Abstract
Recent advances in DNA microarray technology have enabled researchers to comprehensively characterize the complex genomes of higher eukaryotic organisms at an unprecedented level of detail. Array-based comparative genomic hybridization (Array-CGH) has been widely used for detecting DNA copy number alterations on a genomic scale, where the mapping resolution is limited only by the number of probes on the DNA microarray. In this chapter, we present a validated protocol utilizing print-tip spotted HEEBO (Human Exonic Evidence Based Oligonucleotide) microarrays for conducting array-CGH using as little as 25 ng of genomic DNA from a wide variety of sources, including cultured cell lines and clinical specimens, with high spatial resolution and array-to-array reproducibility.
Keywords: DNA microarray, array-CGH, comparative genomic hybridization, HEEBO, post-processing, epoxysilane, whole-genome amplification
1. Introduction
Oligonucleotide-based microarrays consist of thousands of defined oligonucleotide hybridization probes ranging from 25 to 85 nucleotides in length, that are either robotically spotted or synthesized in situ in a pattern of rows and columns on a microscope slide or a chip (1). Oligo-based microarrays have been extensively utilized for genome-wide expression profiling studies in a number of eukaryotic organisms (2–7). However, the recent availability of the complete genome sequences for many of these organisms has extended the utility of oligonucleotide arrays to genomic analysis, including the characterization of genomic DNA copy number aberrations such as gene amplifications and deletions in human cancers (8–12) and high-resolution studies of human genetic disorders (13–15).
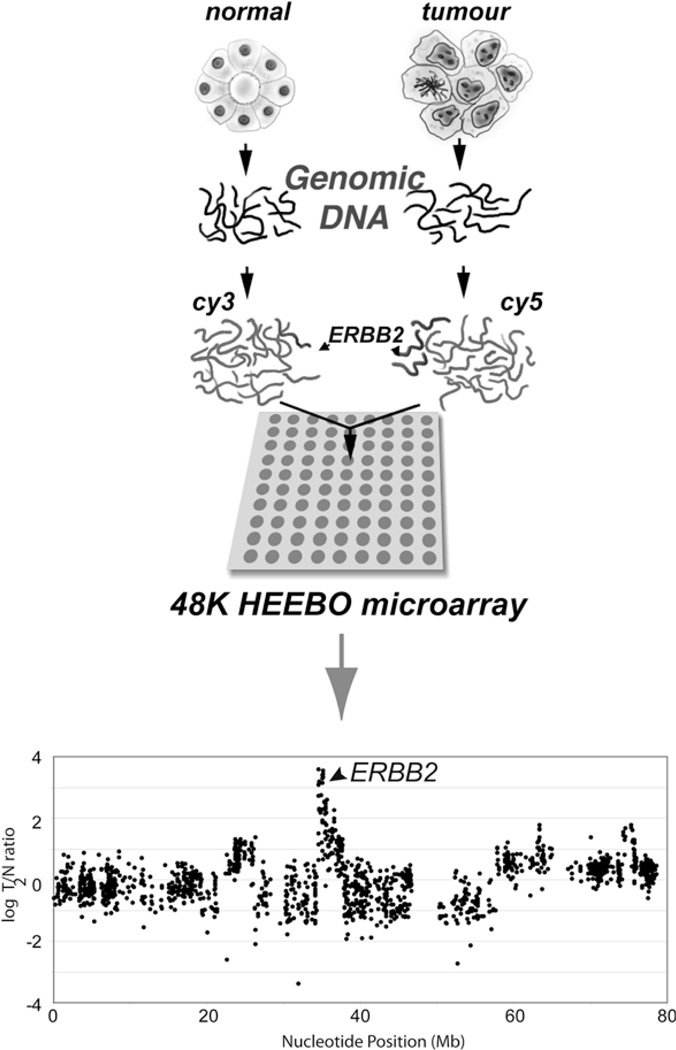
Array-based oligonucleotide comparative genomic hybridization (array-CGH) has rapidly gained acceptance as a technique of choice to characterize DNA copy number alterations at a high resolution. To measure gene copy number, genomic DNAs from two different samples (e.g., tumor and normal) are differentially labeled with fluorescent dyes and co-hybridized to an oligonucleotide microarray that is then scanned, and fluorescent intensities for each oligo element on the array reported. The ratio of fluorescence intensities for each oligo element on the array reflects the relative abundance of the corresponding gene’s (or locus’) copy number between the two samples (Fig. 3.1).
