Abstract
The prognosis of patients with locally advanced pancreatic cancer can be improved if secondary complete (R0) resection is possible. In patients initially staged as unresectable this may be achieved with neoadjuvant treatment which is usually chemoradiotherapy based. We report the case of a 46-year-old patient with an unresectable, locally advanced pancreatic cancer (pT4 Nx cM0 G2) who was treated with a sequential neoadjuvant chemotherapy regimen consisting of 2 cycles of nab-paclitaxel plus gemcitabine followed by 4 cycles of FOLFIRINOX. Neoadjuvant chemotherapy resulted in secondary resectability (R0 resection). After 2 cycles of nab-paclitaxel plus gemcitabine, the patient already had a complete metabolic remission as measured by integrated fludeoxyglucose (18F) positron emission tomography and computerized tomography. After a follow-up of 18 months the patient is alive without progression of disease. We propose to assess the clinical benefit of sequencing the combinations nab-paclitaxel plus gemcitabine and FOLFIRINOX as neoadjuvant therapy for patients with locally advanced and initially unresectable pancreatic cancer in a controlled clinical trial.
Key Words: Pancreatic cancer, Locally advanced disease, Neoadjuvant chemotherapy, Nab-paclitaxel, Gemcitabine, 5-Fluorouracil, Folinic acid, Oxaliplatin, Irinotecan
Introduction
Roughly 30–40% of patients with pancreatic ductal adenocarcinoma (PDAC) have locally advanced disease. Their prognosis is not really different from that of patients with metastatic PDAC [1]. In about one third of the patients, resectability can be achieved with neoadjuvant chemotherapy and/or chemoradiotherapy. If complete (R0) resection is possible, patients have a similar overall survival (OS) as those with an initially resectable PDAC. These are the results of a meta-analysis of 111 trials with a total of 4,394 patients and mainly 5-fluorouracil- or gemcitabine-based neoadjuvant chemoradiotherapy. Initially nonresectable patients reached an estimated median OS of 20.5 months (range 9–62 months) following resection [1].
Recently, 2 large phase III trials in patients with metastatic PDAC demonstrated encouraging improvements in clinical benefit for 2 combination therapies over single-agent gemcitabine [2, 3]. In one trial, the combination of oxaliplatin, 5-fluorouracil, leucovorin and irinotecan (FOLFIRINOX) significantly improved median OS to 11.1 months as compared to 6.8 months for gemcitabine [hazard ratio (HR) 0.57; 95% confidence interval (CI) 0.45–0.73, p < 0.001] and median progression-free survival to 6.4 versus 3.3 months (HR 0.47; 95% CI 0.37–0.59, p < 0.001), respectively. The overall response rate with the combination was 31.6 as compared to 9.4% with gemcitabine alone. There was 1 (0.6%) reported complete response with this combination. The treatment with FOLFIRINOX resulted in significantly more adverse events of grade ≥3 in terms of neutropenia (45.7% for FOLFIRINOX vs. 21.0% for gemcitabine), febrile neutropenia (5.4 vs. 1.2%), thrombocytopenia (9.1 vs. 3.6%), diarrhea (12.7 vs. 1.8%) and peripheral neuropathy (9.0 vs. 0%), while the incidence of alanine aminotransferase elevation was decreased (7.3 vs. 20.8%) [2].
