SUMMARY
Nasal mucosa is innervated by multiple subsets of nociceptive, parasympathetic and sympathetic nerves. These play carefully coordinated roles in regulating glandular, vascular and other processes. These functions are vital for cleaning and humidifying ambient air before it is inhaled into the lungs. The recent recognition of distinct classes of nociceptive nerves with unique patterns of sensory receptors that include seven transmembrane G-protein coupled receptors, new families of transient receptor potential and voltage and calcium gated ion channels, and combinations of neurotransmitters that can be modulated during inflammation by neurotrophic factors has revolutionized our understanding of the complexity and subtlety of nasal innervation. These findings may provide a rational basis for responses to air temperature changes, culinary and botanical odorants (“aromatherapy”), and inhaled irritants in conditions as diverse as idiopathic nonallergic rhinitis, occupational rhinitis, hyposmia, and multiple chemical sensitivity.
Keywords: Nasal mucosa – innervation, Nasal mucosa – physiology, Parasympathetic nervous system – physiology, Sympathetic nervous system – physiology, Ion channels, Neurogenic inflammation
Introduction
Nasal mucosa cleans inhaled air and humidifies, warms or cools the gas to body temperature1. Normal functions of epithelium, glands, and deep venous sinusoids facilitate this cleansing. Their functions depend on local mucosal feedback systems, sensory and autonomic reflexes.
Inhaled air first encounters the vibrissae that protect the anterior nasal valve. This structure is formed by the nasal septum, lateral fleshy wall of the nostril, and anterior tip of the inferior turbinate. Its orifice has a cross-sectional area of 30 to 40 mm2. Air enters by laminar flow at 12 to 18 m/sec, but is rapidly decelerated to turbulent flows of 2 to 3 m/sec in the larger cross-sectional areas posterior to the valve. This change accounts for almost half of the total airflow resistance to the lungs2. The deceleration deposits fine particulate material onto nasal mucus, and promotes the dissolution of water soluble volatile chemicals such as formaldehyde.
Mucus and Epithelium
The epithelial lining fluid is approximately 10 to 15 µm thick. The low viscosity of the sol phase permits efficient, synchronized ciliary motions. Islands of highly adhesive, heavily glycosylated acidic (sialic acid and sulfate) and disulfide bond cross-linked mucins and their associated proteins float on the surface as the gel phase of mucus3. Overall, the mucus consists of 2.5% to 3% mucins, 1% to 2% salts, and 95% water. The fluid has a pH of 5.5 to 6.5. Submucosal gland and plasma proteins extravasated from superficial lamina propria post-capillary venules provide potent antimicrobial protection.
About 1 to 2 L of mucus is produced per day. Free water evaporates to fully humidify the dry inhaled air. The heat lost due to evaporation (enthalpy) is monitored by mucosal cold sensors. Heat loss is proportional to airflow so that the neural output of these cold sensors provides a measure of airway patency. This sensory input enters the brainstem to play a regulatory role for breath-to-breath control of the work of breathing and inspiratory muscle activity. Mucosal vessel autoregulation and countercurrent heating and cooling during inhalation and exhalation maintains the posterior nasal air temperature at ~30 °C and 97% humidity regardless of the inhaled air temperature or absolute humidity.
The cilia skewer the mucin rafts in a coordinated fashion. In the anterior 1 to 2 cm of the nostril, cilia sweep the rafts anteriorly toward the squamous epithelium that lines the nasal orifice. Cilia sweep in a posterior direction in the remainder of the nasal mucosa. Pacemakers in the maxillary sinuses direct ciliary beating upwards against gravity. Cilia beat at about 1,000 times per minute and transport the mucus rafts at 3 to 25 mm per minute4,5. Nearly 100% of particles larger than 4µm in diameter are adsorbed prior to the posterior nasopharynx and are swallowed.
The different populations of epithelial cells secrete proteins such as lipocalin 1 and members of the widely expressed lipocalin subfamily of palate, lung, upper airway, nasal clone (PLUNC) proteins. These bind bacterial polysaccharides and other lipids. Secretoglobins include Clara cell protein (uteroglobin)6. Antimicrobial polypeptides include lacrimal proline rich protein, cystatins, and defensins. Regulatory proteins such as S100A8, S100A9, eotaxin, interleukin (IL)-8, IL-6 and other cytokines may be expressed in normal mucosa and induced under inflammatory conditions. Prolonged exposure to octanal alters lipocalin expression in murine olfactory epithelium7. In contrast, the lipocalin odorant binding protein Ia is down-regulated. Respiratory epithelial goblet cells secrete mucin 5AC. Direct actions of particulate exposure such as changes in osmolarity, toxicity to epithelial or transient leukocytes trafficking through the mucus, and the indirect actions of neurotransmitters released by axon response mechanisms may modulate cellular secretion and the content of epithelial lining fluid.
Post-Capillary Venules
Fenestrated capillaries and post-capillary venules lie beneath the epithelial basement membrane8–10. The latter are important sites for regulation of vascular extravasation, leukocyte adhesion and diapedesis. Endothelial cells have receptors for many inflammatory mediators that promote local vasodilation and edema. The changes in mucosal thickness are small and can only be assessed by microstereometry11. Countercurrent condensation of water from exhaled air may recover 30% of the water and energy used for air humidification during inhalation12. The role(s) of neuropeptides on these vascular processes are controversial because of the relatively low density of innervation in human nasal mucosa. Vascular autoregulation by nitric oxide and hydrogen sulfide (H2S)13 may be relevant to supply plasma water and proteins to the superficial, subepithelial lamina propria region for exudation into the sol phase of epithelial lining fluid.