Fig. 3.1.
Schematic representation of oligonucleotide array-CGH and data visualization. Test (tumor) and reference (normal) samples are differentially labeled with Cy5/Cy3 fluorophores and co-hybridized to a HEEBO microarray containing ~48,000 oligonucleotide probe features. Arrays are scanned and the ratio of fluorescence intensities for each array element indicates the relative gene copy number (i.e., amplification or deletion) between the test and reference samples. Fluorescence ratios for each probe are ordered by genomic position using a genome reference assembly (e.g., UCSC GoldenPath). Here, the ERBB2 gene is shown to be amplified in the tumor sample (indicated by arrow).
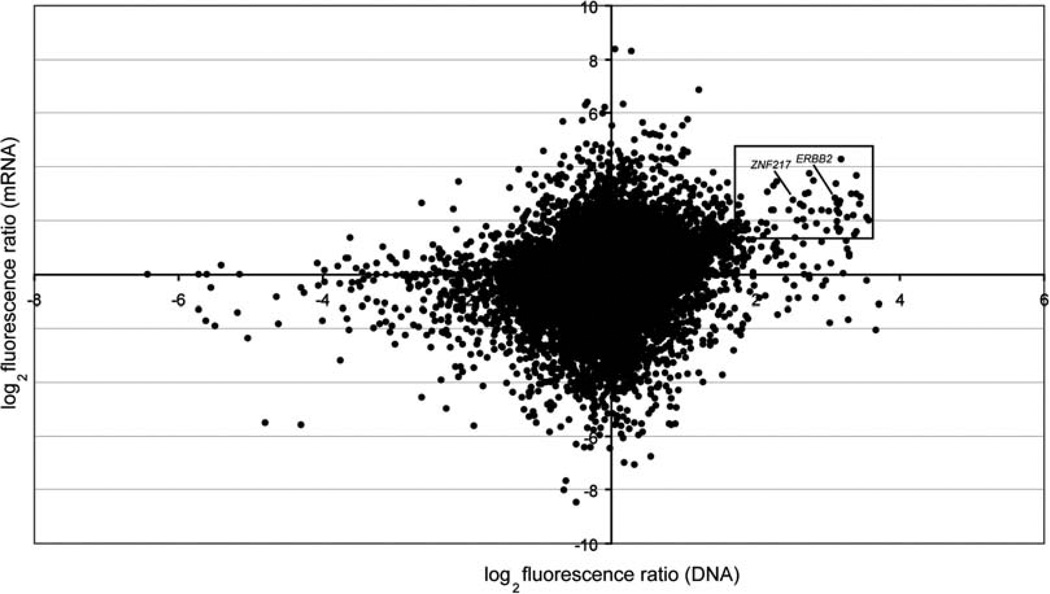
There are several potential advantages of oligo arrays over cDNA and bacterial artificial chromosome (BAC) platforms for genomic analysis. In theory, oligo arrays can be designed for any organism whose genomic sequence is known, while coverage of cDNA and BAC microarrays is limited to the availability of physical clones in dbEST and genomic clone libraries, respectively. Oligo arrays also circumvent some of the technical issues associated with library-based platforms including clone generation, tracking and validation, and offer flexibility in design and synthesis resulting in significant time and cost savings (10). Finally, like cDNA-based arrays, the same oligo microarray may be designed and used for both array-CGH and gene expression analysis which facilitates the comparison of DNA copy number and gene expression data (16) (Fig. 3.2).
Fig. 3.2.
