Abstract
Purpose of review
Ocular surface squamous neoplasia (OSSN) in sub-Saharan countries is an aggressive tumor that affects younger patients and appears to be increasing in incidence. There are data to suggest the association of this disease with solar radiation exposure, HIV, and human papilloma virus (HPV). This trend possibly reflects the association of the high incidence of HIV, concomitant high incidence of exposure to HPV, and the solar radiation exposure that people in this region of the world receive. We undertook a PubMed search with the terms ‘ocular surface squamous neoplasia’, ‘conjunctival carcinoma’, ‘HIV’ and ‘HPV’, and ‘sub-Saharan/Africa’ to ascertain the scope of the problem and to review the available data, with an emphasis on publications of 2009 and the first quarter of 2010.
Recent findings
There is increasing evidence of a significant association between HIV seropositivity and OSSN. The role of HPV as contributing to the cause of OSSN is being investigated.
Summary
Patients with conjunctival cancer in sub-Saharan Africa are typically younger and more than 50% have underlying HIV infection. Initial presentation can be asymptomatic; however, many of these patients have advanced disease before they seek medical help and OSSN appears to have a more aggressive clinical course in sub-Saharan Africa. Treatment in Africa is primarily surgical. Chemotherapy and antiviral agents have been used. A diagnosis of OSSN in younger patients in sub-Saharan Africa should prompt HIV serotesting.
Keywords: AIDS-related malignancy, conjunctival carcinoma, ocular surface squamous neoplasia, sub-Saharan Africa
Introduction
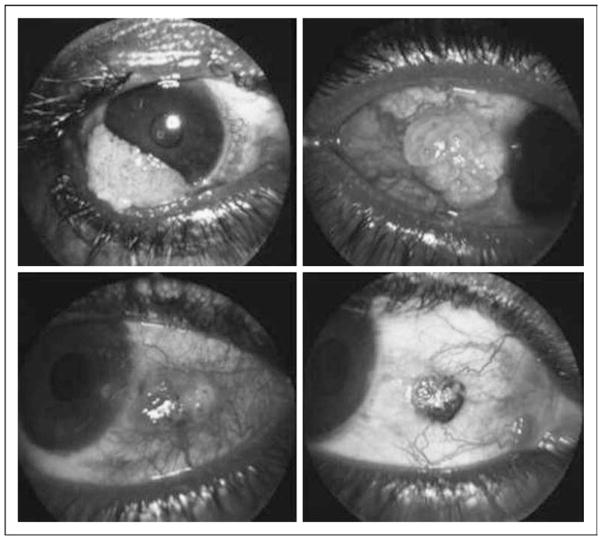
Ocular surface squamous neoplasia (OSSN) is a term that encompasses a spectrum of precancerous and cancerous lesions of the conjunctiva ranging from conjunctival intraepithelial neoplasia (CIN) to frankly invasive tumor with destruction of the orbit and intracranial invasion. Patients may be asymptomatic, but usually present with redness, irritation, and eye pain, which sometimes causes visual impairment. The tumor usually appears as a nodular or gelatinous plaque on the nasal side of the eye at the margin between the cornea and conjunctiva. OSSN can appear as a diffuse red eye closely resembling chronic conjunctivitis (see Fig. 1) [1]. Most lesions are unilateral and can be mistaken for benign conjunctival lesions such as pterygium, pinguecula or squamous papilloma. OSSN can progress in weeks to months [2]. OSSN has historically been a disease of elderly men living in areas of high ambient sunlight. However, with the advent of the AIDS epidemic, and especially in sub-Saharan Africa, the epidemiology of this disease appears to have changed dramatically, with OSSN more often being diagnosed in younger women. The incidence of the tumor is also different in different parts of the world. In the United States, the incidence is reported as 0.03 cases per 100 000 persons [3], whereas a study from Australia reports the incidence as 1.9 cases per 100 000 persons [4]. This is in contrast to much higher incidences reported in sub-Saharan African nations [5,6•]. Additionally, the incidence of OSSN has been increasing in Tanzania and other parts of Africa in the last decade [5]. For example, a study from Kampala, Uganda, reported the incidence of conjunctival squamous cell carcinoma as six per million per year from 1970 until 1988, and it increased to 35 per million per year by 1992 [7]. In a study from Zimbabwe, the median age at diagnosis of OSSN was 35 years, with 70% of the patients being women. This is in contrast with the data from the rest of the world, where the prevalence of OSSN is higher in men. When calculated from a total of 1587 ocular biopsies between 1996 and 2000, the annual prevalence of OSSN in 1996 was 33% in 1996 and 58% in 2000 [8]. In a study done in Nigeria of a total of 43 eye lesions analyzed, OSSN was found in 12% of the patients [6•]. Only one study published in 2010 from Uganda reports a seemingly recent decline in the incidence of OSSN [9•].
