Abstract
Once there was a day when all type C nonmyelinated neurons were indistinguishable. That time of histologic analysis has passed, and we have entered an era of unparalleled technological insight into the mechanisms of pain and pruritus. Since the description of the capsaicin receptor, transient receptor protein vanilloid 1 (TRPV1), in 1997, we have seen the number of related sensor ion channels, G protein–coupled receptors, and signaling proteins explode. Specific nociceptive pathways have been identified based on their sensitivity to mechanical, heat, chemical, and cold stimuli. Pruritus is now recognized to have both histamine-sensitive and histamine-independent afferent arcs. Cross-talk between C-fibre systems and myelinated neural pathways has become more complex, but through complexity, a new reality of sensory coding is emerging. A multitude of novel therapeutics have been and are in planning and production stages. These will almost certainly revolutionize our understanding and treatment of pain and itch by the end of this decade.
Keywords: Pain, Itch, Transient receptor potential, Rhinitis, Nociception, Pruritus
Introduction: Phylogeny of Nociceptors
In the beginning, there was no pain or itch. The need to respond to food and threatening environmental stimuli heralded the evolution of chemical sensors in metazoan animals. The evolution of nociceptors reflected the increasing complexity of neural networks and environmental cues of significance for survival and exploitation of ecological niches. Mechanical nociceptors evolved at the level of Cnidaria (eg, hermit anemone, Cryphonectria parasitica) before bilaterally symmetrical animals appeared [1]. Nematodes (Cnidaria elegans), arthropods (Drosophila. melanogaster), and annelids (eg, medical leech, Hirudo medicinalis) have nonmyelinated neurons and can detect and respond to mechanical, heat, and chemical stimuli. Myelination evolved at the level of teleosts such as rainbow trout (Oncorhynchus mykiss). Cold sensors appeared more recently in mammals and may have been required by warm-blooded animals for temperature regulation. With this long history of evolution, it is likely that many early sensors have evolved into diverse protein families with divergent functions. The increased complexity of sensory input parallels the complexity of the spinal cord and its dorsal horn, connections to the brainstem and telencephalon, and finally to cerebral functions in the sentient mammals. These ion channels, G protein–coupled receptors (GPRs), and other proteins may have unsuspected and novel functions that will make them logical targets for human therapeutic development.
Neuron Classification by Diameter and Sensations
Nonmyelinated type C fibres have the oldest heritage and diversity of sensory detection [1]. They account for about 50% of mammalian peripheral nerve fibres. Narrow diameters on the order of 1 to 5 µm yield conduction velocities of less than 1.5 m/s, with one subset of itch neurons being the slimmest and slowest. Type C neurons fall into four general classes. The group of C-polymodal nociceptive nerves represent about 30% of all neurons and are sensitive to mechanical pressure (threshold, 10 milliNewtons [mN]), a range of cold to hot temperatures, and diverse chemicals and inflammatory autocoids. The CM/MH/MC/H set (20% of neurons) combine mechanical (6 mN) and temperature sensitivities as mechanical (CM), mechanical plus heat (CMH), mechanical plus cold (CMC), and heat (CH) sensitive nociceptors. Type C low-threshold fibers (5% of neurons) respond to very light pressure of less than 0.5 mN. About 5% of neurons are “silent,” heat-sensitive nociceptors that can be activated only in coordination with other neurons. These general patterns of sensory responsiveness are an indication of the combinations of nociceptive sensor proteins that are most commonly associated. However, neural plasticity induced by inflammatory and other stressors can alter the suite of ion channels, GPRs, and other regulatory proteins that are expressed, and thus the threshold for stimulation (electrical depolarization) and sensation (dorsal horn regulation) that may be conveyed. The mechanisms of neural plasticity, central and peripheral sensitization, and induction of neuropathic pain are discussed below.
Type Aδ neurons are approximately 6 µm in diameter with 2-µm thick myelin sheaths and conduction velocities of 2 to 10 m/s. Aδ mechanonociceptors have temperature, mechanical (<5 mN), and other sensory activities. They represent about 12% of neurons. D-hair fibres are highly mechanosensitive (<0.5 mN; 6%), cutaneous Aδ neurons.
Light touch, proprioception, and vibration are essentially mechanical sensations mediated by Aβ fibres. These nerves and sensations are of little importance in the mucosal nasal cavity but may promote referred sensations through other trigeminal neurons. The diameters of Aβ neurons are on the order of 10 to 12 µm, with 2 to 5 µm of myelin. Conduction velocities are greater than 10 m/s. One subset responds to mechanical stimuli with a threshold of about 1.0 mN (10% of neurons). These rapidly adapting mechanosensors have combinations of ion channels that rapidly hyperpolarize the neurons to prevent further neural depolarization and sensory stimulation. The other subset (12%) have higher pressure thresholds for depolarization (1.5 mN) and are slowly adapting mechanoceptors.
Type C neurons terminate in the superficial laminae I and II of Rexed in the substantial gelatinosa of the dorsal horn of the spinal cord [2••]. Trigeminal neurons synapse in the upper three cervical regions and brainstem trigeminal sensory subnucleus caudalis. Aδ and Aδ peripheral neurons terminate in laminae I and V. Postsynaptic interneurons in these laminae have axons that generally cross the midline to ascend in sensation-specific spinothalamic tracts. The presynaptic-postsynaptic neural junction is the site for blockade of peripheral nociceptive input (Gate Theory of Melzack and Wall [3]) under usual circumstances, and of receptor reorganization and microglial interactions during the development of neuropathic pain with hyperalgesia and allodynia (central sensitization). Heightened sensations originating from the nasal mucosa are due to neurotrophins that act on peripheral nerve endings (peripheral sensitization), but in chronic airway inflammation, the central synapse may become more important for the development of persistent nasal discomfort, pain, midfacial pain (“sinus”), postoperative pain syndromes, and other neuralgias.
Thermosensors and Ion Channels
Transient receptor potential ion channels (TRPs) are a large protein family that allow for the flux of cations and anions across membranes. They are indispensable for electrical depolarization, repolarization, and hyperpolarization of neurons.
TRP vanilloid 1 (TRPV1, the capsaicin receptor) was the first of a family of 143 human ion channels to have its activating ligands characterized [4]. Other members of this family include other sensors; voltage-gated sodium channels responsible for cell depolarization; voltage-gated calcium channels that mediate full depolarization and that also induce the functions of neuron, gland, muscle, and other electro-excitable cells; and potassium channels that repolarize and maintain the resting membrane potential of these cells [5, 6]. The basic motif of these transmembrane proteins is a single extracellular loop that dips into, but does not cross, the plasma membrane. Transmembrane α-helices flank the intramembrane loop. Tetramers of these proteins form pores that permit the regulated flow of specific ions into cells. There is the potential for heterotetramers to form that may have modified responses compared with homote-tramers. This greatly extends the ranges of temperature, osmolarity, membrane fluidity, and chemical exposures that may activate these diverse heterotetramers.