Submucosal Glands
Invaginated epithelial ducts lead to tubulo-acinar glands. Mucous cells secrete mucin 5B, microsemino-protein-β14 and the sialydated “deleted in malignant brain tumor 1” scavenger receptor glycoprotein15. Mucous cells are located centrally within the acini, and are surrounded by more distal seromucous “demilunes”. Serous cells are the transport location for locally synthesized IgA. Mucosal plasma cells produce Igα1 and Igα2 heavy chains (3:1 ratio) and κ light chains. The IgA heavy chains are bound by joining or J-chains to form dimers. The dimers bind to the polymeric immunoglobulin receptor (PIGR, secretory component)16 on the interstitial surface of serous cells. The macromolecular complex of PIGR with an IgA dimer is transported by pinocytosis across the serous cell and released as secretory IgA (sIgA). sIgA and serous cell lysozyme each account for ~14% of nasal lavage fluid total protein17. Lactoferrin, secretory leukocyte protease inhibitor (SPLI), lipocalins 1 and 2, palate lung nasal carcinoma (PLUNC) family long PLUNC 1 and 2 (LPLUNC1, LPLUNC2), and short PLUNC1 (SPLUNC1, also called PLUNC)18, additional antimicrobial proteins, neutral mucin 8 that lacks sialic acid, and uric acid19 are also exocytosed. Serous cells may also synthesize cytokines that regulate plasma cell IgA production and the antimicrobial and inflammatory actions of intraluminal leukocytes.
Submucosal gland exocytosis is potently regulated by acetylcholine released by parasympathetic reflexes17. Muscarinic M3 receptors are most responsible20. Endothelin and possibly other constrictor factors activate the circumglandular myoepithelial cells that squeeze the exocytosed tooth paste-like glandular mucus into the duct lumen. A population of small diameter sphenopalatine ganglion parasympathetic cells release vasoactive intestinal peptide and nitric oxide that may contribute to arterial, but not venous, dilation21.
Ducts express aquaporin water channels and ion channels that contribute to the flux of water and ions into the ductal lumen. The ducts are surrounded by a cuff of vessels that provide this plasma ultrafiltrate. The water hydrates the mucins and dissolves the less viscous sol phase proteins22. Chloride ion channels are instrumental in regulating water flux. There are four major Cl− channel families: anoctamin (ANO); bestrophins; GABA/glycine receptors; and the cystic fibrosis transmembrane conductance regulator (CFTR)23. CFTR dysfunction leads to a lower aqueous flux, hypertonic sol phase, poorly hydrated mucin complexes, and the highly tenacious, obstructive mucoclots of cystic fibrosis. ANO channels have been recently identified as calcium-activated Cl− channels. This family has 10 mammalian members that express multiple splice variants. Some lack the ion channel loop and are soluble cytoplasmic proteins. ANO1 appears to be critical for epithelial, glandular and ductal fluid secretion; olfactory and phototransduction; neuron, cardiac and vascular cell excitability; and asymmetric cell division that may lead to pseudostratified respiratory epithelial layering or contribute to tumorigenesis. Intriguingly, IL-4 regulates the expression of the ANO1 protein, TMEM16A, when transfected into the HEK-293 human epithelial cell line24. ANO1 regulation by IL-4 suggests that TH2 lymphocytes contribute to both acquired mucosal immune reactions as well as playing a critical role in the glandular secretion of innate immune antimicrobial proteins.
Pathological glandular hypertrophy represents one subtype of rhinosinusitis25. It remains to be seen if this histological finding contributes to the poorly defined syndrome of “turbinate hypertrophy”.
The Nasal Erectile Apparatus
Venous sinusoids deep in the mucosa determine the thickness of this tissue, and so the cross-sectional area of airflow through the bony box of the nose8–10. Arteriovenous anastomoses carry blood to the sinusoids. Calcitonin gene related peptide receptors are located on these anastomoses suggesting that this potent vasodilator induces an influx of blood with engorgement of the sinusoids, thickening of the mucosa, and decreased airway patency26,27. Histamine, bradykinin, prostaglandins and leukotrienes may also lead to vasodilation in inflammatory conditions.
Neuropeptide tyrosine (NPY) is a vasoconstrictor that also has receptors on arterioles and arteriovenous anastomoses28. NPY is released from a subset of noradrenergic sympathetic neurons. Constriction of the anastomoses and sinusoidal walls leads to deflation of the sinusoids. NPY and norepinephrine may have vasodilator functions on the throttle veins that regulate the flow of blood out of the sinusoids. The loss of blood volume combined with tissue elastic recoil thins the mucosa and increases nasal patency. All subtypes of α1 and α2 adrenergic receptors are expressed on these vessels, although α2C receptors may be most important for vasoconstriction29. Disruption of sympathetic pathways leads to obstinate vasodilation of the deep sinusoidal structures, mucosal thickening, and the loss of nasal patency. Persistent vasodilation follows the disruption of sympathetic innervation following cerebrovascular strokes as in Horner’s syndrome, α-adrenergic antagonist antihypertensives, and chronic overuse of nasal sympathomimetics (rhinitis medicamentosa) with severe rebound vasodilation and obstruction to nasal airflow. Rhinitis medicamentosa has been difficult to test experimentally, and so may occur in a specific subpopulation of humans with a diathesis for this sympathetic-vascular dysfunction. Topical NPY has been reported to block nasal reactions to topical allergen provocations in humans30.
Endothelins are also potential endogenous vasoconstrictors31. Myoepithelial cells form a contractile net around submucosal glands. They have endothelin receptors that may mediate contraction that squeezes the glands to extrude the exocytosed mucus out of the gland ducts. Allergic inflammation appears to upregulate receptors for endothelin-1 and bradykinin on afferent neurons, since both can stimulate nociceptive-parasympathetic reflex arcs in allergic rhinitis, but not in healthy subjects32,33. Endothelin-1 may act through ETB receptors to maintain a proliferative stem cell pool in the developing olfactory epithelium34. This system may be a necessary precursor to the commitment of these cells to the gonadotropin releasing hormone neuronal migratory pathway.
