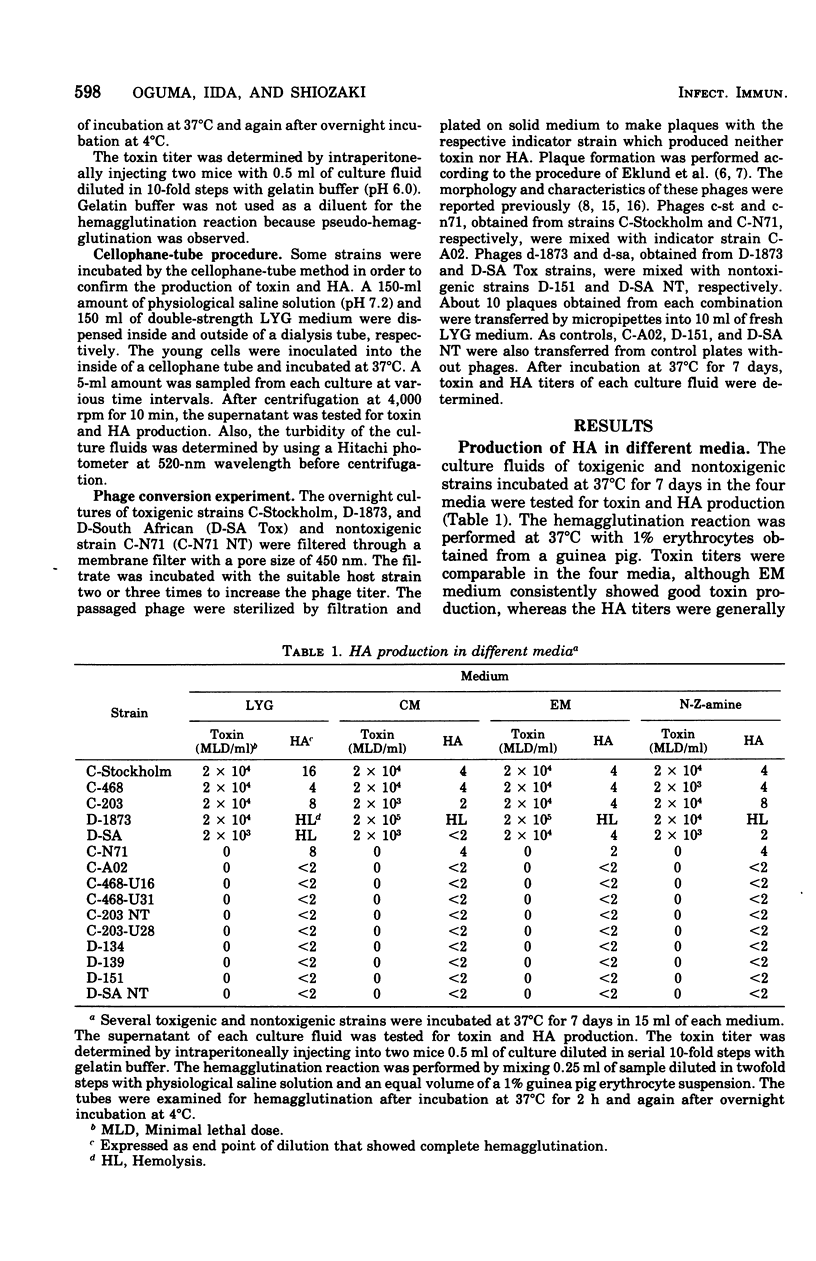
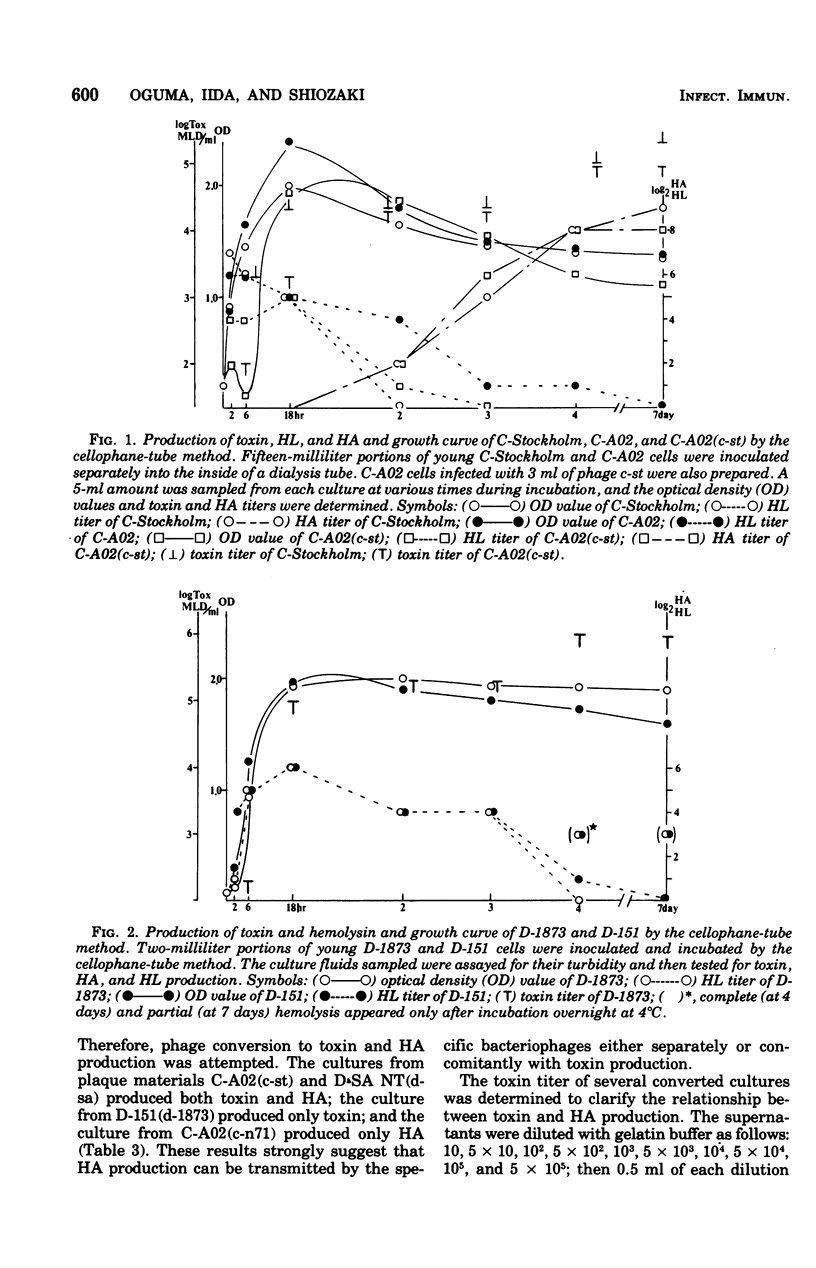
Five toxigenic strains of Clostridium botulinum types C and D were incubated at 37 degrees C for 7 days in 15 ml of the following media: LYG medium, cooked-meat medium, egg meat medium, and N-Z-amine medium. The supernatants of these cultures were tested for hemagglutinin production with 1% erythrocytes obtained from mice, guinea pigs, chickens, sheep, monkeys, and humans. Four toxigenic strains produced hemagglutinin. The highest hemagglutinin titer was obtained with a combination of human erythrocytes and cultures incubated in LYG medium. When the same experiment was carried out with many nontoxigenic strains, hemagglutination was observed in only one strain, C-N71. Strains producing hemagglutinin also produced phages. The phages obtained from toxin- and hemagglutinin-producing strains converted nontoxigenic indicator strains to produce both toxin and hemagglutinin. The phage obtained from a toxin-positive hemagglutinin-negative strain could only induce cultures to produce toxin, and the phage from a toxin-negative hemagglutinin-positive strain could only induce production of hemagglutinin. These studies suggest that the production of hemagglutinin by C. botulinum types C and D is governed by bacteriophages and that hemagglutinin production can be transmitted separately or concomitantly with toxin production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balding P., Gold E. R., Boroff D. A., Roberts T. A. Observations on receptor specific proteins. II. Haemagglutination and haemagglutination-inhibition reactions of Clostridium botulinum types A, C, D and E haemagglutinins. Immunology. 