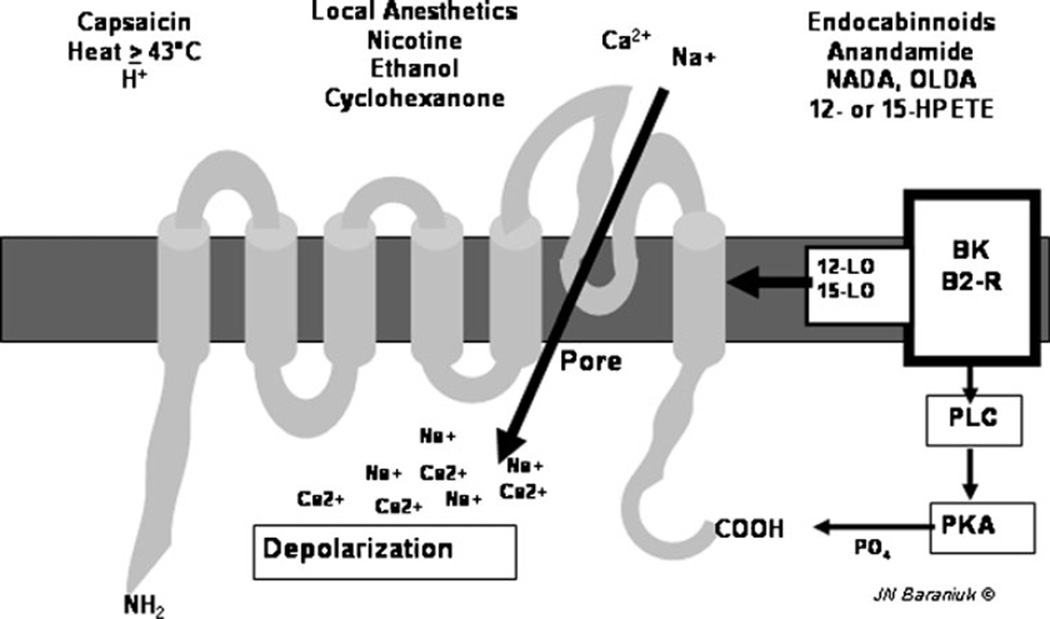
Six thermo TRPs have been characterized. TRPV1 responds to capsaicin, H+, temperatures above 43°C, endocannabinoids, bradykinin, ethanol, and other irritants (Fig. 1). TRPV2 is activated at higher, more painful temperatures. TRPV3 and TRPV4 are osmoreceptors that also respond to nonpainful, ambient warmth. TRP melanostatin 8 (TRPM8) is activated by menthol and nonpainful cool temperatures, while TRP ankyrin 1 (TRPA1) responds to painful cold, mustard oil, and cinnamaldehyde [7]. In each case, temperature or agonists cause conformational changes that open nonselective cation channels that depolarize different populations of neurons. The proteins form hexamers that may involve several different TRP channels with more subtle or wide-ranging sensitivities. Some neurons have combinations of these sensors that may again lead to unanticipated sensitivity patterns and neural depolarization. Their expression can be modulated by neurotrophins and the frequency of nerve depolarization, indicating they can participate in changing neuron function as part of neural plasticity.
Fig. 1.
Schematic transient receptor potential vanilloid 1 (TRPV1) protein with six transmembrane regions and the intramembrane loop that forms the cation pore. Various ligands are shown. Bradykinin (BK) binds to its B2 receptor and activates 12- and 15-lipoxygenase (12-LO, 15-LO) and phospholipase C (PLC) and protein kinase A (PKA) that phosphorylates the carboxy-terminus of the protein. (Used with permission of James N. Baraniuk, MD)
The afferent stimulation of sneezing, itching, and rhinorrhea reflexes is likely to involve these nociceptive proteins. Allergic rhinitis is associated with significant increases in PGP 9.5, substance P, and neuropeptide Y immunoreactive nerve density in the epithelium, subepithelium, glandular, and vascular regions [8]. However, TRPV1 immunoreac-tivity was not changed in allergic rhinitis. This increased nerve density is likely related to increased neurotrophin expression. Nerve growth factor (NGF) has been found in eosinophil granulocytes, the glands, peripheral nerves, and to a lesser extent the epithelium [9]. Brain-derived neurotrophic factor (BDNF) is upregulated in the allergic epithelium. BDNF expression is positively correlated with the maximum increased total nasal symptom score after allergen challenge. The neurotrophin receptors tyrosine kinase A (trkA), pan-neurotrophin receptor p75, and trkB are expressed on endothelium, peripheral nerves, and trkB on nasal mucosal mast cells, respectively. Eosinophils express BDNF, NGF, and these receptors in allergic rhinitis.
Human nasal provocations have been performed with the TRPV1 activators capsaicin, anandamide, and olvanil; TRPA1 activators cinnamaldehyde and mustard oil (allyl isothiocyanate); and TRPM8 activator menthol [10]. These were compared with vehicle (sham) challenges. Symptom scores were compared between provocations performed both before and during pollen seasons. The TRPV1, TRPA1, and TRPM8 activators induced immediate, intense, plus more prolonged, dull pain (first and second pain, respectively), as has been reported for hypertonic saline [11]. During pollen season provocations, the TRPV1 activators induced itch as well as pain. Olvanil was the only agonist to induce rhinorrhea during or prior to the pollen seasons. A TRPV1 antagonist, SB-705498, was synthesized that inhibited capsaicin-induced nasal symptoms. However, daily topical applications had no effect on total symptom score, nasal peak inspiratory flow, or eosinophilic cationic protein levels in allergen-challenged patients with seasonal allergic rhinitis [12]. The individual symptoms, nasal itch or sneezes, were also not affected. These findings may indicate that TRPV1 is not a key mediator of the symptoms in allergic rhinitis. Different responses may have occurred during actual pollen seasons in vivo, or TRPV1 may be a facilitating ion channel for itch during allergic inflammation. For example, sphingosine-1-phosphate is abundantly produced in inflammation, and it may act via S1P1 receptors in concert with TRPV1 to upregulate the sensitivity of some neural populations [13].
