Abstract
Objective
Few studies use longitudinal data to identify predictors of colorectal cancer screening (CRCS). We examined predictors of (1) initial CRCS during the first year of a randomized trial, and (2) repeat CRCS during the second year of the trial among those that completed FOBT in Year 1.
Methods
The sample comprised 1247 participants of the Systems of Support to Increase Colorectal Cancer Screening (SOS) Trial (Group Health Cooperative, August 2008 to November 2011). Potential predictors of CRCS were identified with logistic regression and included sociodemographics, health history, and validated scales of psychosocial constructs.
Results
Prior CRCS (OR 2.64, 95% CI 1.99–3.52) and intervention group (Automated: OR 2.06 95% CI 1.43–2.95; Assisted: OR 4.03, 95% CI 2.69–6.03; Navigated: OR 5.64, 95% CI 3.74–8.49) were predictors of CRCS completion at Year 1. For repeat CRCS at Year 2, prior CRCS at baseline (OR 1.97, 95% CI 1.25–3.11), intervention group (Automated: OR 9.27, 95% CI 4.56–18.82; Assisted: OR 11.17, 95% CI 5.44–22.94; Navigated: OR 13.10, 95% CI 6.33–27.08), and self-efficacy (OR 1.32, 95% CI 1.00–1.73) were significant predictors.
Conclusion
Self-efficacy and prior CRCS are important predictors of future screening behavior. CRCS completion increased when access barriers were removed through interventions.
Keywords: Colorectal cancer, Cancer screening, Longitudinal study, Behavioral intervention research
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed and leading cause of cancer death in the U.S. (Siegel et al., 2013). Despite the evidence that colorectal cancer screening (CRCS) with the fecal occult blood test (FOBT), colonoscopy, and/or sigmoidoscopy decreases CRC incidence and mortality (Zauber et al., 2008), screening rates remain below target levels (Centers for Disease Control, Prevention (CDC), 2012).
Interventions that promote the uptake of CRCS must address modi-fiable determinants or predictors of screening behavior. However, most of the literature is cross-sectional studies of correlates of past screening. Few studies use longitudinal data to examine prospective predictors of CRCS (McQueen et al., 2007). Relying on results from cross-sectional studies when designing interventions may overlook important factors because there may be differences in correlates and predictors of cancer screening behaviors (Bastani et al., 1996; McQueen et al., 2007). For example, a study comparing cross-sectional and prospective predictors of mammography found a number of important variables related to future screening (i.e., predictors) that were not associated with past screening (i.e., correlates) (Bastani et al., 1996). Many of the significant predictors were psychosocial or attitudinal variables. Similarly, for CRCS, a study of initiation and maintenance revealed that there were differences in correlates and predictors (McQueen et al., 2007). The results of these studies call into question the usefulness of targeting or tailoring interventions based on cross-sectional data. A better understanding of the prospective predictors of CRCS may inform development of interventions that target those behaviors.
There also has been limited research on repeat CRCS. Although a number of trials to evaluate the efficacy of CRCS have reported rates of repeat screening (Hardcastle et al., 1986, 1989, 1996; Mandel et al., 1999), very few studies have examined psychosocial predictors of regular screening. Studies of repeat FOBT conducted in community settings report completion rates between 14 and 54% among persons who had previously completed an FOBT on schedule (Fenton et al., 2010; Gellad et al., 2011; Liss et al., 2013; Myers et al., 1993). Receipt of a prior preventive health examination, younger age, lesser comorbidity, and a greater number of physician visits were significantly associated with repeat CRCS (Fenton et al., 2010; Liss et al., 2013; Myers et al., 1993). We found only one study that examined social cognitive factors in relation to repeat CRCS (Duncan et al., 2014). On-schedule screening is particularly important for FOBT because its effectiveness may be reduced when patients do not adhere to a regular schedule (Hardcastle et al., 1986, 1989, 1996; Mandel et al., 1999).
To address these gaps in the literature, we conducted a secondary analysis of data from a randomized trial to increase CRCS in adults (Green et al., 2013) and examined prospective predictors of (1) CRCS completion during the first year of the trial, and (2) repeat, on-schedule CRCS during the second year of the trial among those that completed an FOBT in Year 1.
Methods
This research was conducted as part of the Systems of Support to Increase Colorectal Cancer Screening (SOS) Trial (clinicaltrials.gov registration number NCT00697047). Details of the study design (Green et al., 2010), recruitment (Green et al., 2012), and findings (Green et al., 2013) have been reported. Briefly, the trial compared the effectiveness of stepped increments of centralized interventions to increase CRCS and was delivered through 21 medical centers of Group Health Cooperative, a large nonprofit integrated healthcare delivery system in Washington State. Participants were recruited between August 2008 and November 2009.