Figure 1. Examples of ocular surface squamous neoplasia.
Note often nodular and/or gelatinous appearance of lesion which is most commonly unilateral and located on the nasal side of the eye. Occasionally, OSSN may also present with diffuse conjunctival erythema suggestive of conjunctivitis. OSSN, ocular surface squamous neoplasia. Reproduced with permission from [1].
The increasing prevalence of OSSN in sub-Saharan Africa could be due to a confluence of multiple risk factors. These include ultraviolet radiation exposure –sunlight, HIV infection, and HPV co-infection. The impact of these three most important risk factors for the development of OSSN is discussed below.
Sunlight exposure
Africa is almost bisected by the equator and consequently receives abundant sunshine. Solar ultraviolet radiation decreases with increase in latitude. In an elegant study, Newton et al. [10] showed that the incidence of OSSN declined by 49% for each 10 degree increase in latitude (P <0.0001) from more than 12 cases per million per year in Uganda (latitude 0°) to less than 0.2 cases per million per year in the United Kingdom at latitude greater than 50°. This translated into a 29% decrease in the incidence of squamous cell carcinoma of the eye per unit reduction in ultraviolet exposure (P <0.0001). Thus, there is a link between increased ultraviolet exposure and OSSN, although other factors contribute to the increased risk in Africa, such as HIV and HPV infection. Increased TP53 gene mutations were seen in OSSN samples when compared with benign conjunctival lesions from patients in Uganda. TP53 mutations may arise due to increased sunlight exposure, thereby contributing to the increased relative risk of OSSN [11]. In a study from Uganda, 318 patients with OSSN and 762 controls were studied. The control patients consisted of patients who were being evaluated for other ophthalmic conditions and patients from a voluntary HIV testing service without eye examinations to increase the recruitment of HIV-positive patients. In this study, the risk of OSSN was 1.7% [95% confidence interval (CI) 1.2–2.4] in patients reporting 2–4 h of sunlight exposure and 1.8% (95% CI 1.1–3.2) in patients reporting more than 5 h of sunlight exposure. Previous injury to the affected eye increased the risk of OSSN [odds ratio (OR) 2.4; 95% CI 1.2–4.5] [12•].