Parallel analysis of DNA copy number and mRNA expression in breast cancer cell line SKBR3 using HEEBO microarrays. Log2 fluorescence ratios for DNA and mRNA measurements for each gene are plotted. Genes with high-level DNA copy number and mRNA expression values are highlighted. Known breast cancer oncogenes (ERBB2, ZNF217), whose expression is driven by underlying DNA amplification, are indicated.
In this chapter, we present details requisite to successful application of the array-CGH technique on spotted “home-made” HEEBO microarrays, including (1) preparation, labeling, and hybridization of genomic DNA, (2) processing of microarrays, and (3) post-hybridization protocols, including microarray washing, imaging, and data analysis. While described for the HEEBO array platform, this is a generic protocol that should be equally applicable to any other spotted oligonucleotide microarray platform.
2. Materials
2.1. HEEBO Microarrays
2.1.1. About HEEBO Microarrays
High-quality spotted HEEBO 70-mer microarrays covering a majority of human genes and providing an average genome mapping resolution of ~35 kb are readily available from various academic microarray core facilities (e.g., Stanford Functional Genomics Facility) or commercial sources for immediate use in microarray experiments. Complete details regarding HEEBO array design and feature annotations are available at http://www.microarray.org/sfgf/heebo.do (see Note 1).
2.1.2. Processing of HEEBO Arrays
2X SSC.
0.1% Triton X-100.
1 mM HCl.
100 mM KCl.
1X Blocking Solution: 50 mM ethanolamine, 0.1% SDS in 0.1 M TrisHCl, pH 9.0.
Humidifying slide chambers (Sigma-Aldrich).
Metal slide rack and glass staining dishes (Wheaton Science).
Temperature-controlled shaking water bath (Bellco Glass).
Desktop centrifuge with microtiter plate adaptors (Beckman Allegra GS-6R or equivalent).
Slide warmer (LabScientific).
2.2. Amplification and Labeling of Genomic DNA
Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare).
DpnII restriction enzyme and supplied 10X restriction enzyme buffer (New England Biolabs).
BioPrime Array CGH Genomic Labeling Module (Invitrogen): contains 10X dCTP nucleotide labelingmix, high-concentration exo-minus Klenow DNA polymerase (40 U/µL), 2.5X random primers (octamers), and stop solution (0.5 M EDTA).
Cy5-dCTP, Cy3-dCTP (GE Healthcare).
2.3. DNA Clean-Up and Hybridization
TE pH 7.4.
Microcon YM-30 filters (42410; Fisher Scientific).
Human Cot-1 DNA (1 µg/µL) (15279-011; Invitrogen).
Oligo aCGH/ChIP-on-ChIP Hybridization Kit (contains 2X Hi-RPM Hybridization Buffer and 10X Lyophilized Blocking Agent) (Agilent Technologies).
SureHyb Hybridization Chambers (Agilent Technologies).
Hybridization Slide Gaskets (Agilent Technologies).
Microarray Hybridization Oven (Agilent Technologies).
Rotator Rack for Hybridization Oven (Agilent Technologies).
2.4. Post-hybridization Washing of Microarrays
Wash solution 1: 2X SSC/0.03% SDS.
Wash solution 2: 2X SSC.
Wash solution 3: 1X SSC.
Wash solution 4: 0.2X SSC.
Temperature-controlled shaking water bath (Bellco Glass).
Metal slide racks (2) and glass staining dishes (Wheaton Science).
Desktop centrifuge with microtiter plate adaptors (Beckman Allegra GS-6R or equivalent).
2.5. Microarray Imaging, Data Acquisition and Analysis
Dual-laser confocal microarray scanner (GenePix 4000B;Molecular Devices or equivalent) for microarray image acquisition.
Feature extraction software (SpotReader 1.3; Niles Scientific or equivalent) for automatic identification and extraction of fluorescent ratios from array elements.
Database software (e.g., Microsoft Excel or other customized server-based microarray databases) for data storage, manipulation, and analysis.