TRPA1 ion channels are expressed on type C and Aδ neurons that can be activated by cold and inflammatory stimuli. Cinnamaldehyde and to a lesser extent allyl isothiocyanate (component of garlic and mustard oil) are agonists. It is not clear if cold temperatures induce osmotic or mechanical changes in epithelial cell shape, shearing forces with the release of autocoids similar to cinnamaldehyde, or TRPA1 protein conformational changes in nerve endings that lead to cation permeability and neural depolarization. TRPA1 immunoreactivity was limited in allergic and nonallergic rhinitis and control tissues [14]. TRPA1 may also play a role in cough during respiratory infections, asthma, and chronic obstructive pulmonary disease [15••].
TRPA1 may have dorsal horn functions [16]. Cinnamaldehyde may promote signal transduction by type C nociceptive neurons (presynaptic effect). This agonist may promote neurotransmission by selected dorsal horn neurons in the first and second layers of the substantia gelatinosa that project in vertical and radial directions, suggesting a postsynaptic effect as well.
TRPA1 may also be a model of drug hypersensitivity. For example, oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation [17]. Cisplatin can upregulate purinergic P2×3 ion channels and acid-sensing ion channel 3 (ASIC3), which may promote inflammatory responses and pain [18].
TRPM8 ion channels on cold-sensitive sensory neurons are activated by menthol and to a lesser extent by eucalyptol. Menthol is incorporated into cigarettes, potentially to activate TRPM8 neurons that induce counterirritant effects on cigarette smoke components such as acrolein, acetic acid, and cyclohexanone [19]. In human nasal mucosa, TRPM8 immunoreactive nerves were present around deep venous sinusoids, and to a lesser extent subepithelial lamina propria [14]. The distribution was not different among allergic rhinitis, nonallergic rhinitis, and control tissues.
Acid-Sensing Ion Channels (Proton-Activated Acid-Sensing Ion Channels)
Tissue acidosis due to the release of protons is one of the first features of tissue injury. Extracellular protons activate ASIC3 on peripheral nociceptive type C neurons [20]. ASIC3 may form heteromeric complexes with TRPV1 and purinergic X3/4 ion channels that have increased sensitivity to adenosine triphosphate (ATP) and acidosis. ASIC3 may also join complexes that transduce mechanosensation and mechanonociception. These complexes have been most studied in the gastrointestinal tract and suggest the potential for involvement in irritable bowel syndrome and other disorders of hollow viscus organs. mRNAs for ASIC3, TRPV1, and PX3/4 are upregulated in peripheral blood leukocytes of a subpopulation of chronic fatigue syndrome (CFS) patients, but not healthy controls, following a moderate exercise provocation (25 min of bicycle exercise at 70% of maximum predicted heart rate) [21]. It remains to be demonstrated if a similar upregulation occurs in nociceptive neurons leading to systemic hyperalgesia, allodynia, or excessive fatigue in CFS. This is of interest given the neurological nonallergic rhinopathy of CFS patients [11].
ASIC1a on some dorsal horn cells may be activated by µ-opiate receptor activation and block pain transmission [20]. Agonists of ASIC1a may have the potential to be analgesics for a broad range of pain conditions.
Purinergic Neurotransmission
ATP has long been recognized as a neurotransmitter, but only more recently has it been identified as a cotransmitter with glutamate, noradrenaline, γ-aminobutyric acid, acetylcholine, and dopamine [22••]. ATP is also released quickly by dying cells and is a stimulus for rapid inflammatory cell recruitment and tissue repair. Hence, it is not surprising that it can also activate nociceptive neurons. ATP, adenosine diphosphate, adenosine, and uracil nucleotides can act via P2X ion channels and P1 and P2Y G protein-coupled receptors. The receptors induce fast signaling in neurotransmission; act in microglial neuromodulation; and provide long-term (trophic) signaling for cell proliferation, differentiation, and death. P2Y1 regulates polymodal C-fiber thermal thresholds and appears to maintain TRPV1 expression during peripheral inflammation [23]. Novel interactions have come to light, such as P2Y12 receptor mediation of leukotriene E4-induced pulmonary inflammation [24], and the important role of purinergic systems in the protection and repair of olfactory mucosa and its function [25]. mRNA for P2X (P2×3, P2×4, P2×7) and P2Y (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12) receptors were identified in human nasal epithelial cells [26]. P2Y2, P2Y6, and P2Y11 receptors have been shown to play roles in mucin and fluid secretion in these cells. The P2Y2 receptor is involved in ATP-induced MUC5AC gene expression following lipo-polysaccharide activation of human airway epithelial cells in vitro [27]. Adenosine can act through its own receptors. A (2B) adenosine receptor signaling is altered in asthma and chronic obstructive pulmonary disease and may be necessary for adequate cystic fibrosis transmembrane conductance regulator function to maintain epithelial lining fluid water volume [28].
Another potential ATP receptor is “MAS-related GPR member D” (Mrgprd) [29]. Murine dorsal root ganglion neurons expressing Mrgprd responded to ATP in a fashion similar to P2×3 channels. They had long action potentials, tetrodotoxin-resistant sodium currents, and calcium currents that were inhibited by µ.-opioid agonists. These properties are characteristic of nociceptive nerves. The neurons had essentially no sensitivity to capsaicin, cinnamaldehyde, menthol, pH 6.0, or glutamate, indicating the absence of TRPV1, TRPA1, TRPM8, ASIC, or glutaminergic ion channels and receptors. However, more recent and detailed studies have suggested a role for Mrgprd neurons in itch (see below).
Molecular Mechanisms for Neural Conduction of Different Sensations
Heat is not generally considered a stimulus for nasal complaints, but hot, dry (desert) or moist (jungle) air may induce newcomers to those environmental conditions, or during appropriate nasal provocations. Based on studies in the skin [2••], increased temperatures may activate TRPV3 and TRPV4 ion channels on non-neuronal keratinocytes or other cells that then release autocoids or other mediators, or shape changes that lead to mechanical stress on mechanicosensitive nociceptive neurons. The temperature elevation may directly activate TRPV1, TRPV2, and other nonselective cation channels to directly depolarize the nerve ending. Decreased flux of K+ through TREK and potentially TRAAK potassium channels may prolong depolarization so that an action potential can be developed.
Cold on the skin appears to be mediated solely by nerve endings with increased cation flux through TRPM8 and TRPA1, with a requirement for the Nav1.8 sodium channel. Again, potassium flux via TRAAK and/or TREK-1 and possibly other ion channel actions helps initiate the action potential.
TRPA1 (cinnamaldehyde) and TRPM8 (menthol-activated) ion channels may respond to mucosal temperature fluctuations caused by evaporation of water from the epithelial lining fluid. High airflow through a patent nostril leads to increased evaporation to hydrate inhaled air. Evaporation of high kinetic energy water molecules reduces the mucosal temperature. The temperature changes may activate TRPA1 and TRPM8 on Aδ neurons that synapse in the trigeminal dorsal horn association areas, leading to signals to brainstem respiratory centers that reduce the overall effort and work of breathing.