These processes come together in the normal nasal cycle. The cross-sectional area for airflow undulates inversely between the left and right nostrils on a 1- to 4-h cycle35,36. This brainstem and autonomic biorhythm is poorly understood. However, its effects may be magnified or nullified in infectious, allergic, and nonallergic irritant conditions that increase lamina propria thickness and secreted glandular mucus volumes.
Type C Neurons and Sensory Receptors
Irritants such as carbon dioxide, powdered mannitol, adenosine, hypertonic saline (HTS) solution and other nociceptive agents stimulate trigeminal neural responses that are distinct from olfactory sensations. Inhalation of CO2, a relatively specific activator of trigeminal nociceptive nerves, activates limited brain cortical regions such as the cingulate gyrus37. H2S stimulates olfactory neurons that activate frontal, entorhinal, occipital, and cerebellar cortical regions. Capsaicin activates laryngeal and pharyngeal vagal and glossopharyngeal afferents that activate the urge to cough. Functional magnetic resonance imaging demonstrates activation of the primary and visceral insular sensory cortices; and the anterior midcingulate and orbitofrontal cortices responsible for planning and executive functions such as the execution or repression of an active cough via subsequent actions on the supplementary motor area and cerebellum38.
HTS sprayed onto the inferior turbinate generates a rapid-onset, sharp burning sensation (“first pain”) that is likely due to activation of fast conducting, thinly myelinated Aδ nerve fibers39. A paresthetic, “second pain” tingling sensation is then appreciated. Both the intensity of the 1st pain and duration of the 2nd pain were correlated to the HTS dose40. Substance P was released during the initial three minutes, and was followed by a strong, HTS dose-related secretion from glands between 3 to 5 minutes. Ipratropium did not block the response indicating that central parasympathetic reflexes were not recruited. Nasal lavage fluid albumin concentrations did not change indicating no alteration of vascular permeability in normal, acute allergic rhinitis, or acute and chronic rhinosinusitis subjects41. Tachykinin NK1 receptors were localized to gland acini suggesting that substance P or co-localized neurokinin A released from type C neurons near glands may have initiated glandular exocytosis40. Gastrin releasing peptide (GRP) may also play a role since it is co-localized in afferent nociceptive neurons and has receptors on submucosal glands and the epithelium42. A population of GRP-containing neurons may also mediate the sensation of itch since GRP receptors are restricted to lamina I of the dorsal spinal cord43. Point mutations in GRP receptors in mice lead to reduced scratching behavior in response to pruritogenic stimuli, but intact thermal, mechanical, neuropathic and inflammatory pain responses.
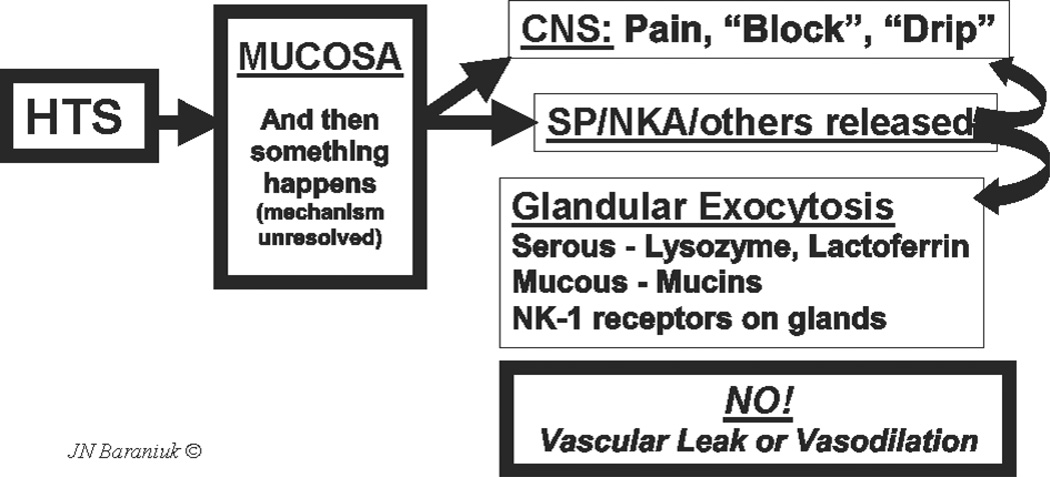
These findings suggest that HTS-induced axon responses in humans induce copious glandular exocytosis with no significant vascular component (Fig. 1). Thus, human nasal mucosal neurogenic inflammation is a rapid-onset mucosal defense mechanism designed to stimulate secretion of submucosal gland mucins and antimicrobial proteins into the nasal epithelial lining fluid. These proteins will replenish gel and sol phase components to aid in the adsorption of inhaled irritants and particulate material and to kill or neutralize microbes and their toxins.
Fig. 1.
Axon response. Hypertonic saline (HTS) stimulates as yet unknown mechanisms in the nasal mucosa that lead to nociceptive nerve depolarization. This leads to central nervous system perceptions of pain, blockage or drip, and the local mucosal release of substance P (SP), neurokinin A (NKA) and potentially other neurotransmitters. Receptors for these tachykinins are localized to glands. This suggests that tachykinins released by the axon response mechanism near submucosal glands stimulate exocytosis of serous and mucous cell products. No changes in vascular permeability or vasodilation were detected indicating the absence of a vascular component to human airway axon responses.
Histamine nasal provocation causes itch, vascular permeability, and cholinergic-reflex mediated glandular secretion. These effects are consistent with allergic rhinoconjunctivitis. Histamine H1 receptors have been localized to a population of very narrow diameter neurons that are localized to distinct spinal cord dorsal horn regions, ascending pathways, thalamus, and thalamocortical radiations44. This route is distinct from the neural pathways activated by capsaicin-sensitive neurons. Histamine H3 and H4 receptors on sensory and autonomic neurons act as inhibitory autoreceptors to hyperpolarize neurons and prevent their depolarization45. H2 receptors are present on epithelium and glands. Histamine-induced axon responses have not been clearly demonstrated in human airway mucosa.