HIV infection and association with AIDS
It is recognized that immunosuppression is associated with an increased risk of developing cancer. In a meta-analysis by Grulich et al. [13], the relative risk of cancer was higher in both HIV patients and in patients who were on chronic immunosuppression from organ transplantation. The incidence of OSSN in Uganda has increased more than three-fold with the onset of the AIDS epidemic. In a study done in Uganda and Malawi [14], 71 and 86% of the patients with OSSN were HIV-seropositive in these countries, respectively. Nkomazana and Tshitswana [15] reported that in sub-Saharan Africa, OSSN is seen in 4–8% of persons with HIV infection (which represents a 5–6% increase over patients not infected with HIV), with reported recurrence rates of 3.2–31.2% after surgery. In a report from Zimbabwe, the relative risk of being HIV-seropositive among patients with OSSN was 3.6 (95% CI 1.5–8.6) [16]. In this study, the mean CD4+ lymphocyte cell count level was significantly lower among patients with OSSN when compared with patients with other ocular pathologies. Similar findings are reported from the United States, where the overall standardized incidence rates (SIRs) were elevated for OSSN in patients with HIV infection (SIR 12.2; 95% CI 6.8–20.2), and this increase was greater in patients with greater sunlight exposure [17]. Data from two Kenyan hospitals where 409 patients with HIV infection were screened showed the prevalence of OSSN was 7.8% and the surgical recurrence rates were high [18]. A case–control study of 302 adult patients with various cancers in Kampala, Uganda, showed that HIV infection was associated with a significantly (P <0.05) increased risk of OSSN (OR 10.9; 95% CI 3.1–37.7) based on 22 cases [19]. Data from a German study conducted on samples from Kinshasa, Congo, found no difference in frequency of CIN between HIV-seropositive or HIV-seronegative patients, but HIV-seropositive patients with OSSN were on average 17 years younger [20]. In Malawi, patients with a diagnosis of OSSN were offered HIV testing in a study. Of the patients who agreed to have HIV testing, 79% tested positive and none of them were aware of the infection. No other signs or symptoms of HIV disease were seen in 21% of these patients [21]. It is important to point out that a study that looked at AIDS-related malignancy rates in Germany did not report any OSSN, which is in contrast to reports from Africa [22•]. In summary, there is an increase in the incidence of OSSN in sub-Saharan Africa since the onset of the AIDS epidemic and there is epidemiologic evidence of an association between these two diseases. It may be prudent to verify HIV-serostatus for all patients with OSSN in sub-Saharan Africa if their HIV-serostatus is otherwise unknown.
Human papilloma virus co-infection
Africa has the highest prevalence of HPV infection in the world, with an age-adjusted prevalence rate of 26% in women aged 15–74 years as tested by HPV PCR [23]. In a case–control study done in Uganda, HPV prevalence by PCR assay was tested on frozen biopsies from 94 squamous cell conjunctival carcinoma patients (79 HIV-seropositive), 39 dysplasia cases (34 HIV-seropositive), and 285 hospital controls (128 HIV-seropositive) having other eye conditions that required surgery. Cutaneous HPV types were detected in 45% of the cases of squamous cell carcinoma of the conjunctiva, 41% of dysplasia cases, and 11% of controls [24•]. HPV 5 and 8 were the most common types noted in squamous cell carcinoma of the conjunctiva, often occurring in combination with other types. The risk of OSSN was increased with multiple HPV genotype infection (OR 18.3; 95% CI 6.2–54.4) versus single HPV genotype infection (OR 2.3; 95% CI 1.2–4.4) [24•]. In a small study of 10 consecutive patients with CIN done in the United States, HPV 16 or 18 DNA and mRNA corresponding to the E6 region was detected in all 10 specimens, and in none of the control specimens [25]. This is consistent with previous observations with cervical carcinoma in which the E6 protein encoded region of HPV 16 and 18 