Mechanical sensors are the most ancient, and so there are multiple potential molecular mechanisms for transducing this sensation. TRPV4 on keratinocytes or nasal epithelial cells may become activated, as it may detect osmotic stimuli through cellular shape changes. Transduction of this initial stimulus to the nerve ending leads to a cation influx and neural depolarization (Fig. 2). Potential roles for increased cation flux through TRPV1, TRPV4, and TRPA1 have been proposed. An as-yet-undefined rapidly adapting mechanosensor appears to induce a rapid burst of depolarization, followed by accommodation with hyperpolarization. Intermediate-adapting and slow-adapting mechanonociceptor mechanisms are also proposed that lead to slower induction of peripheral nerve ending hyperpolarization. Downregulation of potassium ion channels such as K,3.4; K,4.3; TRAAK; and TREK-1 may reduce the threshold for further neural depolarization or the ability to repolarize or hyperpolarize the neuron. The result would be increased sensitivity to mechanical stimuli and hyperalgesia. Nasal mechanoceptors may gauge ciliary mucoid raft flux in mucus hypersecretion, deep venous sinusoid wall stretching during vasodilation and turbinate engorgement, or deformation of mucosal thickness in occluded ethmoid sinuses or sinus ostia during inflammation or infection.
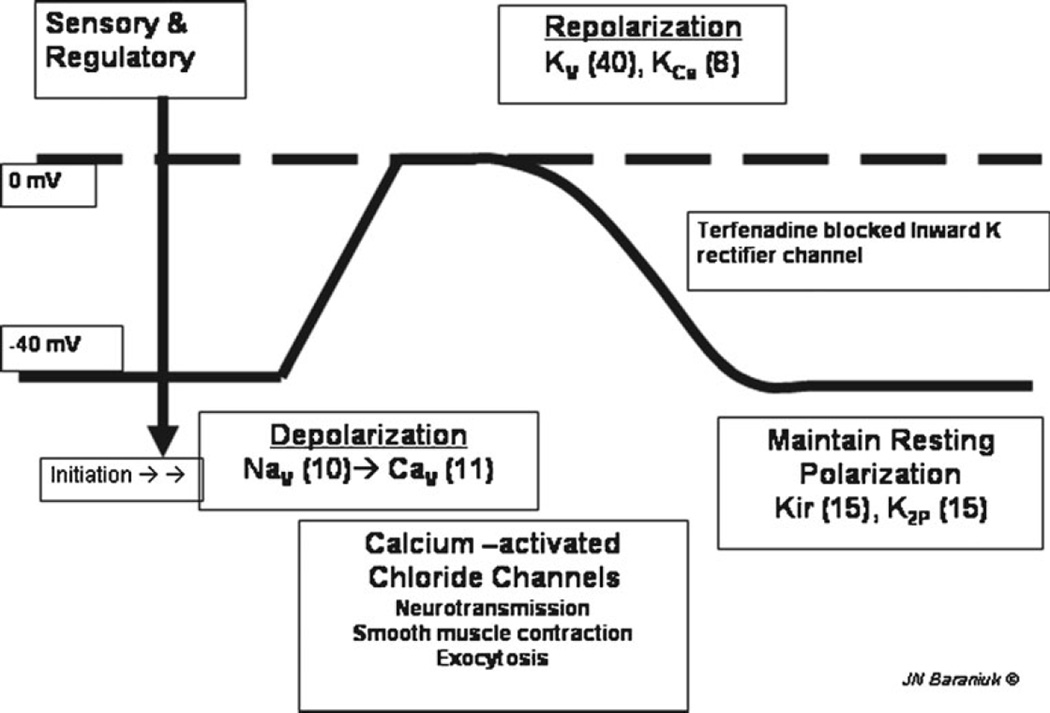
Fig. 2.
Schematic nerve depolarization curve. Resting membrane potential is maintained at about −40 mV by potassium channels (Kir, K2P). Sensory and regulatory channels initiate depolarization. Voltage-gated sodium channels (Nav) and calcium channels (Cav) induce full depolarization to 0 mV. The cell is repolarized by voltage- and calcium-activated potassium channels (Kv and KCa). The numbers of recognized members for each type of channel are in parentheses. (Used with permission of James N. Baraniuk, MD)
Cross-Talk
Single and simultaneous application of warm and cold temperatures to the skin by a “thermal grill” has shown that distinct populations of neurons interact at the dorsal horn level to allow perception of specific sensations (Table 1) [30••]. Cold activates a population of type C cold neurons that cause a cold sensation that also has a “hot-burning” quality [31]. Coactivation of cold-responsive Aδ nerves leads to a faster appreciation of cold sensation and modulation of C-cold neuron effects so that a “pure cold” sensation is perceived. Warm temperatures stimulate C-warm neurons that inhibit the Aδ-cold transmission to its postsynaptic dorsal horn cell. When a grid of warm and cold temperatures is applied to the skin, then a hierarchy of inhibitory crosstalk occurs. The C-warm fibres generate a warm sensation and inhibit Aδ-cold transmission. However, the cold-activated Aδ-cold fibres can still modulate C-cold transmission. The result is a paradoxical hot burning pain sensation mediated by C-cold plus C-warm presynaptic interactions following simultaneous warm and cold stimulation. The dual temperature thermal grill was perceived to be more unpleasant than painful, but with more intense sensations than the warm or cold applied alone. In functional MRI studies, both warm and cold stimuli activated bilateral anterior insulae and frontoparietal regions [32]. In contrast, the thermal grill activated the contralateral thalamus, unlike the individual temperature stimuli, and activation of the contralateral mid/anterior insula was weakly correlated with perceived unpleasantness. These results suggest that the bimodal thermoreceptive properties of the C-cold neurons may contribute to several puzzling psychophysical phenomena, such as “innocuous cold nociception,” “paradoxical heat,” and some neuropathic pains.
Table 1.