The population of neurons that are solely “itch specific” may be small. Other H1-receptor bearing neurons appear to express the capsaicin receptor that is responsible for the burning heat sensation. This multimodal sensory receptor and ion channel is classified as the transient receptor potential vanilloid 1 (TRPV1) protein. Other distinct combinations of proteins such as purinergic P2X receptors and acid sensing ion channel 3 (ASIC3) may also be present on subsets of these neurons that would then respond to ATP, adenosine, H+, K+, and Ca+2 that may be released by cellular injury or during inflammation46. ASIC can be blocked by amiloride and aspirin. Neurons with TRPV1, ASIC3 and P2X receptors may have particularly important functions in the detection of visceral pain or other conditions47. Inflammation with the release of leukotriene B4, nerve growth factor (TrkA receptor), brain derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4; TrkB receptor), and NT-3 (TrkC receptor) can lead to significant neurotransmitter, sensory receptor, and inhibitory autoreceptor plasticity that alters the sensations or other functions within sets of neuron subpopulations48,49.
Chlorine gas is another trigeminal irritant. Low dose chlorine does not induce any sensory perception, and can induce neurogenic nasal airflow obstruction without neuropeptide release50. This suggests that a local mucosal mechanism may be involved.
Cold dry air inhalation causes a dose dependent obstruction of nasal airflow in humans with idiopathic non-allergic rhinitis51. Healthy controls do not respond. Bradykinin also had no effect in idiopathic rhinitis52. Again, this was distinct from the nasal hyperresponsiveness and recruitment of parasympathetic reflexes found in allergic rhinitis33.
Voltage-Gated Ion Channel Protein Family
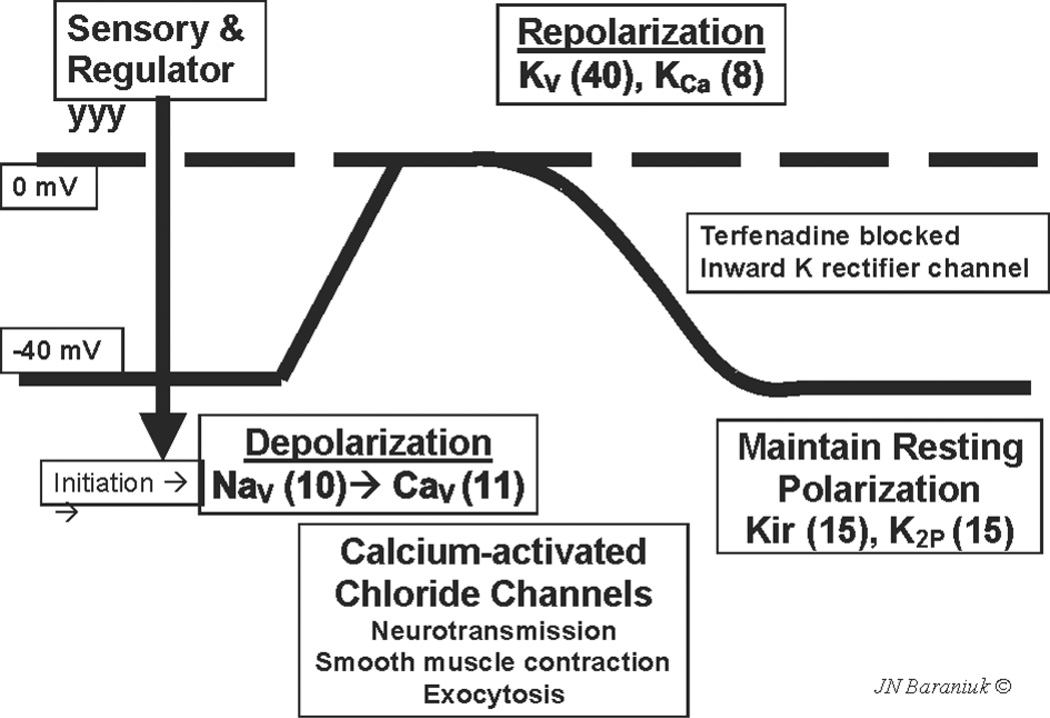
TRPV1 was the first of a family of 143 human ion channels to have its activating ligands characterized53. Other members of this family include other sensors; voltage-gated sodium channels responsible for cell depolarization; voltage-gated calcium channels that mediate full depolarization and that also induce the functions of neuron, gland, muscle and other electro-excitable cells; and potassium channels that repolarize and maintain the resting membrane potential of these cells54,55 (Fig. 2). The basic motif of these transmembrane proteins is a single extracellular loop that dips into, but does not cross, the plasma membrane. Transmembrane alpha-helices flank the intramembrane loop. Tetramers of these proteins form pores that permit the regulated flow of specific ions into cells. There is the potential for heterotetramers to form that may have modified responses compared to homotetramers. This greatly extends the ranges of temperature, osmolarity, membrane fluidity, and chemical exposures that may activate these diverse heterotetramers.
Fig. 2.
Schematic nerve depolarization curve. Resting membrane potential is maintained at about −40 mV by potassium channels (Kir, K2P). Sensory and regulatory channels initiate depolarization. Voltage gated sodium channels (Nav) and calcium channels (Cav) induce full depolarization to 0 mV. The cell is repolarized by voltage and calcium activated potassium channels (Kv and KCa). The numbers of recognized members for each type of channel are in brackets. Used with permission of the copyright holder.