3. Methods
3.1. Processing of HEEBO Microarrays
Microarrays will need to be “post-processed” (i.e., processed after printing) to be activated for use in hybridization. Post-processing entails (1) hydration of microarrays to enable covalent immobilization of oligonucleotides to reactive epoxysilane moieties on slide surface; (2) washing of unbound oligos and residue from array printing; and (3) blocking of reactive groups in non-printed areas of the microarray.
3.1.1. DNA Immobilization
Warm 100 mL of 2X SSC solution to ~50°C in a microwave oven and pour solution in a humidifying chamber.
Carefully place microarrays (printed side down) into the slots of the chamber and cover with lid. Hydrate for at least 1 h at room temperature (see Note 2).
Proceed to washing and blocking.
3.1.2. Washing and Blocking of Microarrays
Transfer arrays to a metal slide rack and wash in 0.1% Triton X-100 for 5 min on an orbital shaker at room temperature (see Note 3).
Wash arrays 2X in 1 mM HCl solution for 2 min each on an orbital shaker at room temperature.
Incubate arrays in 100 mM KCl solution for 10 min on an orbital shaker at room temperature.
Wash arrays for 2 min in ddH2O on an orbital shaker at room temperature.
Incubate arrays in pre-warmed 1X Blocking Solution for 20 min in a shaking water bath preset to 50°C.
Wash arrays in ddH2O for 5 min at room temperature on an orbital shaker.
Quickly transfer slide rack containing arrays to a desktop centrifuge with microtiter adaptors and centrifuge at 500g for 5 min.
Check individual arrays under ambient light to ensure that there are no residues on array surface (see Note 4).
Incubate arrays at 37°C in a slide warmer until ready for use.
3.2. Genomic DNA
3.2.1. Isolation of Genomic DNA
Isolation of high-quality genomic DNA from cultured cell lines and tissue (see Note 5) is routinely done using commercially available kits standard in many laboratories. Anion-exchange column-based kits (e.g., Blood and Cell Culture Kit; DNeasy/QiaAmp Tissue Kit; Qiagen) are good alternatives to traditional genomicDNA isolation methods. Isolated genomic DNA should be run out on an agarose gel to assess quality (see Note 6), and quantified using ultraviolet spectrophotometry. If less than 3 µg of genomic DNA input is available for subsequent labeling, then follow the optional whole-genome amplification (WGA) protocol below.
3.2.2. Amplification of Genomic DNA
In separate microfuge tubes, dilute test and reference genomic DNA in ddH2O to a concentration of 25 ng/µL (see Note 7).
Add 1 µL diluted genomic DNA from test and reference samples into separate microfuge tubes and mix with 9 µL of Sample Buffer (this and other reagents are provided with the Illustra GenomiPhi V2 DNA Amplification Kit).
Heat denature samples in a boiling water bath for 3 min, and immediately snap-cool samples on ice.
For each amplification reaction, prepare a master mix containing 9 µL Reaction Buffer and 1 µL Enzyme Mix, and add 10 µL to each test and reference DNA sample. Mix well and briefly spin-down in a microcentrifuge (see Note 8).
Incubate samples at 37°C for 1.5 h.
Heat-inactivate enzyme at 65°C for 10 min.
Quantify amplified DNA yield by UV spectrophotometry and check quality (confirming adequate DNA size) by agarose gel electrophoresis of a small aliquot (see Note 9).
3.2.3. Labeling of Genomic DNA
In separate microfuge tubes, aliquot and digest 3 µg of (amplified) test and reference genomic DNAs using DpnII restriction enzyme as per manufacturer’s directions (see Note 10).
Heat-inactivate digestion reaction by incubation at 65°C for 20 min.
Add 20 µL 2.5X random primer mix (this and other reagents are provided with the BioPrime ArrayCGHGenomic Labeling Module) to each tube and place samples in a boiling water bath for 5 min, then snap-cool samples on ice for another 5 min (see Note 11)
To each tube, add 5 µL 10X dCTP nucleotide labeling mix, 3 µL Cy5-dCTP (for test/tumor DNA) or Cy3-dCTP (for reference/normal DNA), and 1 µL high-concentration Klenow enzyme.