The combination of nab-paclitaxel plus gemcitabine was studied in the pivotal phase III trial MPACT. A total of 861 patients with metastatic PDAC received first-line chemotherapy either with the combination of nab-paclitaxel at 125 mg/m2 and gemcitabine at 1,000 mg/m2, both administered on days 1, 8, 15, 29, 36, and 43 of a 56-day cycle with subsequent cycles on days 1, 8, and 15 of a 28-day cycle, or with gemcitabine monotherapy at 1,000 mg/m2 weekly for 7 out of 8 weeks in the first cycle and in subsequent cycles on days 1, 8, and 15 of a 28-day cycle. Median OS significantly increased to 8.7 months (95% CI 7.89–9.69) as compared to 6.6 months (95% CI 6.01–7.20) with gemcitabine alone (HR 0.72; 95% CI 0.620–0.825, p < 0.0001) and median progression-free survival was 5.5 versus 3.7 months (HR 0.69; 95% CI 0.58–0.82, p < 0.001), respectively. The combination of nab-paclitaxel plus gemcitabine resulted in an overall response rate of 23 versus 7% with gemcitabine monotherapy (p < 0.001) [3, 4]. There was 1 (0.2%) reported complete response with this combination. The most frequently observed adverse events of grade ≥3 were neutropenia (38% for nab-paclitaxel and gemcitabine vs. 27% for gemcitabine alone), leukopenia (31 vs. 16%), fatigue (17 vs. 7%) and neuropathy (17 vs. 1%) [3]. The results of this trial led to the approval of the combination of nab-paclitaxel and gemcitabine in the European Union as the first-line treatment for patients with metastatic pancreatic adenocarcinoma in January 2014 [5].
Thus, both combinations offer a markedly increased efficacy compared to prior standard systemic therapy in the metastatic setting of PDAC. Based on these encouraging data we treated a patient with an initially unresectable, locally advanced PDAC with a novel neoadjuvant chemotherapy consisting of a sequence of these combination regimens, i.e. nab-paclitaxel plus gemcitabine followed by FOLFIRINOX.
Case Presentation
In December 2012, a 46-year-old female patient presented to our center with jaundice. She was otherwise asymptomatic and in an excellent performance status. She reported no comorbidities and neither alcohol nor nicotine abuse nor prior diabetes mellitus. Family history concerning malignancies was also negative. Laboratory findings were typical of cholestasis with an elevated concentration of total serum bilirubin to 8.4 mg/dl. The level of the tumor marker CA 19-9 was increased to 171 U/ml. Abdominal ultrasound revealed a dilation of the ductus hepatocholedochus (DHC) but no detectable tumor mass. Endoscopic retrograde cholangiopancreatography showed a malignant stenosis of the DHC. A stent was placed into the DHC to allow biliary drainage, and as a result total serum bilirubin decreased to a normal value within a few days. Endosonography revealed a hypoechoic lesion in the pancreatic head between DHC and the portal vein in the vicinity of the celiac trunk as well as suspicious locoregional lymph nodes. There were, however, no tumor cells in the bioptic material gained repeatedly during this session. Integrated fludeoxyglucose (18F) positron emission tomography (PET)-computed tomography (CT) found a PET-avid tumor in the pancreatic head largely adjacent to the celiac trunk and a PET-avid regional lymph node metastasis (fig. 1). There were no signs of peritoneal carcinomatosis or distant metastasis. An exploratory laparotomy confirmed the diagnosis of PDAC by intraoperatively frozen sections and the suspected unresectability which was due to an encasement of the celiac trunk (>180°) by the pancreatic tumor. Histology detected a moderately differentiated pancreatic adenocarcinoma surrounded by stromal desmoplasia. Thus, the locally advanced PDAC could be classified as pT4 Nx cM0 G2. During surgery, the patient received a biliodigestive anastomosis using Roux-en-Y anastomosis and a central venous port system.
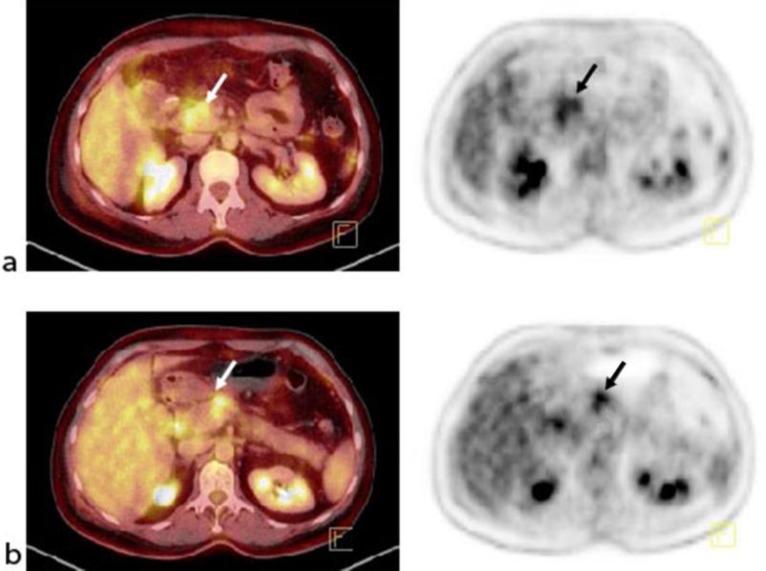
Fig. 1.