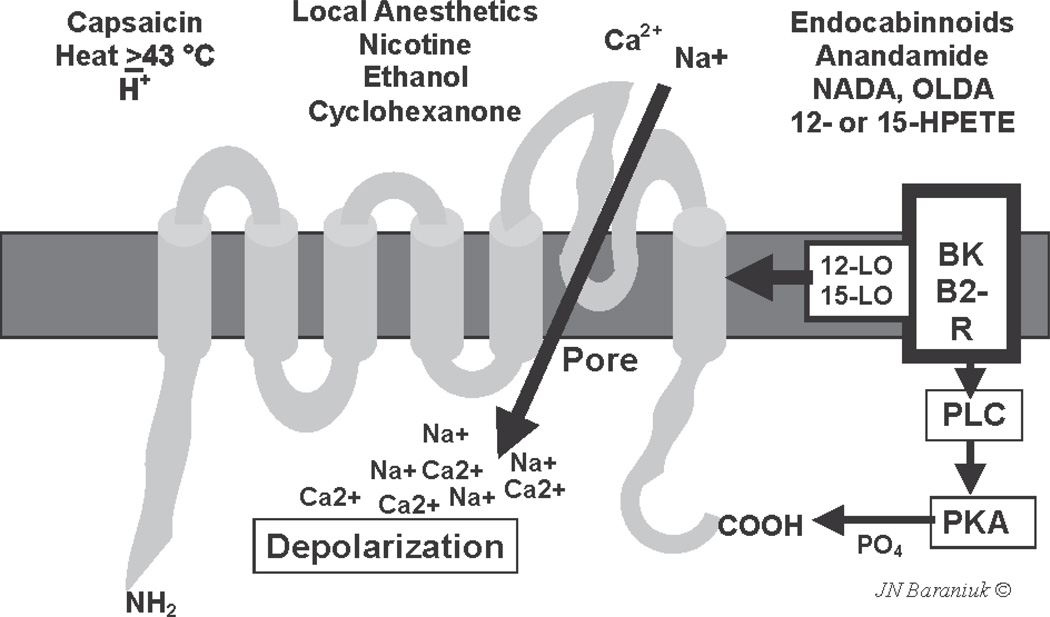
TRPV1 is a highly promiscuous polymodal chemoreceptor (Fig. 3). Capsaicin, temperatures above 43 °C, local anesthetics, nicotine, ethanol, clotrimazole, the endocabinnoid family of arachidonic acid metabolites, and products of 12- and 15-lipoxygenases may all activate specific regions of TRPV156,57. The peptide regions involved have been well illustrated using protein point mutation studies. Specific intracellular phosphokinases can phosphorylate the C-terminal and alter the sensitivity of the ion channel to these stimuli. Opening the tetrameric ion pore allows a rapid influx of Na+ and Ca2+56. This influx will initiate depolarization that is accelerated by both voltage- and calcium-dependent sodium and calcium ion channels.
Fig. 3.
Schematic TRPV1 protein with 6 transmembrane regions and the intramembrane loop that forms the cation pore. Various ligands are shown. Bradykinin binds to its B2 receptor and activates 12- and 15-lipoxygenase (12-LO, 125-LO) and phospholipase C (PLC) and protein kinase A (PKA) that phosphorylates the carboxy-terminus of the protein. Used with permission of the copyright holder.
TRPV1 is also present on airway epithelium and keratinocytes. Expression of TRPV1 and probably other, related sensors on epithelium greatly expands the potential for complex interactions between these cells, intermediate messenger molecules, and Type C neurons. Human 293t embryonic kidney cells transfected with TRPV1 exhibited calcium ion influx indicative of depolarization when stimulated with capsaicin and cyclohexanone58. Both wild type and transfected cells responded to acetic acid, R-(−)-carvone and S-(+)-carvone indicating the presence of at least one other distinct sensor system in these cells. However, other trigeminal irritants including amyl acetate, toluene, benzaldehyde, (−)-nicotine, and R-(+)-limonene had no effect, indicating that their irritant properties must be mediated by additional sets of sensor systems.
TRP Thermometer and Aromatherapy
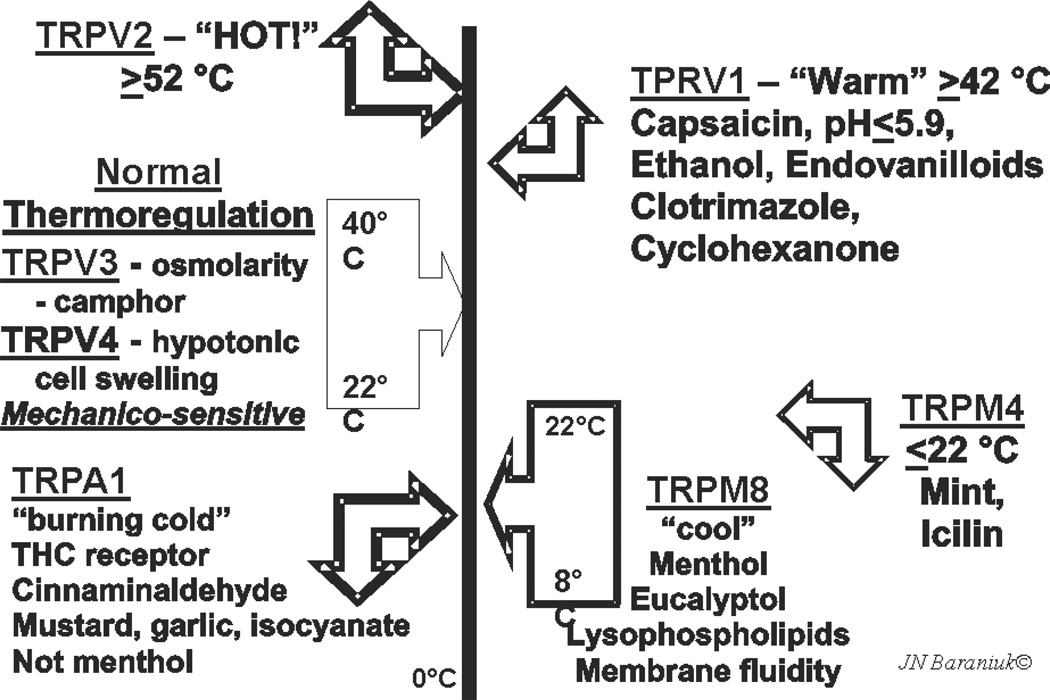
TRPV1 is the only capsaicin-sensitive channel. However, other culinary spices have played major roles in the discovery of TRP protein families54,55. Many of these proteins are multimodal and respond to specific temperature and osmolality ranges. A “TRP thermometer” can be constructed to illustrate this diversity (Fig. 4). TRPV3 and TRPV4 respond to ambient temperatures. They are also mechanicosensitive and are activated by changes in osmotic cell swelling. Temperatures ≥42 °C activate TRPV1, while TRPV2 responds to dangerously high, tissue damaging temperatures ≥52 °C. Lower temperatures are sensed by TRP melanostatin 4 (TRPM4). This receptor also responds to mint and the odorless, topical coolant icilin found in many cutaneous ointments. TRPM8 menthol receptors respond to temperatures of about 8 °C to 22 °C. TRPM8 are sensitized by lysophospho-inositol, -choline, and -serine. These lysophospholipids determine overall membrane fluidity. The apparent temperature sensitivity may be a reflection of the “melting” of the lipid bilayer by higher temperatures that will also be reflected by the membrane constituents. TRPM8-induced neurotransmission may inform the brain about changes in the relative “stiffness” of the membrane. “Very cold” temperatures, mustard oil and garlic isocyanate compounds, and tetrahydrocannibinol activate the TRPA1 (ankryn) receptor and “cold pain”. Stimulation of muscarinic M1 receptors and their G-coupled proteins activates phospholipase C and a phosphokinase that can increase the sensitivity of TRPA1. Responses of trigeminal cold receptors are modulated by bis-phosphoinositol phosphate (PIP2) and are abolished by phospholipase A2 inhibitors that prevent the release of arachidonic acid59.