Incubate samples at 37°C for 2 h.
Stop the reactions by adding 5 µL of the stop solution to each tube.
3.3. DNA Clean-Up and Hybridization
Pool paired test and reference samples together in a Microcon YM-30 filter with 400 µL TE (pH 7.4) and spin at 12,000g in a microcentrifuge for 10–12 min.
Discard the flowthrough and add an additional 500 µL TE (pH 7.4) to each sample, re-centrifuge at 12,000g for 10–12 min. Carefully measure the remaining volume in the filter (should be <20 µL). Discard flowthrough (see Note 12).
Invert filter to a clean microfuge tube and spin at 12,000g for 1 min to collect the labeled probe mixture.
For each sample, bring up the volume to 150 µL with ddH2O. Add 50 µL of Human Cot-1 DNA, 50 µL of 10X Agilent Blocking Agent, and 250 µL of 2X Hi-RPM Hybridization Buffer (see Notes 13 and 14). Mix gently (to avoid bubbles) and boil hybridization mixture in a heated water bath for 3 min.
Incubate samples at 37°C for 30 min.
Place ~500 µL of hybridization mixture onto the slide gasket (assembled in hybridization chamber as per manufacturer’s instructions) and place microarray with printed side facing the hybridization mix to form an “array sandwich”.
Load hybridization chambers into a rotating oven set at 65°C with a rotation speed of 20 rpm for 30–40 h (see Note 15).
3.4. Post-hybridization Washing of Microarrays
Washing of microarrays following hybridization serves to remove unbound labeled nucleic acid.
In separate glass staining chambers, prepare 400 mL of each of the four wash solutions, and preheat wash solutions 1 and 2 to 65°C (see Note 16).
Place arrays in a slide rack, and gently agitate for 3 min each sequentially in wash solution 1 (at 65°C), 2 (at 65°C), 3 (at room temperature), and 4 (at room temperature).
After the final wash, spin dry microarrays using a desktop centrifuge with microtiter plate adaptors at 500g for 5 min.
3.5. Microarray Imaging, Data Acquisition, and Analysis
Hybridized microarrays should be immediately scanned in a dual-color microarray scanner (e.g., GenePix 4000B) or equivalent (see Note 17). After image acquisition, fluorescence intensity ratios are extracted from each array feature using a feature extraction software (e.g., GenePix Pro 6.0; Molecular Devices, Spot-Reader v1.3; Niles Scientific; BlueFuse; Cambridge Blue Gnome). To account for differences in sample input and labeling efficiency, feature-extracted raw data are normalized by setting the average log2 ratio for each array to 0. Resulting microarray data can be stored in a number of databases, ranging from Microsoft Excel to more specialized solutions designed for storage, retrieval, and analysis of large microarray datasets (see Note 18). For visualization and analysis of HEEBO array-CGH data, academic and commercial solutions are available that map fluorescence ratios of each oligonucleotide on the array to its physical genomic location based on genome assemblies such as UCSC GoldenPath (http://genome.ucsc.edu) (see Note 19).
Footnotes
For array-CGH, it is highly recommended that oligo arrays be printed on microscope slides derivatized with epoxysilane functional groups for best performance.
Prior to end of hydration, turn on water bath to 50°C and prepare fresh 1X Blocking Solution. Incubate 1X Blocking Solution in water bath until ready for use.
All washing solutions should be made fresh and 0.22-µm sterile-filtered prior to use. Microarrays must be completely submerged in solution at all times during the washing and blocking steps; thus prepare enough of each wash/blocking solution (depending on the size of vessel used) to ensure microarrays are not exposed to air for prolonged periods of time. Triton X-100 solution should be completely dissolved before use; if not dissolved, place in a 50°C water bath for 5 min.
It is important to remove any traces of blocking residue from the array surface, as it will cause noticeable fluorescent background when arrays are scanned. If hazy or “swirl” residue is visible on the array surface, wash arrays in molecular biology grade 70% ethanol for 5 min and spin-dry. This wash step should eliminate any remaining surface residue on the array.