1973 Nov;25(5):773–782. [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Nyberg S., Höglund S. Electron microscopy of the toxin and hemagglutinin of type A Clostridium botulinum. Infect Immun. 1972 Dec;6(6):1003–1007. doi: 10.1128/iai.6.6.1003-1007.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Boroff D. A. Separation of toxin and hemagglutinin from crystalline toxin of Clostridium botulinum type A by anion exchange chromatography and determination of their dimensions by gel filtration. J Biol Chem. 1968 Mar 10;243(5):1065–1072. [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T. Activation of a toxic component of Clostridium botulinum types C and D by trypsin. Appl Microbiol. 1972 Jul;24(1):108–113. doi: 10.1128/am.24.1.108-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T. Interconversion of type C and D strains of Clostridium botulinum by specific bacteriophages. Appl Microbiol. 1974 Jan;27(1):251–258. doi: 10.1128/am.27.1.251-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M. Bacteriophage and the toxigenicity of Clostridium botulinum type D. Nat New Biol. 1972 Jan 5;235(53):16–17. doi: 10.1038/newbio235016a0. [DOI] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M., Smith C. A. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971 Apr 30;172(3982):480–482. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- Inoue K., Iida H. Bacteriophages of Clostridium botulinum. J Virol. 1968 May;2(5):537–540. doi: 10.1128/jvi.2.5.537-540.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Iida H. Conversion of toxigenicity in Clostridium botulinum type C. Jpn J Microbiol. 1970 Jan;14(1):87–89. doi: 10.1111/j.1348-0421.1970.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Inoue K., Iida H. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1971 Feb;24(1):53–56. [PubMed] [Google Scholar]