has been shown to interact with p53 tumor suppressor genes to promote carcinogenesis, which may point to a role of HPV 16 and 18 in the development of OSSN [25]. In a study that evaluated 38 OSSN specimens from Kenyan and Ugandan HIV-infected patients, HPV 18 was seen by PCR analysis in 23 of 38 (61%) specimens and double genotype HPV 16 in another six (16%) tumor specimens [26•]. These samples were also tested for epithelial growth factor receptor (EGFR) activation. Nuclear and cytoplasmic expression of phosphorylated and total EGFR and two downstream effectors of the EGFR signal-transduction pathway, MAPK and Akt, were assessed in tissue specimens by immunohistochemistry. Immunohistochemistry and qPCR data suggest that activation and expression of the EGFR signaling pathway is related to disease progression of conjunctival carcinoma. The associations between cytoplasmic p-MAPK, cytoplasmic p-Akt, and tumor invasiveness were significant (P = 0.05 or P = 0.028) [26•]. Nuclear p-EGFR appeared only in invasive tumors. A significant positive association between EGFR expression and disease invasiveness was observed (P = 0.01). This could point to a role for EGFR antagonists in the treatment of OSSN. In another study done on autopsy specimens in Tanzania, where there is a high prevalence of HPV disease, 14 samples of conjunctival carcinoma were tested for HPV by highly sensitive nonradioactive in-situ hybridization technique. HPV 6/11, HPV 16, and HPV 18 were found in 84% of cases [27]. There have also been studies that do not show a relationship between HPV disease and OSSN. In one study that was done to find out whether HIV infection was associated with HPV infection in the conjunctiva, 136 lesion-free conjunctival biopsies were tested for HPV in patients who died of or had HIV infection, other infectious disease, chronic disease, or trauma. No excess of HPV infection in lesion-free conjunctiva was found at autopsy in patients who had died of HIV infection, when compared with the other groups [28]. In a German study, using PCR, Guthoff et al. [29•] reported that all of the 32 patients with OSSN were negative for HPV infection. Newton et al. [30] showed that the presence of anti-HPV-16 antibodies was not significantly associated with OSSN (OR 1.5; 95% CI 0.5–4.3; P = 0.5), but that there was a 10-fold increased risk of OSSN in HIV-infected individuals. The evidence linking OSSN to HPV and HIV co-infection in patients is evolving and further studies are needed to confirm the association (see Table 1).
Table 1.
Association of human papilloma virus with ocular surface squamous neoplasia
| Study details | HPV positivity
|
||
|---|---|---|---|
| Intraepithelial neoplasia | Invasive carcinoma | Control | |
| HPV was tested by PCR in 418 patients from Uganda. The risk of OSSN was increased with multiple HPV genotype infection (OR 18.3; 95% CI 6.2–54.4) versus single HPV genotype infection (OR 2.3; 95% CI 1.2–4.4). HPV 5 and 8 were the most common serotypes detected [24•] | 41% cutaneous HPV* | 45% cutaneous HPV* | 11% cutaneous HPV* |
| Ten consecutive patients with conjunctival intraepithelial neoplasia in the United States [25] | 100% (50% HPV 16 and 50% HPV 18) | NA | NA |
| Thirty-eight HIV-infected patients with OSSN from Kenya and Uganda tested for HPV by PCR [26•] | 61% HPV 18 and 16% double genotype HPV 16 + 18 | ||
| Fourteen conjunctival carcinoma samples at autopsy tested for HPV by highly sensitive nonradioactive in-situ hybridization technique in a study from Tanzania [27] | 93% HPV 18, 86% HPV 16 and HPV 6/11 | ||
| Thirty-two patients with OSSN from Germany tested for HPV by PCR [29•] | 0% | ||
| Report of 60 patients from Uganda with conjunctival carcinoma, compared to 1214 controls tested for anti-HPV 16, 18 and 45 antibodies. The presence of anti-HPV-16 antibodies was not significantly associated with OSSN (OR 1.5; 95% CI 0.5–4.3; P = 0.5) [30] | NA | 21% HPV 16. 10% HPV 18 and 5% HPV 45 | 10% HPV 16, 4% HPV 18 and 6% HPV 45 |
CI, confidence interval; HPV, human papilloma virus; OR, odds ratio; OSSN, ocular surface squamous neoplasia.