Interactions of warm and cold thermoreceptor neuron populations and the “thermal grill” illusion
| Temperature of skin stimulation |
Neurons activated |
Dorsal horn cross-talk and inhibition |
Perception | Brain blood flow increased (fMRI) |
|---|---|---|---|---|
| Warm (41°C) | C-warm | Inhibit Aδ-cold | Warm | Contralateral mid/anterior insula |
| Cold (18°C) | Aδ-cold | Mask C-cold–induced sensation | Cold | Contralateral mid/anterior insula |
| Cold (18°C) | C-cold | – | Cold with a stinging quality |
Contralateral mid/anterior insula |
| Warm + cold (thermal grid of 41°C and 18°C) |
C-warm + Aδ- cold + C-cold |
Aδ-cold blocked so it cannot mask C-cold “stinging” sensation |
Hot, burning pain from C-warm + C-cold |
Contralateral thalamus and mid/anterior insula |
fMRI, functional MRI
Increased cutaneous sensitivity to cold (cold allodynia) has been demonstrated in healthy individuals by determining the threshold for cold-induced pain and then applying innocuous cold stimuli (5°C above the threshold) and noxious cold stimuli (5°C below the threshold) [33]. Cold allodynia was induced by applying topical menthol (TRPM8 activator) and then measuring the increase in cold pain threshold. Innocuous cold, noxious cold, and cold allodynia significantly activated the bilateral insular, frontal, and anterior cingulate cortex regions. Differences in the intensity of activation were found, with innocuous cold having more activation of the ipsilateral anterior insular cortex, and noxious cold the posterior insula. In contrast, the cold allodynia condition caused significantly greater activation in the bilateral anterior insulae and dorsolateral prefrontal cortices and ipsilateral parabrachial nucleus of the midbrain. This suggests that these additional brain areas are involved in central nociceptive sensitization processes. Prior experience with cold-induced pain or allodynia [34] and affective dysfunction [35] may also worsen the intensity of pain perceptions and these other central responses. It will be of interest to determine if similar findings occur in nonallergic rhinopathy defined by environmental or experimental cold air sensitivity [36].
Pruritus
Itch is the unpleasant sensation that evokes a desire to scratch. Pruritus precedes the skin lesions of atopic dermatitis and contributes to many other skin, systemic, and nervous system disorders. Antihistamines have been the foundation of antipruritic therapy for decades, but in many instances, pathological itch is insensitive to antihistamine treatment. This suggests that several pathways may convey this sensation. It is unclear which invertebrate animals perceive itch. At first blush, this leaves the evolutionary tree of pruritic sensors bare except for histamine H1 receptors (H1R).
H1R are present on endothelium, vascular smooth muscle, glands, and epithelium [37]. Histamine nasal provocation causes itch, vascular permeability, and cholinergic reflex-mediated glandular secretion. These effects are consistent with allergic rhinoconjunctivitis. H1R have been localized to a small population of very narrow diameter neurons that are localized to distinct spinal cord dorsal horn regions, ascending pathways, thalamus, and thalamocortical radiations [38]. This route is distinct from the neural pathways activated by capsaicin-sensitive nociceptive neurons. Histamine H4R agonists may also induce itch under conditions of H1R blockade. H3R on sensory and autonomic neurons generally act as inhibitory autoreceptors to hyperpolarize neurons and prevent their depolarization but may also indirectly permit itch under some circumstances. H2R are present on epithelium and glands. Histamine-induced axon responses have not been clearly demonstrated in human airway mucosa.
The population of neurons that are solely “itch specific” may be small. Some H1R–bearing neurons appear to express the TRPV1. Other distinct combinations of proteins, such as purinergic P2X receptors and ASIC3, may also be present on subsets of these neurons that would then respond to ATP, adenosine, H+, K+, and Ca 2+ that may be released by cellular injury or during inflammation [39]. ASIC can be blocked by amiloride and aspirin. Neurons with TRPV1, ASIC3, and P2X receptors may have particularly important functions in detection of visceral pain or other conditions [40]. The sensors and neurons that mediate the itch sensation that occurs as wounds heal are not known.
Histamine-independent itch may be associated with several distinct neural pathways. As mentioned previously, ATP may activate members of the Mas-related G protein-coupled receptor (MRGPR) family [29]. MRGPRA3 and MRGPRC11 are receptors for the pruritogens chloroquine and BAM8–22, respectively. It has now been established that TRPA1 is required for MRGPR-mediated signaling [41]. Sensory neurons from TRPA1-deficient mice had minimal responses to chloroquine and BAM8–22, and these mice had negligible scratching in response to these pruritogens. This suggests that ATP, endogenous agonists of MRGPR receptors, and TRPA1 activators may mediate itch in illnesses that do not involve mast cells, such as uremia and severe hepatic failure. These proteins may be targets for novel antipruritic agents.
Itch may be two subtle sensations. To trained observers, histamine induces a burning itch. In contrast, the dart-like spicules of the cowhage plant (Mucuna pruriens) induce a “stinging/pricking” itch. In addition, this pruritogen does not induce a large flare response, as seen with histamine [42]. The itch is due to the mucunain protease [43], which can cleave protease receptor 2 (PAR2) or PAR4 on populations of PAR2/4+ neurons that are also 1) mechano-heat sensitive or 2) heat sensitive but with histamine-insensitive nerve endings [44]. Mast cell tryptase is a PAR2 agonist that may induce histamine-independent itch in humans.
Davidson and Giesler [45•] consolidated these mechanisms and proposed that two pathways mediate itch sensations. First, H1R and H4R bearing C mechanically insensitive nerve terminals mediate histaminergic pruritus. They propose that MRGPRA3 may also be present on these peripheral neurons, and thus mediates ATP-induced itch. TRPV1 may also be present on these neurons. H1R activation stimulates phospholipase A2 (PLA2) and phospholipase Cβ3 (PLCβ3), which release arachidonic acid, diacylglycerol, and other acyl glycerol phosphate moieties that activate TRPV1 ion channels on the same neurons, and induce a sudden influx of calcium that depolarizes the neuron. Bradykinin B1 and B2 receptors may also be present. The wave of depolarization spreads throughout the extensively dendritic network of peripheral nerve endings to generate the axon response (Fig. 3). These endings have swellings or varicosities that release calcitonin gene-related peptide (CGRP), gastrin-releasing peptide (GRP), substance P, neurokinin A, and possibly glutamate into the surrounding interstitial spaces. CGRP is responsible for the vasodilation of the flare response. GRP and potentially the tachykinins may cause the glandular exocytosis found in the human nasal mucosa. The central axon synapses on its association interneuron in the dorsal horn, and the itch signal is conveyed to the thalamus and then relayed to higher centers.