January 2013. Tumor lesions in the primary tumor and lymph nodes prior to chemotherapy. a Fused PET-CT and PET of the primary tumor. b Fused PET-CT and PET of the regional lymph node metastases.
Three weeks after surgery, intensified neoadjuvant chemotherapy was started with the objective to achieve secondary resectability. The patient was given 2 cycles of a combination of nab-paclitaxel plus gemcitabine. Nab-paclitaxel was given at a dose of 125 mg/m2 on days 1, 8 and 15, gemcitabine at a dose of 1,000 mg/m2 on days 1, 8 and 15, both repeated on day 29. Chemotherapy was tolerated well. The patient developed grade 2 neutropenia and grade 1 fatigue. Treatment resulted in a complete metabolic remission. The follow-up PET-CT showed no more PET-avid lesions (fig. 2).
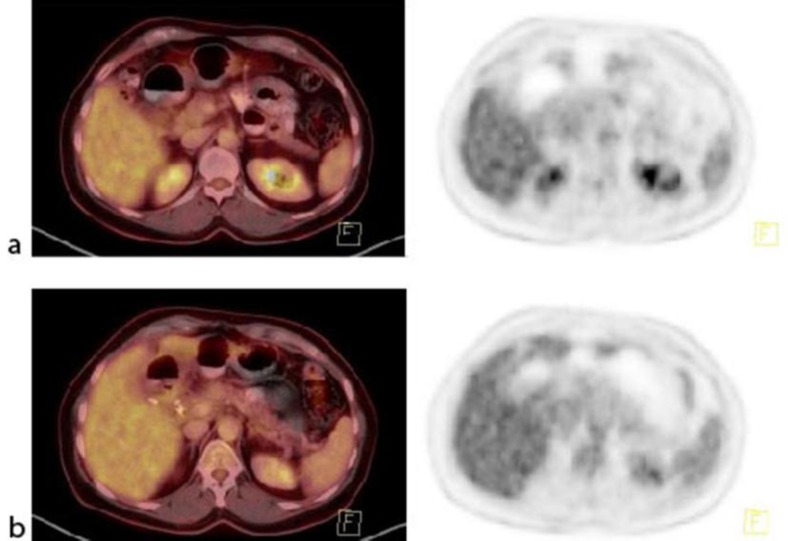
Fig. 2.
March 2013. Tumor lesions in the primary tumor and lymph nodes after 2 cycles of nab-paclitaxel plus gemcitabine. a Fused PET-CT and PET of the primary tumor. b Fused PET-CT and PET of the regional lymph node metastases.
Chemotherapy was continued with 4 cycles of FOLFIRINOX. Oxaliplatin was administered at a dose of 85 mg/m2 on day 1, leucovorin at a dose of 400 mg/m2 on day 1, and irinotecan at a dose of 180 mg/m2 on day 1 followed by continuous infusion of 5-fluorouracil over 46 h at a dose of 2,400 mg/m2. Cycles were repeated every 2 weeks. Side effects were grade 1 neutropenia, grade 2 fatigue, grade 1 diarrhea and grade 1 peripheral neuropathy.
The follow-up PET-CT confirmed the complete metabolic remission; yet, it also showed a persistent tumor abutment of the celiac trunk, formally still unresectable according to NCCN guidelines (fig. 3).