Treatment
Surgery is the primary modality of treatment for this disease in sub-Saharan Africa. Other treatments that have been used include 5-fluorouracil (1% solution), topical mitomycin C (0.02% solution), radiotherapy, and interferon alpha-2b. The primary objective of surgery is to achieve complete removal of the tumor in order to decrease the chance of recurrence. OSSN was treated by enucleation in up to 60% of cases in a study from Nigeria [6•]. OSSN was seen in 4–8% of HIV-infected patients in South Africa and Botswana and the recurrence rate after surgery ranged over 3.2–32% [15]. A study of 29 consecutive patients compared those treated with surgery with wide surgical margins to those treated with topical interferon alfa 2b [31]. Fifteen received topical interferon and 14 were surgically excised. Two patients in the interferon group subsequently underwent surgical excision for apparent lack of response to interferon. No patient in either group developed a recurrence during the study period. Mean disease-free follow-up was 35.6 months in the interferon group (95% CI 21.9–49.3) and 35.6 months in the surgery group (95% CI 22.9–48.3). The authors concluded that both surgery and topical interferon were effective for primary OSSN [31]. Microscopically controlled excision of conjunctival squamous cell carcinoma was found effective in one study [32]. A Ugandan study enrolled 476 patients between 1995 and 2001 to evaluate treatment modalities for corne-conjunctival squamous neoplasia [33]. Ninety-seven percent of patients had eye-conserving surgery. A total of 414 patients had OSSN (230 intraepithelial neoplasia and 184 invasive carcinoma lesions). The recurrence rate after a median follow-up of 32 months (range 0–81) was 3.2%. Sixty-four percent of these patients were HIV-seropositive. In a retrospective study of 55 patients with OSSN in Turkey (with 26 intraepithelial neoplasia and 31 invasive carcinoma lesions) who were treated with excision and cryosurgery with nitrous oxide probe, the success rate for patients with intraepithelial and invasive carcinomas was 88.5 and 87.1%, respectively, with no difference in recurrence rates between the two groups by log rank test (P = 0.68) [34]. Topical mitomycin C (0.02%) has also been used successfully to treat CIN. In three patients followed for 12 months, CIN resolved with mitomycin C treatment [35]. In a study of five patients with recurrent OSSN in India who were treated with surgery, cryosurgery, and mitomycin C applied to the eye, no recurrence was seen after 1 year [36]. Potential therapeutic strategies that may evolve include a role for HPV vaccination and use of EGFR inhibitors in the prevention and treatment of OSSN, although neither has been studied [37].
Conclusion
OSSN is on the rise in sub-Saharan Africa and contributes to significant morbidity and mortality in HIV-infected patients. Clinicians should be aware of the link between OSSN and HIV infection and should actively monitor HIV-infected patients for OSSN so that treatment could be instituted as soon as possible. In the same vein, it is also reasonable to recommend that the African patients diagnosed with OSSN should have HIV-serostatus evaluated. OSSN also appears to be more aggressive in the setting of HIV disease. Recurrence rates are higher among patients with OSSN in sub-Saharan Africa. The initial therapeutic approach to this disease is surgery. Additionally, cryotherapy and mitomycin C are also potentially good therapeutic options and should be made available to these patients. A simple intervention to decrease sunlight exposure, such as the use of sunglasses that filter ultraviolet light, is recommended. There may be a role for HPV vaccination in preventing OSSN because of the possible link between these two diseases. Further studies are necessary to clarify this association and whether providing the HPV vaccination can decrease the incidence of this disease. Randomized clinical trials comparing different treatments regimens for OSSN in sub-Saharan Africa are needed as well.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 527–528).