Microdissection techniques (e.g., laser-capture microscopy) can be used to increase the enrichment of certain populations of interest (e.g., tumor cells) from heterogeneous tissue mixtures.
Genomic DNA isolated from archival tissue specimens (e.g., ethanol- or formalin-fixed paraffin-embedded samples) can vary in quality depending on the age of tissue and the method of fixation, and may produce variable hybridization results. In our experience, samples where most DNA is <1 kb (by agarose gel electrophoresis) indicates substantial and biased DNA degradation, and produces noisy array-CGH data. It is also recommended that a test hybridization be performed on a representative archival specimen(s) to assess data quality prior to commencement of a large-cohort microarray study.
To increase the amount of starting input material for hybridization, a WGA approach is utilized. This technique takes advantage of several unique properties of the Φ29 phage DNA polymerase, including extreme template processivity, multiple-strand displacement capabilities, and high fidelity. Typical yields of amplified genomic DNA range from 4 to 6 µg, starting from 1 to 25 ng of input. To minimize potential amplification biases, similar amounts of test and reference genomic DNAs should be amplified in parallel.
The master mix contains all the necessary components (e.g., salts, random primers, dNTPs, DNA polymerase) to initiate the amplification reaction.
The size range of pre-amplification and post-amplification DNAs should be compared by agarose gel electrophoresis. Following a successful amplification reaction, the DNA size range should be ~2–10 kb. Smaller sizes indicate degraded input DNA or unsuccessful amplification.
The total volume of the digestion reaction should be <25 µL; thus, input DNA may need to be concentrated first by using a SpeedVac or other methods. Other four-base cutting restriction enzymes (e.g., AluI or RsaI) may be used in lieu of DpnII. Restriction digestion of starting genomic DNA serves to increase labeling efficiency.
Boiling of sample ensures complete denaturation of DNA, and subsequent snap-cooling of samples on ice allows random primers to bind to complementary single-stranded DNA template.
The additional washing step is necessary to remove unincorporated fluorescent nucleotides. If the remaining volume in the filter is >20 µL, centrifuge samples in additional 1 min increments until the volume is <20 µL. Visualization of a purple-colored probe mixture on the filter is indicative of a successful labeling reaction.
Human Cot-1 and 10X Blocking Agent are used for blocking repetitive elements in target DNA and non-specific hybridization, respectively. 10X Blocking Agent should be re-suspended to a final volume of 1,250 µL in ddH2O before use.
The Agilent hybridization buffer is convenient and in our experience performs comparably or better than other aqueous hybridization solutions. Also, our experience is that high-volume hybridizations carried out with mixing of hybridization solution (as detailed here) yields stronger fluorescence hybridization signal-to-noise compared to protocols using low-volume hybridizations under a glass coverslip.
The optimal length of time for efficient hybridization depends on the hybridization kinetics and buffering of the nucleic acid mixture. Hybridization times less than 30 h lead to incomplete nucleic acid binding (e.g., low feature signals) and >40 h lead to high signals but increased background levels.
Time can be saved by using preheated ddH2O (by microwaving) to make wash solutions 1 and 2. Heated wash solutions should be placed in a water bath set to 65°C for the duration of washing.
Cyanine-based dyes, especially Cy5, are extremely sensitive to atmospheric ozone. Exposure to 5–10 ppb of ozone for short periods (e.g., 30 min) can adversely affect Cy5 signal and data reproducibility. It is recommended that measures be taken to mitigate this effect, such as the use of an ozone eliminator (e.g., Ozone Interceptor; Ozone Solutions, Inc.) or a homemade ozone scrubber (details at http://cmgm.stanford.edu/pbrown/protocols/Ozone_Prevention.pdf).
Specialized academic and commercial microarray databases designed for data storage, retrieval, and basic analysis include Stanford Microarray Database (SMD), AMAD (Another MicroArray Database;UCSF), andGenePix Acuity (Molecular Devices).