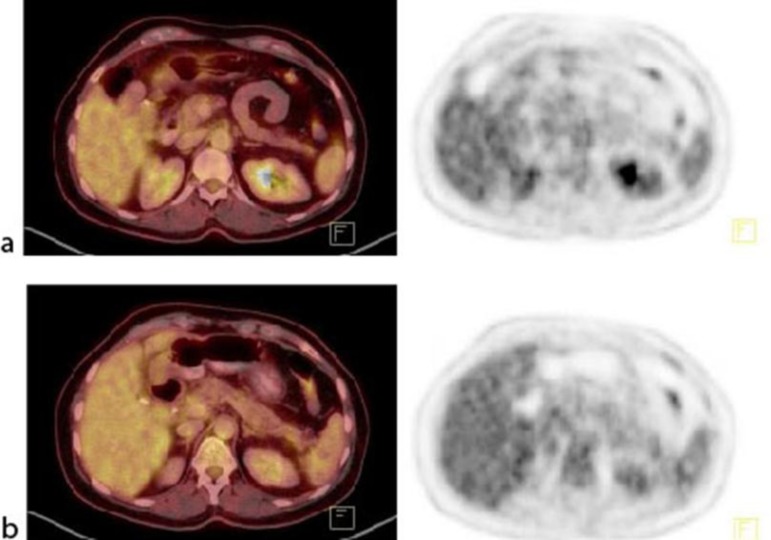
Fig. 3.
June 2013. Tumor lesions in the primary tumor and lymph nodes after 4 cycles of FOLFIRINOX. a Fused PET-CT and PET of the primary tumor. b Fused PET-CT and PET of the regional lymph node metastases.
In June 2013, a second laparotomy was performed. No vital tumor cells were found in the biopsies taken intraoperatively (frozen sections) of the tissue surrounding the celiac trunk and the DHC stump. Thus, a pylorus-preserving resection of the pancreatic head, regional lymphadenectomy, pancreaticojejunostomy and antecolic duodenojejunostomy were performed.
Surgery resulted in resection-free margins (R0 resection). The pancreatic tumor was 2.2 cm in diameter. Of the 33 lymph nodes that were resected, 2 showed micrometastases. Thus, the postoperative pathology result was ypT3 (2.2 cm) ypN1 (2/33; 1 mm) L0 V0 Pn1, local R0. Less than 10% vital tumor cells were found in the resection specimen, corresponding to Evans Regression Score grade III [6].
Subsequently, the patient was given 2 cycles of adjuvant chemotherapy with gemcitabine from July to September 2013. The patient was alive 18 months after the primary diagnosis at the last follow-up visit in April 2014. She showed a good performance status and had no signs of recurrent disease.
Discussion
Our case shows that secondary R0 resection can be achieved with neoadjuvant chemotherapy utilizing modern combination regimens. This is relevant as patients with an initially unresectable locally advanced PDAC have a similar prognosis to patients with a primarily resectable pancreatic cancer if R0 resection can be achieved [1]. However, it needs to be stressed that the concept of neoadjuvant therapy in locally advanced unresectable PDAC and its actual clinical benefits still have to be proven, since evidence from prospective, randomized trials is still lacking.
After a follow-up of 18 months, our patient was still progression free. The neoadjuvant chemotherapy we chose was actually a sequence of the 2 most active regimens for metastatic PDAC known today. The patient tolerated this intensified chemotherapy well. However, she already showed complete metabolic remission after 2 cycles of nab-paclitaxel plus gemcitabine. In the pivotal trial in metastatic PDAC, patients with a biochemical response after nab-paclitaxel plus gemcitabine had a better outcome than patients without a PET-response [7]. There are also data suggesting that a metabolic PET response may be of predictive value for patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy [8].
It is unclear to what extent the sequential treatment with FOLFIRINOX improved clinical outcome or whether neoadjuvant chemotherapy with the combination of nab-paclitaxel plus gemcitabine alone would have sufficed. This will be the objective of the randomized phase II trial NEOLAP that will start in September 2014. In this trial, patients with locally advanced, initially unresectable or borderline-resectable PDAC will either receive neoadjuvant chemotherapy with 4 cycles of nab-paclitaxel plus gemcitabine or 2 cycles of nab-paclitaxel plus gemcitabine followed by 4 cycles of FOLFIRINOX. The R0 resection rate will be the primary endpoint.