- 1.Guramatunhu S. Squamous cell carcinoma in HIV/AIDS. J Comm Eye Health. 2003;16:37. [PMC free article] [PubMed] [Google Scholar]
- 2.Pe’er J. Ocular surface squamous neoplasia. Ophthalmol Clin North Am. 2005;18:1, 13, vii. doi: 10.1016/j.ohc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Sun EC, Fears TR, Goedert JJ. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:73–77. [PubMed] [Google Scholar]
- 4.Lee GA, Hirst LW. Incidence of ocular surface epithelial dysplasia in metropolitan Brisbane. A 10-year survey. Arch Ophthalmol. 1992;110:525–527. doi: 10.1001/archopht.1992.01080160103042. [DOI] [PubMed] [Google Scholar]
- 5.Poole TR. Conjunctival squamous cell carcinoma in Tanzania. Br J Ophthalmol. 1999;83:177–179. doi: 10.1136/bjo.83.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Ogun GO, Ogun OA, Bekibele CO, Akang EE. Intraepithelial and invasive squamous neoplasms of the conjunctiva in Ibadan, Nigeria: a clinicopathological study of 46 cases. Int Ophthalmol. 2009;29:401–409. doi: 10.1007/s10792-008-9257-8. A contemporary study that reported a 12% incidence of OSSN among 43 eye lesions. [DOI] [PubMed] [Google Scholar]
- 7.Ateenyi-Agaba C. Conjunctival squamous-cell carcinoma associated with HIV infection in Kampala, Uganda. Lancet. 1995;345:695–696. doi: 10.1016/s0140-6736(95)90870-6. [DOI] [PubMed] [Google Scholar]
- 8.Pola EC, Masanganise R, Rusakaniko S. The trend of ocular surface squamous neoplasia among ocular surface tumour biopsies submitted for histology from Sekuru Kaguvi eye unit, Harare between 1996 and 2000. Cent Afr J Med. 2003;49:1–4. [PubMed] [Google Scholar]
- 9•.Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. doi: 10.1002/ijc.24838. Study reported declining OSSN incidence rates since the mid-1990s. [DOI] [PubMed] [Google Scholar]
- 10.Newton R, Ferlay J, Reeves G, et al. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. Lancet. 1996;347:1450–1451. doi: 10.1016/s0140-6736(96)91685-2. [DOI] [PubMed] [Google Scholar]
- 11.Ateenyi-Agaba C, Dai M, Le Calvez F, et al. TP53 mutations in squamous-cell carcinomas of the conjunctiva: evidence for UV-induced mutagenesis. Mutagenesis. 2004;19:399–401. doi: 10.1093/mutage/geh048. [DOI] [PubMed] [Google Scholar]
- 12•.Waddell K, Wehangana JK, Johnston WT, et al. A case–control study of ocular surface squamous neoplasia (OSSN) in Uganda. Int J Cancer. 2010;127:427–432. doi: 10.1002/ijc.25040. The study reviews risk of sunlight exposure and OSSN. [DOI] [PubMed] [Google Scholar]
- 13.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 14.Waddell KM, Lewallen S, Lucas SB, et al. Carcinoma of the conjunctiva and HIV infection in Uganda and Malawi. Br J Ophthalmol. 1996;80:503–508. doi: 10.1136/bjo.80.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkomazana O, Tshitswana D. Ocular complications of HIV infection in sub-Sahara Africa. Curr HIV/AIDS Rep. 2008;5:120–125. doi: 10.1007/s11904-008-0019-z. [DOI] [PubMed] [Google Scholar]
- 16.Chinogurei TS, Masanganise R, Rusakaniko S, Sibanda E. Ocular surface squamous neoplasia (OSSN) and human immunodeficiency virus at Sekuru Kaguvi eye unit in Zimbabwe: the role of operational research studies in a resource poor environment? Cent Afr J Med. 2006;52:56–58. [PubMed] [Google Scholar]
- 17.Guech-Ongey M, Engels EA, Goedert JJ, et al. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer. 2008;122:2590–2593. doi: 10.1002/ijc.23384. [DOI] [PubMed] [Google Scholar]
- 18.Chisi SK, Kollmann MK, Karimurio J. Conjunctival squamous cell carcinoma in patients with human immunodeficiency virus infection seen at two hospitals in Kenya. East Afr Med J. 2006;83:267–270. doi: 10.4314/eamj.v83i5.9432. [DOI] [PubMed] [Google Scholar]
- 19.Newton R, Ziegler J, Beral V, et al. A case–control study of human immunodeficiency virus infection and cancer in adults and children residing in Kampala, Uganda. Int J Cancer. 2001;92:622–627. doi: 10.1002/1097-0215(20010601)92:5<622::aid-ijc1256>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Timm A, Stropahl G, Schittkowski M, et al. Association of malignant tumors of the conjunctiva and HIV infection in Kinshasa (D. R. Congo) First results Ophthalmologe. 2004;101:1011–1016. doi: 10.1007/s00347-003-0960-6. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer MS, Batumba NH, Chirambo T, et al. Ocular surface squamous neoplasia as the first apparent manifestation of HIV infection in Malawi. Clin Exp Ophthalmol. 2008;36:422–425. [PubMed] [Google Scholar]