There are a number of applications that can be utilized to visualize and analyze HEEBO array-CGH data. Some of these applications are dedicated for visualization or analys is only, while others offer a comprehensive solution for the end-user. These include Java Caryoscope (for visualizing aCGH data) (http://dahlia.stanford.edu:meme/caryoscope/index.html) and CGH-Miner (automated gain/loss calling and output visualization) (http://www-stat.stanford.edu/_wpll/CGH-Miner). Comprehensive commercial solutions include CGH Analytics (Agilent Technologies), CGH Nexus (BioDiscovery), and Partek Genomics Suite (Partek).
References
- 1.Ylstra B, van den Ijssel P, Carvalho B, Brakenhoff RH, Meijer GA. BAC to the future! or oligonucleotides: a perspective for micro array comparative genomic hybridization (array CGH) Nucleic Acids Res. 2006;34:445–450. doi: 10.1093/nar/gkj456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 3.Wodicka L, Dong H, Mittmann M, Ho MH, Lockhart DJ. Genomewide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- 4.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 5.Hill AA, Hunter CP, Tsung BT, Tucker-Kellogg G, Brown EL. Genomic analysis of gene expression in C. elegans. Science. 2000;290:809–812. doi: 10.1126/science.290.5492.809. [DOI] [PubMed] [Google Scholar]
- 6.Hughes TR, Mao M, Jones AR, Burchard J, Marton MJ, Shannon KW, et al. Expression profiling using microarrays fabricated by an ink-jet oligonucleotide synthesizer. Nat. Biotechnol. 2001;19:342–347. doi: 10.1038/86730. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 8.Lucito R, Healy J, Alexander J, Reiner A, Esposito D, Chi M, et al. Representational oligonucleotide microarray analysis: a high-resolution method to detect genome copy number variation. Genome Res. 2003;13:2291–2305. doi: 10.1101/gr.1349003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett MT, Scheffer A, Ben-Dor A, Sampas N, Lipson D, Kincaid R, et al. Comparative genomic hybridization using oligonucleotide microarrays and total genomic DNA. Proc. Natl. Acad. Sci. U S A. 2004;101:17765–17770. doi: 10.1073/pnas.0407979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho B, Ouwerkerk E, Meijer GA, Ylstra B. High resolution microarray comparative genomic hybridisation analysis using spotted oligonucleotides. J. Clin. Pathol. 2004;57:644–646. doi: 10.1136/jcp.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Li C, Paez JG, Chin K, Janne PA, Chen TH, et al. An integrated view of copy number and allelic alterations in the cancer genome using single nucleotide polymorphism arrays. Cancer Res. 2004;64:3060–3071. doi: 10.1158/0008-5472.can-03-3308. [DOI] [PubMed] [Google Scholar]
- 12.Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27:3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman JM, Baross A, Delaney AD, Ally A, Arbour L, Armstrong L, et al. Oligonucleotide microarray analysis of genomic imbalance in children with mental retardation. Am. J. Hum.Genet. 2006;79:500–513. doi: 10.1086/507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ming JE, Geiger E, James AC, Ciprero KL, Nimmakayalu M, Zhang Y, et al. Rapid detection of submicroscopic chromosomal rearrangements in children with multiple congenital anomalies using high density oligonucleotide arrays. Hum. Mutat. 2006;27:467–473. doi: 10.1002/humu.20322. [DOI] [PubMed] [Google Scholar]
- 15.Balciuniene J, Feng N, Iyadurai K, Hirsch B, Charnas L, Bill BR, et al. Recurrent 10q22–q23 deletions: a genomic disorder on 10q associated with cognitive and behavioral abnormalities. Am. J. Hum. Genet. 2007;80:938–947. doi: 10.1086/513607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinkel D, Albertson DG. Array comparative genomic hybridization and its applications in cancer. Nat. Genet. 2005;37(Suppl):S11–S17. doi: 10.1038/ng1569. [DOI] [PubMed] [Google Scholar]