The primary tumor of our patient showed extensive stromal desmoplasia which is a typical feature of PDAC. The concept of stromal depletion has been identified as a promising therapeutic route in the treatment of pancreatic cancer [9]. Preclinical data show that the treatment with nab-paclitaxel results in depleting desmoplastic stroma and reducing stromal density of pancreatic tumor tissue [10, 11]. The effect of stromal depletion was highest with the combination of nab-paclitaxel and gemcitabine [11]. There is also clinical evidence from a neoadjuvant trial in 16 patients with resectable pancreatic cancer treated with 2 cycles of nab-paclitaxel plus gemcitabine before surgical resection. Of the 12 patients who completed treatment, 7 were R0 resected with major pathological regressions. A significant decrement in tumor stiffness was measured by endoscopic ultrasound elastography. Histology showed a change in the architecture of the tumor stroma as indicated by markedly disorganized collagen and a very low density of cancer-associated fibroblasts, which was not observed in the untreated or conventionally treated control groups. A preclinical, co-clinical study in a mouse model showed that these effects were specific of nab-paclitaxel and not of gemcitabine [12]. The precise mechanism of stroma depletion by nab-paclitaxel is currently not known and has to be defined in further preclinical and clinical studies. Among the multitude of stromal components, the matricellular glycoprotein secreted protein acidic and rich in cysteine (SPARC) has attracted particular interest. However, despite encouraging data from previous phase I/II trials, extensive SPARC analysis within a phase III trial (MPACT trial) failed to demonstrate any prognostic or predictive role of stromal and/or tumor SPARC expression in the metastastic setting [13].
In conclusion, chemotherapy with nab-paclitaxel plus gemcitabine followed by FOLFIRINOX was an effective and well-tolerated neoadjuvant treatment in a patient with initially irrresectable, locally advanced PDAC which resulted in secondary resectability (R0 resection). Not only the clinical benefit of sequencing both combination regimens as neoadjuvant therapy for patients with locally advanced PDAC, but also the general concept of neoadjuvant treatment in locally advanced pancreatic cancer needs to be assessed in future prospective clinical trials.
Disclosure Statement
The authors received editorial support in the preparation of this paper from Dr. Susanne Hell, funded by Celgene GmbH. Theyare fully responsible for the entire content and all editorial decisions relating to this paper.
References
- 1.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena F, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein D, El-Maraghi RH, Hammel P, et al. Analyses of updated overall survival (OS) and prognostic effect of neutrophil-to-lymphocyte ratio (NLR) and CA 19-9 from the phase III MPACT study of nab-paclitaxel (nab-P) plus gemcitabine (Gem) versus Gem for patients (pts) with metastatic pancreatic cancer (PC) (abstract 4027) J Clin Oncol. 2014;32(suppl):5s. [Google Scholar]
- 5.Abraxane® prescribing information. October 2013. http://abraxane.com/
- 6.Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ, Ames FC. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin TJ, Arena FP, et al. Results of a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas with PET and CA19–9 correlates (abstract 4005) J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 8.Topkan E, Parlak C, Kotek A, Yapar AF, Pehlivan B. Predictive value of metabolic 18FDG-PET response on outcomes in patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy. BMC Gastroenterol. 2011;11:123. doi: 10.1186/1471-230X-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40:118–128. doi: 10.1016/j.ctrv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awasthi N, Zhang C, Schwarz AM, Hinz S, Wang C, Williams NS, Schwarz MA, Schwarz RE. Comparative benefits of nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34:2361–2369. doi: 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidalgo M, Plaza C, Illei PB, et al. SPARC analysis in the phase III MPACT trial of nab-paclitaxel plus gemcitabine vs. Gem alone for patients with metastatic pancreatic cancer (abstract O-0004) 2014. 16th World Congress on Gastrointestinal Cancer, Barcelona.