Fig. 4.
TRP thermometer and aromatherapy in the trigeminal chemosensory nervous system. Used with permission of the copyright holder.
Voltage-Gated Sodium Channels: Na(v)
Once nerves or other electrosensitive cells are activated, the wave of depolarization is conducted via voltage-gated sodium and other channels. The Na(v) family is of interest for their putative roles in pain transmission60. Genomic studies have linked mutation of the Na(v)1.1 protein to familial hemiplegic migraine headaches. A point mutation in Na(v)1.7 (F1449V) leads to primary erythermalgia, a congenital disorder of severe pain and flushing61. This protein is expressed in dorsal root ganglia with Na(v)1.8. The mutant causes a gain of function phenotype with hyperexcitability of nociceptive nerves62. In contrast, sympathetic neurons do not express Na(v)1.8. In these cells, the mutant ion channel leads to a loss of function situation with decreased sympathetic activity. This selectivity of Na(v)1.8 for dorsal root ganglion cells may explain why its antagonists appear to be highly selective analgesics.
Conclusions
Many of the functions of the nasal mucosa and their regulation by neural mechanisms are reasonably well understood63. The regulation of these functions by distinct subclasses of neurons is a new and exciting development. The exchanges between neurotransmitters and cellular signaling molecules may generate bilateral trophic effects that regulate epithelial cell, superficial lamina propria vascular, submucosal gland, deep venous sinusoid, and neural activities. In turn, neurotransmitter release is likely to have effects on immune responses given the high density of neuropeptide receptors on cells such as lymphocytes. The recent recognition of the many different classes of sensor proteins, their wide ranges of ligands, promiscuous interactions between the transient receptor potential proteins, and expression of these proteins on airway epithelial cells opens new possibilities for deeper understanding of mucosal neuron activation, electrosensitive cell depolarization, and the potential that new, highly novel analgesics may modulate dysfunctional actions and symptoms in rhinosinusitis and other airway syndromes. These new concepts also provide new insights into mucosal axon responses. These rapidly recruited neural responses lead to glandular secretion in human nasal mucosa. The discovery that several aspects of mucosal innate immunity such as the calcium-activated chloride channel ANO1 can be induced by IL-4 supplements the existing hypothesis that the primary function of TH2 lymphocytes is mucosal defense against extracellular pathogens too large to be phagocytosed.
Acknowledgment
The figures have been used with the permission of the copyright holder. Supported by Public Health Service Awards 1 RO1 ES015382 and P50 DC006760, and Department of Defense Award W81XWH-07-1-0618.