- LOWENTHAL J. P., LAMANNA C. Characterization of botulinal hemagglutination. Am J Hyg. 1953 Jan;57(1):46–59. doi: 10.1093/oxfordjournals.aje.a119562. [DOI] [PubMed] [Google Scholar]
- Oguma K., Iida H., Inoue K. Bacteriophage and toxigenicity in Clostridium botulinum: an additional evidence for phage conversion. Jpn J Microbiol. 1973 Sep;17(5):425–426. doi: 10.1111/j.1348-0421.1973.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Oguma K., Iida H., Inoue K. Observations on nonconverting phage, c-n71, obtained from a nontoxigenic strain of Clostridium botulinum type C. Jpn J Microbiol. 1975 Jun;19(3):167–172. doi: 10.1111/j.1348-0421.1975.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Oguma K., Iida H., Shiozaki M., Inoue K. Antigenicity of converting phages obtained from Clostridium botulinum types C and D. Infect Immun. 1976 Mar;13(3):855–860. doi: 10.1128/iai.13.3.855-860.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K. The stability of toxigenicity in Clostridium botulinum types C and D. J Gen Microbiol. 1976 Jan;92(1):67–75. doi: 10.1099/00221287-92-1-67. [DOI] [PubMed] [Google Scholar]
- STERNE M. Hemagglutination by Clostridium botulinum type D. Science. 1954 Apr 2;119(3092):440–441. doi: 10.1126/science.119.3092.440. [DOI] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Purification and crystallization of Clostridium botulinum type C toxin. Jpn J Vet Res. 1972 Jun;20(1):19–30. [PubMed] [Google Scholar]