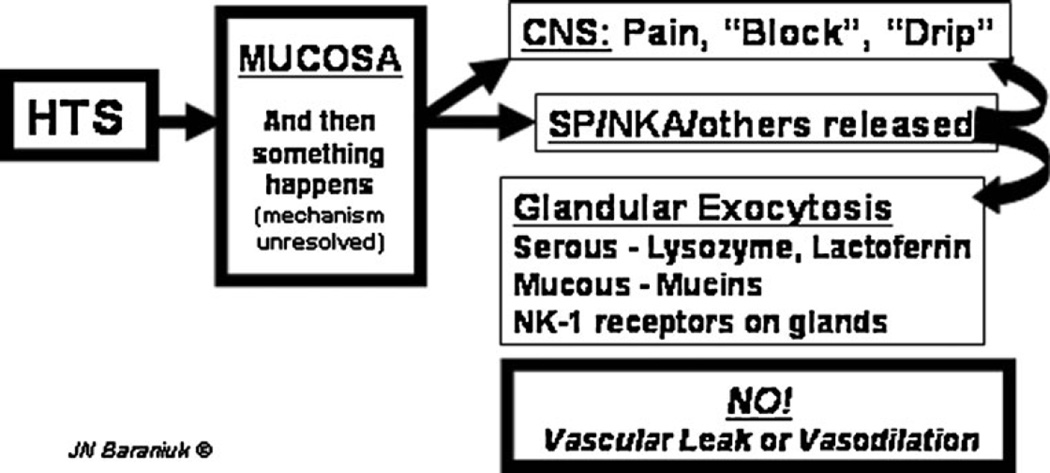
Fig. 3.
Axon response. Hypertonic saline (HTS) stimulates as-yet-unknown mechanisms in the nasal mucosa that lead to nociceptive nerve depolarization. This leads to central nervous system (CNS) perceptions of pain, blockage, or drip and the local mucosal release of substance P (SP), neurokinin A (NKA), and potentially other neurotransmitters. Receptors for these tachykinins are localized to glands. This suggests that tachykinins released by the axon response mechanism near submucosal glands stimulate exocytosis of serous and mucous cell products. No changes in vascular permeability or vasodilation were detected, indicating the absence of a vascular component to human airway axon responses. (Used with permission of James N. Baraniuk, MD)
Davidson and Giesler [45•] proposed that PAR2 and PAR4 G protein-coupled receptors are activated by proteases on C-polymodal nerve terminals. PLC releases lipids that directly activate TRPV1 and other unknown mediators that activate TPRA1 on these neurons. These mediators inhibit voltage-gated potassium channels, preventing repolarization of these neurons and termination of neural depolarization. They propose that CGRP, GRP, substance P, and neurokinin A are colocalized in these neurons as well. However, PAR2/4 neurons are not thought to have large vasodilatory flare reactions. This implies that these itch neurons are not highly branched, do not release the vasodilator CGRP in the periphery, and thus may not possess axon responses. The central axons synapse on a different set of lamina I dorsal horn interneurons that cross the midline and ascend in the spinothalamic tract to the ventrolateral thalamus. The stinging itch signal is then conveyed to the appropriate cerebral cortex regions.
GRP-containing neurons are proposed to convey the sensation of itch to the dorsal horn because GRP receptors are restricted to itch-selective postsynaptic neurons in lamina I of the substantia gelatinosa [46]. Point mutations in GRP receptors in mice lead to reduced scratching behavior in response to pruritogenic stimuli but intact thermal, mechanical, neuropathic, and inflammatory pain responses. The peripheral nerve ending receptors that depolarize GRP-immunoreactive neurons are not defined. In human nasal mucosa, GRP is colocalized with CGRP, substance P, and neurokinin A in afferent nociceptive neurons and has receptors on submucosal glands and the epithelium [47] Because it is a secretagogue, GRP or a related peptide such as neuromedin B may mediate the nociceptive neuron axon response in human nasal mucosa [11].
This dual neuron system may be too simplistic (Table 2). Others have proposed as many as six peripheral neural pathways for itch [48•]. T lymphocytes release interleukin (IL)-31, which can induce itch. It activates the IL-31 receptor heterodimer of IL-31 receptor A plus oncostatin M [49]. Staphylococcal enterotoxin B and superantigen stimulate IL-31 production. The PAR2, PAR4, and MRGPR itch systems may be independent. There is also debate about the suite of receptors on different sets of touch- and heat-sensitive and insensitive pruritinergic neurons. Some of these combinations may reflect studies in rodents, healthy humans, or in the presence of preexisting pruritus or affective states that modulate the patterns of signaling pathways in the nerves, and regions of central sensitization and neurogenic pruritus. Itch affecting normal-appearing skin or mucosa may be mediated by dramatically different neural pathways compared with itch related to lesional skin, as in atopic dermatitis, prurigo nodularis, or allergic rhinitis.
Table 2.
Potential neural pathways and mechanisms of pruritus
| Nerve subtypes | Peripheral activating ligands | Neural receptors |
|---|---|---|
| CMi: C-fibres, unmyelinated, insensitive to mechanical stimulation |
Histamine | H1R |
| Capsaicin and other vanilloids Endocannabinoids? |
TRPV1 | |
| CMH: C-fibres, unmyelinated, mechanical and heat sensitive |
Tryptase | PAR2 |
| Cowhage mucunain protease | (PAR4) | |
| Vespid, dust mite, and other proteases? | TRPV1 | |
| Stinging nettles and other plant toxins | ||
| Capsaicin and other vanilloids | ||
| Heat | ||
| Endocannabinoids? | ||
| C-fibres: mechanical and heat insensitive | Proteases | PAR2/4 |
| C-fibres: not further differentiated | Histamine | H4R? |
| Other endogenous ligands? | ||
| C-fibres: not further differentiated | GRP and related peptides, such as neuromedin B and bombesin |
GRPR |
| C-fibres: not further differentiated | CGRP | CGRP R1, R2, or related receptors |
| Substance P/neurokinin A | NK1, NK2 | |
| C-fibres: not further differentiated | ATP | MRGRP subtypes |
| Chloroquine | ||
| BAM8–22 | TRPA1 | |
| Opioid peptides (γ2-melanocyte-stimulating hormone |
||
| C-fibres: not further differentiated | Interleukin-31 | Interleukin-31 receptor A—oncostatin M heterodimer |
| Aδ fibres? | – | – |
| ? | ? | Heterohexameric TRP protein sensors |
ATP adenosine triphosphate; CGRP calcitonin gene-related peptide; CMH type C mechanical plus heat nociceptors; CMi C mechano-insensitive nerve fibre; GPR G protein–coupled receptor; GRP gastrin-releasing peptide; GRPR GRP receptor; H1R histamine receptor 1; H4R histamine receptor 4; MRGRP MAS-related GPR member P; NK1 neurokinin receptor 1; NK2 neurokinin receptor 2; PAR protease-activated receptor; TRP transient receptor potential; TRPA1 TRP ankyrin 1; TRPV1, TRP vanilloid 1
Pain and Pruritic Cross-Talk: How Does Scratching Stop an Itch?