Abbreviations
- ANO
anoctamin
- ASIC
acid sensing ion channel
- CFTR
cystic fibrosis transmembrane regulator
- GRP
gastrin releasing peptide
- H2S
hydrogen sulfide
- HTS
hypertonic saline
- Ig
immunoglobulin
- IL
interleukin
- Na(v)
voltage-activated sodium channel
- NK1
neurokinin 1 receptor
- NPY
neuropeptide tyrosine
- PIGR
polymeric immunoglobulin receptor
- PLUNC
palate lung nasal carcinoma
- SLPI
secretory leukocyte protease inhibitor
- TRP
transient receptor potential
- TRPV1
transient receptor potential vanilloid 1 (capsaicin receptor)
References
- 1.Naclerio RM, et al. Observations on the ability of the nose to warm and humidify inspired air. Rhinology. 2007;45:102–111. [PubMed] [Google Scholar]
- 2.Swift DL, Proctor DF. Access of air to the respiratory tract. In: Brain JD, Proctor DF, Reid LM, editors. Respiratory defense mechanisms. New York, NY: Marcel Dekker; 1977. [Google Scholar]
- 3.Widdicombe J. The physiology of the nose. Clin Chest Med. 1986;7:159–170. [PubMed] [Google Scholar]
- 4.Cole P. Pathophysiology and treatment of airway mucociliary clearance. A moving tale. Minerva Anestesiol. 2001;67:206–209. [PubMed] [Google Scholar]
- 5.Stannard W, O’Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med. 2006;19:110–115. doi: 10.1089/jam.2006.19.110. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds SD, et al. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med. 2002;166:1498–1509. doi: 10.1164/rccm.200204-285OC. [DOI] [PubMed] [Google Scholar]
- 7.Barbour J, et al. New insight into stimulus-induced plasticity of the olfactory epithelium in Mus musculus by quantitative proteomics. J Proteome Res. 2008;7:1594–1605. doi: 10.1021/pr7005796. [DOI] [PubMed] [Google Scholar]
- 8.Cauna N, Hinderer KH. Fine structure of blood vessels of the human nasal respiratory mucosa. Ann Otol Rhinol Laryngol. 1969;78:865–879. doi: 10.1177/000348946907800418. [DOI] [PubMed] [Google Scholar]
- 9.Cauna N. Electron microscopy of the nasal vascular bed and its nerve supply. Ann Otol Rhinol Laryngol. 1970;79:443–450. doi: 10.1177/000348947007900303. [DOI] [PubMed] [Google Scholar]
- 10.Cauna N. Blood and nerve supply to the nasal lining. In: Proctor DF, Anderson IB, editors. The nose: upper airway physiology and the atmospheric environment. Amsterdam: Elsevier Biomedical Press BV; 1982. pp. 45–69. [Google Scholar]
- 11.Hallen H, Graf P. Evaluation of rhinostereometry compared with acoustic rhinometry. Acta Otolaryngol. 1999;119:921–924. doi: 10.1080/00016489950180298. [DOI] [PubMed] [Google Scholar]
- 12.Scherer PW, Hahn II, Mozell MM. The biophysics of nasal airflow. Otolaryngol Clin North Am. 1989;22:265. [PubMed] [Google Scholar]
- 13.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiological vasorelaxant: hypertension in mice with deletion of cystathionine ã-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogushi F, et al. Identification and localization of immunoglobulin binding factor in bronchoalveolar lavage fluid from healthy smokers. Am J Respir Crit Care Med. 1995;152:2133–2137. doi: 10.1164/ajrccm.152.6.8520786. [DOI] [PubMed] [Google Scholar]
- 15.Kang W, Reid KB. DMBT1, a regulator of mucosal homeostasis through the linking of mucosal defense and regeneration? FEBS Lett. 2003;540:21–25. doi: 10.1016/s0014-5793(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 16.Brandtzaeg P. Immune functions of human nasal mucosa and tonsils in health and disease. In: Bienenstock J, editor. Immunology of the lung and upper respiratory tract. New York: McGraw-Hill; 1984. pp. 28–95. [Google Scholar]
- 17.Baraniuk JN. Mechanisms of rhinitis. In: Lasley MV, Altman LC, editors. Rhinitis. Immunology and Allergy Clinics of North America, 20. Philadelphia: WB Saunders; 2000. May, pp. 245–264. [Google Scholar]
- 18.Casado B, Viglio S, Baraniuk JN. Proteomics of sinusitis nasal lavage fluid. In: Thongboonkerd V, editor. Proteomics of human body fluids: principles, methods, and applications. New York: Humana Press; 2006. [Google Scholar]
- 19.Peden DB, et al. Uric acid is a major antioxidant in human nasal airway secretions. Proc Natl Acad Sci U S A. 1990;87:7638–7642. doi: 10.1073/pnas.87.19.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baraniuk JN, Kaliner MA, Barnes PJ. Localization of m3 muscarinic receptor mRNA in human nasal mucosa. Am J Rhinol. 1992;6:145–148. [Google Scholar]
- 21.Riederer A, et al. Histochemical and immunocytochemical study of nitrergic innervation in human nasal mucosa. Ann Otol Rhinol Laryngol. 1999;108:869–875. doi: 10.1177/000348949910800909. [DOI] [PubMed] [Google Scholar]
- 22.Assanasen P, et al. Natural and induced allergic responses increase the ability of the nose to warm and humidify air. J Allergy Clin Immunol. 2000;106:1045–1052. doi: 10.1067/mai.2000.110472. [DOI] [PubMed] [Google Scholar]
- 23.Hartzell HC. CaCl-ing channels get the last laugh. Science. 2008;322:534–535. doi: 10.1126/science.1165668. [DOI] [PubMed] [Google Scholar]
- 24.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 25.Malekzadeh S, et al. Density of middle turbinate subepithelial mucous glands in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127:190–195. doi: 10.1067/mhn.2002.126800. [DOI] [PubMed] [Google Scholar]
- 26.Baraniuk JN, et al. Calcitonin gene related peptide (CGRP) in human nasal mucosa. Am J Physiol. 1990;258:L81–L88. doi: 10.1152/ajplung.1990.258.2.L81. [DOI] [PubMed] [Google Scholar]
- 27.Rinder J. Sensory neuropeptides and nitric oxide in nasal vascular regulation. Acta Physiol Scand Suppl. 1996;632:1–45. [PubMed] [Google Scholar]
- 28.Baraniuk JN, et al. Neuropeptide Y (NPY) in human nasal mucosa. Am J Respir Cell Mol Biol. 1990;3:165–173. doi: 10.1165/ajrcmb/3.2.165. [DOI] [PubMed] [Google Scholar]