- 22•.Hensel M, Goetzenich A, Hanhoff E, et al. Cancer incidence in HIV-positive patients in Germany: A nation-wide survey from 2000 to 2007. J Clin Oncol. 2009;27(Suppl):abstract e22115. A nationwide survey in Germany did not identify any cases of OSSN. [Google Scholar]
- 23.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 24•.Ateenyi-Agaba C, Franceschi S, Wabwire-Mangen F, et al. Human papillomavirus infection and squamous cell carcinoma of the conjunctiva. Br J Cancer. 2010;102:262–267. doi: 10.1038/sj.bjc.6605466. The study reports increased odds of occurrence of OSSN with HPV multiple genotype infection versus single HPV genotype in patients with HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–547. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 26•.Yu JJ, Fu P, Pink JJ, et al. HPV infection and EGFR activation/alteration in HIV-infected east African patients with conjunctival carcinoma. PLoS One. 2010;5:e10477. doi: 10.1371/journal.pone.0010477. A study reported 61% of 38 OSSN samples infected with HPV 18; another 16% infected with double genotype HPV 16 and 18. This study also demonstrated activation and expression of EGFR signaling pathway is related to disease progression. The authors postulate a potential role for inhibitors of EGFR as a potential therapeutic pursuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moubayed P, Mwakyoma H, Schneider DT. High frequency of human papillomavirus 6/11, 16, and 18 infections in precancerous lesions and squamous cell carcinoma of the conjunctiva in subtropical Tanzania. Am J Clin Pathol. 2004;122:938–943. doi: 10.1309/T189-UWWV-B71M-9VRC. [DOI] [PubMed] [Google Scholar]
- 28.Ateenyi-Agaba C, Weiderpass E, Tommasino M, et al. Papillomavirus infection in the conjunctiva of individuals with and without AIDS: an autopsy series from Uganda. Cancer Lett. 2006;239:98–102. doi: 10.1016/j.canlet.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 29•.Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–671. doi: 10.1080/02713680903007162. HPV was not detected in 32 cases of OSSN. [DOI] [PubMed] [Google Scholar]
- 30.Newton R, Ziegler J, Ateenyi-Agaba C, et al. The epidemiology of conjunctival squamous cell carcinoma in Uganda. Br J Cancer. 2002;87:301–308. doi: 10.1038/sj.bjc.6600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturges A, Butt AL, Lai JE, Chodosh J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology. 2008;115:1297–1302. 1302.e1. doi: 10.1016/j.ophtha.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Buus DR, Tse DT, Folberg R, Buuns DR. Microscopically controlled excision of conjunctival squamous cell carcinoma. Am J Ophthalmol. 1994;117:97–102. doi: 10.1016/s0002-9394(14)73021-1. [DOI] [PubMed] [Google Scholar]
- 33.Waddell KM, Downing RG, Lucas SB, Newton R. Corneo-conjunctival carcinoma in Uganda. Eye (Lond) 2006;20:893–899. doi: 10.1038/sj.eye.6702043. [DOI] [PubMed] [Google Scholar]
- 34.Peksayar G, Altan-Yaycioglu R, Onal S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye (Lond) 2003;17:228–232. doi: 10.1038/sj.eye.6700331. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Lopez JF, Martinez-Costa Perez R, Cisneros Lanuza AL, et al. Treatment of conjunctival intraepithelial neoplasia with topical mitomycin C 0.02% Arch Soc Esp Oftalmol. 2004;79:375–378. doi: 10.4321/s0365-66912004000800004. [DOI] [PubMed] [Google Scholar]
- 36.Khokhar S, Soni A, SinghSethi H, et al. Combined surgery, cryotherapy, and mitomycin-C for recurrent ocular surface squamous neoplasia. Cornea. 2002;21:189–191. doi: 10.1097/00003226-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Hughes DS, Powell N, Fiander AN. Will vaccination against human papillomavirus prevent eye disease? A review of the evidence. Br J Ophthalmol. 2008;92:460–465. doi: 10.1136/bjo.2007.135038. [DOI] [PubMed] [Google Scholar]