It should be clear that there now appear to be multiple subtypes of nociceptive and pruritic neurons with distinct ligands for activation based on their specific suite of receptor proteins and sensor ion channels. The interactions between pain and itch can be simplified to some extent by recognizing that the dorsal horn of the spinal cord has several regulatory mechanisms for controlling both sensations. One critical mechanism involves itch-inhibitory interneurons. In general, simultaneous presentation of itch plus nociceptive stimuli leads to pain. This is because the nociceptive neurons stimulate the interneuron to inhibit the postsynaptic transmission of itch. The details of neurotransmitters and other mediators of this process have not been described.
If itch is activated and perceived (insula) without pain, then executive decision-making (anterior cingulate and posterior parietal) regions plan and execute the motor functions (S1 and S2 motor cortex) of scratching. This tactile, mechanical stimulus activates nociceptive neurons that stimulate the dorsal horn itch-inhibitory interneurons, and replaces the conveyed sensation of itch with hedonistic mild pain (insula, prefrontal cortex).
In the event of stronger pain with a secondary itch stimulus, the pain may induce periaqueductal gray regions to release enkephalin, norepinephrine, and serotonin near the dorsal horn postsynaptic nociceptive spinothalamic neuron. This descending antinociceptive system is designed to block the pain. In such a case, the presynaptic nociceptive neurons may not be able to activate the antipruritic interneuron. By default, the sensation conveyed to the thalamus and cortex would be one of pruritus. Administration of opiates can induce generalized pruritus by inhibiting the synaptic transmission of the nociceptive signal plus inhibiting the antipruritic interneuron. Again, itch is the default sensation to be conveyed. More intense pain will overcome this opioid blockage and again inhibit the pruritic pathway.
As more is learned regarding the subtypes of nociceptive and pruritic pathways, more diverse and selective drugs may be developed to more specifically block these sensations. Their benefit will not be to eliminate the original cause of the pain or itch, but rather to palliate the perceptions and affective and cognitive dysfunction that pain and itch induce. Additional therapy will be required to eliminate the peripheral inflammatory, uremic, hepatic, or other inciting pathophysiologic process.
Psychometric Assessments
Although pain is not typically considered a significant symptom in rhinitis, it is a common feature of acute sinusitis. However, the milder forms of nociceptive stimuli mentioned above may have the capacity to lead to significant, prolonged discomfort and morbidity in allergic and nonallergic rhinopathies. One way to bridge these molecular sensations with patient perceptions is to follow the lead of Melzack and Casey [50] in their characterization of three dimensions of pain: 1) “sensory-discriminative” (sense of the intensity, location, quality, and duration of the pain), 2) “affective-motivational” (unpleasantness and urge to escape the unpleasantness), and 3) “cognitive-evaluative” (cognitions such as appraisal, cultural values, distraction, and hypnotic suggestion). Perceptions of pain intensity and unpleasantness may not be related to the magnitude of the nasal discomfort but may be significantly modified by cognitive activities. Distracting activities, suggestion, or placebo may act via their cognitive-evaluative dimension to decrease the appreciation of nasal congestion, “sinus discomfort,” and mid-facial pain. The compassionate physician may take advantage of these affective, motivational, and cognitive aspects to reinforce patient satisfaction and treatment responses. Dissatisfaction or misunderstanding of medical explanations and treatments may have the opposite effect on this sensory dimension, leading to chronic, unremitting rhinitis complaints and disgruntlement.
The same logic may be applied to itch and its systemic consequence in allergic rhinitis: sneezing. Neuropathic itching and pruritus from systemic illnesses may show different patterns of dimensions from atopy.
This type of dimensional nociceptive and itch approach may be augmented by using rhinitis questionnaires analogous to the Multidimensional Pain Inventory [51] to assess the psychosocial state of the individual with chronic rhinitis. Turk and Rudy [51] defined three classes of chronic pain patients: 1) dysfunctional (people who perceived the severity of their pain to be high reported that pain interfered with much of their lives, reported a higher degree of psychological distress caused by pain, and reported low levels of activity), 2) interpersonally distressed (people with a common perception that significant others were not very supportive of their pain problems), and 3) adaptive copers (patients who reported high levels of social support, relatively low levels of pain and perceived interference, and relatively high levels of activity).
Conclusions
Animal and human microneurography of peripheral neurons and dorsal horn neurons is clarifying the multiple and varied subtypes of C-fibres. General patterns are emerging of interactions between populations of these peripheral neurons, interactions with their dorsal horn spinothalamic neurons, the roles of regulatory interneurons, and descending central inhibitory innervation. The patterns of cross-talk and inhibition are beginning to reveal a general coding system that leads to the sensations that we perceive. The multiplicity of mechanisms is a great opportunity to develop novel antagonists, ligands, and ion channel-active drugs that will provide relief of pain and itch. There is every expectation that these will have benefit in airway diseases such as allergic rhinoconjunctivitis and asthma.
Acknowledgments
The figures are used with the permission of the copyright holder. Support was provided by Congressionally Directed Medical Research Program awards W81XWH-07-1-0618 and W81XWH-09-1-0526, and the Georgetown University-Howard University Clinical and Translational Science Award.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Smith ES, Lewin GR. Nociceptors: a phylogenetic view. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;1952:1089–1106. doi: 10.1007/s00359-009-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. This is an excellent summary of one classification of pain-mediating sensor systems and their interactions.
- 3.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Yu FH, Catterall WA. The VGL-Chanome: a protein superfamily specialized for electrical signaling and ionic homeostatis. Science STKE. 2004:re15. doi: 10.1126/stke.2532004re15. www.stke.org/cgi/content/full/sigtrans;2004/253/re15. [DOI] [PubMed] [Google Scholar]
- 6.Belmone C, Viana F. Molecular and cellular limits to somatosensory specificity. Molecular Pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hanlon S, Facer P, Simpson KD, Sandhu G, Saleh HA, Anand P. Neuronal markers in allergic rhinitis: expression and correlation with sensory testing. Laryngoscope. 2007;117:1519–1527. doi: 10.1097/MLG.0b013e3180ca7846. [DOI] [PubMed] [Google Scholar]
- 9.Raap U, Braunstahl GJ. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2010;10:8–13. doi: 10.1097/ACI.0b013e328334f5de. [DOI] [PubMed] [Google Scholar]
- 10.Alenmyr L, Högestätt ED, Zygmunt PM, Greiff L. TRPV1-mediated itch in seasonal allergic rhinitis. Allergy. 2009;64:807–810. doi: 10.1111/j.1398-9995.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 11.Baraniuk JN, Petrie KN, Le U, Tai CF, Park YJ, Yuta A, Ali M, Vandenbussche CJ, Nelson B. Neuropathology in rhinosinusitis. Am J Respir Crit Care Med. 2005;171:5–11. doi: 10.1164/rccm.200403-357OC. [DOI] [PubMed] [Google Scholar]
- 12.Alenmyr L, Greiff L, Andersson M, Sterner O, Zygmunt PM, Högestätt ED. Effect of mucosal TRPV1 inhibition in allergic rhinitis. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00803.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubí-Robles M, Quarta S, Biasio W, Kuner R, Gibbins IL, Kress M, Haberberger RV. Genetic evidence for involvement of neuronally expressed S1P1 receptor in nociceptor sensitization and inflammatory pain. PLoS One. 2011;6:e17268. doi: 10.1371/journal.pone.0017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology. 2011;49:453–457. doi: 10.4193/Rhino11.089. [DOI] [PubMed] [Google Scholar]
- 15. Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2011;24:286–288. doi: 10.1016/j.pupt.2010.11.002. This is an excellent review of cough mechanisms and the potential role of TRPA1 receptors and nerve populations.