- 29.Stafford-Smith M, Bartz R, Wilson K, Baraniuk JN, Schwinn DA. Alpha-adrenergic mRNA subtype expression in the human nasal turbinate. Can J Anaesth. 2007;54:549–555. doi: 10.1007/BF03022319. [DOI] [PubMed] [Google Scholar]
- 30.Lacroix JS, Mosimann BL. Attenuation of allergen-evoked nasal responses by local pretreatment with exogenous neuropeptide Y in atopic patients. J Allergy Clin Immunol. 1996;98:611–616. doi: 10.1016/s0091-6749(96)70095-7. [DOI] [PubMed] [Google Scholar]
- 31.Baraniuk JN, et al. Endothelin and the airway mucosa. Pulm Pharmacol Ther. 1998;11:113–123. doi: 10.1006/pupt.1998.0125. [DOI] [PubMed] [Google Scholar]
- 32.Riccio MM, et al. Effects of intranasal administration of endothelin-1 to allergic and nonallergic individuals. Am J Respir Crit Care Med. 1995;152:1757–1764. doi: 10.1164/ajrccm.152.6.8520734. [DOI] [PubMed] [Google Scholar]
- 33.Baraniuk JN, et al. Ibuprofen augments bradykinin-induced glycoconjugate secretion by human nasal mucosa in vivo. J Allergy Clin Immunol. 1992;89:1032–1039. doi: 10.1016/0091-6749(92)90226-r. [DOI] [PubMed] [Google Scholar]
- 34.Romanelli RG, et al. Role of endothelin-1 in the migration of human olfactory gonadotropin-releasing hormone-secreting neuroblasts. Endocrinology. 2005;146:4321–4330. doi: 10.1210/en.2005-0060. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa M, Kern EB. The human nasal cycle. Mayo Clin Proc. 1977;52:28. [PubMed] [Google Scholar]
- 36.Kennedy DW, et al. Physiologic mucosal changes within the nose and the ethmoid sinus: imaging of the nasal cycle by MRI. Laryngoscope. 1988;98:928. doi: 10.1288/00005537-198809000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Bensafi M, et al. Neural coding of stimulus concentration in the human olfactory and intranasal trigeminal systems. Neuroscience. 2008;154:832–838. doi: 10.1016/j.neuroscience.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 38.Mazzone SB, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- 39.Dray A, Urban L, Dickenson A. Pharmacology of chronic pain. TiPS. 1994;15:190–197. doi: 10.1016/0165-6147(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 40.Baraniuk JN, et al. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med. 1999;160:655–662. doi: 10.1164/ajrccm.160.2.9805081. [DOI] [PubMed] [Google Scholar]
- 41.Baraniuk JN, et al. Neuropathology in rhinosinusitis. Am J Respir Crit Care Med. 2005;171:5–11. doi: 10.1164/rccm.200403-357OC. [DOI] [PubMed] [Google Scholar]
- 42.Baraniuk JN, et al. Gastrin-releasing peptide in human nasal mucosa. J Clin Invest. 1990;85:998–1005. doi: 10.1172/JCI114577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swain MG. Gastrin-releasing peptide and pruritus: more than just scratching the surface. J Hepatol. 2008;48:681–683. doi: 10.1016/j.jhep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Andrew D, Craig AD. Spinothalamic lamina 1 neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 45.Nakaya M, Takeuchi N, Kondo K. Immunohistochemical localization of histamine receptor subtypes in human inferior turbinates. Ann Otol Rhinol Laryngol. 2004;113:552–557. doi: 10.1177/000348940411300707. [DOI] [PubMed] [Google Scholar]
- 46.Vaughan RP, et al. Adeonsine sensory transduction pathways contribute to activation of the sensory irritation response to inspirited irritant vapors. Toxicol Sci. 2006;93:411–421. doi: 10.1093/toxsci/kfl061. [DOI] [PubMed] [Google Scholar]
- 47.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- 48.Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp. 2008;289:222–233. doi: 10.1002/9780470751251.ch18. [DOI] [PubMed] [Google Scholar]
- 49.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 50.Shusterman D, et al. Chlorine inhalation procedure produces nasal airflow limitation in allergic rhinitis subjects without evidence of neuropeptide release. Neuropeptides. 2004;38:351–358. doi: 10.1016/j.npep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Braat JP, et al. Intranasal cold dry air is superior to histamine challenge in determining the presence and degree of nasal hyperreactivity in nonallergic noninfectious perennial rhinitis. Am J Respir Crit Care Med. 1998;157:1748–1755. doi: 10.1164/ajrccm.157.6.9701016. [DOI] [PubMed] [Google Scholar]
- 52.Sheahan P, et al. Subjects with non-allergic non-infectious perennial rhinitis do not show nasal hyper-responsiveness to bradykinin. Eur Arch Otorhinolaryngol. 2007;264:33–37. doi: 10.1007/s00405-006-0161-4. [DOI] [PubMed] [Google Scholar]
- 53.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 54.Yu FH, Catterall WA. The VGL-Chanome: a protein superfamily specialized for electrical signaling and ionic homeostatis. Science STKE. 2004 doi: 10.1126/stke.2532004re15. re15 www.stke.org/cgi/content/full/sigtrans;2004/253/re15. [DOI] [PubMed]
- 55.Belmone C, Viana F. Molecular and cellular limits to somatosensory specificity. Molecular Pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 57.Silver WL, et al. TRPV1 receptors and nasal trigeminal chemesthesis. Chem Senses. 2006;31:807–812. doi: 10.1093/chemse/bjl022. [DOI] [PubMed] [Google Scholar]
- 58.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophosopholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waxman SG, Dib-Haji SD. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11:555–562. doi: 10.1016/j.molmed.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Waxman SG, Dib-Haji SD, Lui S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypo-excitability in different types of neurons. Proc Natl Acad Sci USA. 2006;103:8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tai C-F, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol. 2002;2:11–19. doi: 10.1097/00130832-200202000-00003. [DOI] [PubMed] [Google Scholar]