- 16.Uta D, Furue H, Pickering AE, Rashid MH, Mizuguchi-Takase H, Katafuchi T, Imoto K, Yoshimura M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci. 2010;31:1960–1973. doi: 10.1111/j.1460-9568.2010.07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 18.Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain. 2010;149:393–405. doi: 10.1016/j.pain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099–1112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. This is an excellent review of purinergic receptors and their functions in the central nervous system.
- 23.Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron pheno-typic switching during peripheral inflammation. Pain. 2011 Nov 30; doi: 10.1016/j.pain.2011.10.042. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassenklöver T, Schwartz P, Schild D, Manzini I. Purinergic signaling regulates cell proliferation of olfactory epithelium progenitors. Stem Cells. 2009;27:2022–2031. doi: 10.1002/stem.126. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Kim SS, Choi JY, Shin JH, Kim JY, Namkung W, Lee JG, Lee MG, Yoon JH. Membrane-specific expression of functional purinergic receptors in normal human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L835–L842. doi: 10.1152/ajplung.00285.2003. [DOI] [PubMed] [Google Scholar]
- 27.Song KS, Kim HJ, Kim K, Lee JG, Yoon JH. Regulator of G-protein signaling 4 suppresses LPS-induced MUC5AC overproduction in the airway. Am J Respir Cell Mol Biol. 2009;41:40–49. doi: 10.1165/rcmb.2008-0280OC. [DOI] [PubMed] [Google Scholar]
- 28.Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, Lethem MI, Donaldson SH, Tarran R. A2B adenosine receptors regulate the mucus clearance component of the lung’s innate defense system. Am J Respir Cell Mol Biol. 2008;39:190–197. doi: 10.1165/rcmb.2007-0450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120(11):3773–3778. doi: 10.1172/JCI43426. This is an important introduction to the patterns of C-fibre interactions that code for different sensations.
- 31.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindstedt F, Johansson B, Martinsen S, Kosek E, Fransson P, Ingvar M. Evidence for thalamic involvement in the thermal grill illusion: an FMRI study. PLoS One. 2011;6(11):e27075. doi: 10.1371/journal.pone.0027075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifert F, Maihöfner C. Representation of cold allodynia in the human brain—a functional MRI study. NeuroImage. 2007;35:1168–1180. doi: 10.1016/j.neuroimage.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Krämer HH, Stenner C, Seddigh S, Bauermann T, Birklein F, Maihöfner C. Illusion of pain: pre-existing knowledge determines brain activation of ‘imagined allodynia. J Pain. 2008;9:543–551. doi: 10.1016/j.jpain.2008.01.340. [DOI] [PubMed] [Google Scholar]
- 35.Boettger MK, Schwier C, Bär KJ. Sad mood increases pain sensitivity upon thermal grill illusion stimulation: implications for central pain processing. Pain. 2011;152:123–130. doi: 10.1016/j.pain.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Braat JP, Mulder PG, Fokkens WJ, van Wijk RG, Rijntjes E. Intranasal cold dry air is superior to histamine challenge in determining the presence and degree of nasal hyperreactivity in nonallergic noninfectious perennial rhinitis. Am J Respir Crit Care Med. 1998;157:1748–1755. doi: 10.1164/ajrccm.157.6.9701016. [DOI] [PubMed] [Google Scholar]
- 37.Nakaya M, Takeuchi N, Kondo K. Immunohistochemical localization of histamine receptor subtypes in human inferior turbinates. Ann Otol Rhinol Laryngol. 2004;113:552–557. doi: 10.1177/000348940411300707. [DOI] [PubMed] [Google Scholar]
- 38.Andrew D, Craig AD. Spinothalamic lamina 1 neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan RP, Szewczyk MT, Jr, Lanosa MJ, Desesa CR, Gianutsos G, Morris JB. Adenosine sensory transduction pathways contribute to activation of the sensory irritation response to inspirited irritant vapors. Toxicol Sci. 2006;93:411–421. doi: 10.1093/toxsci/kfl061. [DOI] [PubMed] [Google Scholar]
- 40.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2XandTRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- 41.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shelley WB, Arthur RP. Studies on cowhage (Mucuna pruriens) and its pruritogenic proteinase, mucunain. AMA Arch Derm. 1955;72:399–406. doi: 10.1001/archderm.1955.03730350001001. [DOI] [PubMed] [Google Scholar]
- 44.Handwerker HO. Microneurography of pruritus. Neurosci Lett. 2010;19(470):193–196. doi: 10.1016/j.neulet.2009.06.092. [DOI] [PubMed] [Google Scholar]
- 45. Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. This is a useful distillation of pruritic mechanisms to two pathways and demonstration of the interactions of pain and itch systems.
- 46.Swain MG. Gastrin-releasing peptide and pruritus: more than just scratching the surface. J Hepatol. 2008;48:681–683. doi: 10.1016/j.jhep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Baraniuk JN, Lundgren JD, Goff J, Peden D, Merida M, Shelhamer J, Kaliner M. Gastrin-releasing peptide in human nasal mucosa. J Clin Invest. 1990;85:998–1005. doi: 10.1172/JCI114577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ständer S, Raap U, Weisshaar E, Schmelz M, Mettang T, Handwerker H, Luger TA. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9(6):456–463. doi: 10.1111/j.1610-0387.2011.07585.x. This is a comprehensive examination of mechanisms of itch, including newer potential pathways.
- 49.Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 50.Melzack R, Casey KL. Sensory, motivational, central control determinants of chronic pain: a new conceptual model. In: Kenshalo DR, editor. The skin senses: proceedings of the first international symposium on the skin senses. Springfield: Thomas; 1968. [Google Scholar]
- 51.Turk DC, Rudy TE. Toward an empirically derived taxonomy of chronic pain patients: integration of psychological assessment data. J Consult Clin Psychol. 1988;56:233–238. doi: 10.1037//0022-006x.56.2.233. [DOI] [PubMed] [Google Scholar]