The interventions targeted constructs from the Preventive Health Model (Myers et al., 1994). Trial participants were randomized to usual care or one of three intervention groups: automated mailed interventions (automated), mailed interventions plus medical assistant telephone support (assisted), or both automated and assisted interventions plus nurse navigation (navigated). The usual care group received preventive services as part of routine care. Intervention participants received FOBT Hemoccult SENSA® cards, simplified instructions, and a postage-paid envelope for returning them. Interventions were repeated in Year 2 for those due for screening (i.e., completed FOBT or were not screened in Year 1). Compared with the usual care group, participants in the intervention groups were more likely to be current for CRCS in both trial years with significant increases by intervention intensity (usual care, 26.3%; automated, 50.8%; assisted, 57.5%; and navigated, 64.7%) (Green et al., 2013).
We extend the findings of the trial by examining predictors of CRCS in a sub-sample of participants who completed a supplementary baseline survey that included measures of behavioral and psychosocial constructs. Approximately 30% (n = 1364) of participants randomized to the trial (n = 4664) were randomly selected to complete the survey. The sample for this analysis consisted of 1247 study participants that responded to the survey (91.4% response rate).
Measures
Outcome variables
Two binary dependent variables assessing screening completion were examined: (1) CRCS completion during the first year of the study, and (2) repeat, on-schedule CRCS during the second year of the study among those that completed an FOBT in Year 1. Screening completion included completion of FOBT, sigmoidoscopy, or colonoscopy. Electronic Health Record (EHR) or claims data were used to assess screening completion.
Predictor variables
Sociodemographic, health history, and psychosocial variables were examined as predictors of both screening outcomes. Variables were obtained from automated data (e.g., EHR and claims data) and patient self-reported data on the survey. Sociodemographic variables included age, sex, race/ethnicity, marital status, education, employment, and insurance type.
Health history variables included family history of CRC (first-degree relative), smoking status, overall self-rated health, comorbidity, continuity of care, CRCS test preference (no test preference vs. preference for colonoscopy/ sigmoidoscopy/FOBT), physician recommendation for CRCS, and prior CRCS at baseline. The Johns Hopkins Adjusted Clinical Group case-mix system measured comorbidity based on age, gender, and the number and types of ICD-9 diagnostic codes during 12 months prior to randomization (Starfield et al., 1991; Weiner et al., 1991). Patient comorbidity was defined based on expected resource utilization needs and classified as low, moderate, or high (Green et al., 2010). Continuity of care was evaluated using the Usual Provider Continuity Index, calculated as the proportion of primary care visits to a patient's most frequently visited physician (Breslau and Reeb, 1975).
The survey contained 33 items that measured five psychosocial constructs: pros, cons, self-efficacy, social influence, and cancer worry (Green et al., 2010; Myers et al., 1994). Items and scales were adapted from those used in other CRCS trials (Vernon et al., 2011; Tilley et al., 1999) and were validated in diverse settings (McQueen et al., 2008; Murphy et al., 2013; Rawl et al., 2001; Ritvo et al., 2008; Tiro et al., 2005). Pros measured positive aspects of CRCS (7 items, α = 0.83), and cons measured negative aspects (10 items, α = 0.86). Self-efficacy assessed confidence in performing aspects of CRCS (10 items, α = 0.93). Social influence measured norms of friends, family, and physicians related to CRCS (3 items, α = 0.61). Cancer worry measured perceived consequences of completing CRCS (3 items, α = 0.68). All items were measured on a five-point scale ranging from “strongly disagree” (1) to “strongly agree” (5). Scores were set to missing if participants did not answer more than four items for 10-item scales, three items for the 7-item pros scale, and two items for 3-item scales. Scale scores were standardized by dividing the total scale score by the number of items answered.
Statistical analysis
Pearson chi-square or Fisher exact tests were used to compare categorical characteristics of screeners and nonscreeners, and Student's t-tests or Wilcoxon rank-sum tests were used to compare continuous characteristics. Variables with a p-value less than 0.25 in univariable analysis were included in a multivariable logistic regression model (Hosmer et al., 2013) with CRCS completion at Year 1 and repeat CRCS at Year 2 as the dependent variables. Intervention group assignment (usual care, automated, assisted, or navigated) was retained in all analyses.
Because the intervention had a statistically significant effect in the primary outcomes analysis (Green et al., 2013), we conducted exploratory analyses to examine whether the intervention moderated the association of screening with baseline predictors. We used a conservative approach and included variables with p < 0.10 in univariable analysis. To test for moderation, we fit multivariable logistic models that included main effects terms for the individual intervention groups, the predictor of interest, and the interaction term between the predictor variable and the combined intervention groups (usual care vs. any intervention). This method allowed the association between the predictor and screening outcomes to differ between the usual care group and combined intervention groups. An interaction term with p < 0.05 suggested the association differed between the usual care and combined intervention groups. Continuous variables were centered by subtracting the mean from each observation before being included in the regression models.
No variable had more than 1% missing data. Chi-square tests (p < 0.05) indicated that respondents with incomplete data (n = 199, 16%) were more likely to be older (≥65 years), retired, have prior CRCS at baseline, have Medicare or basic health insurance, and have lower scores on the social influence scale; however, there was no statistically significant difference in screening completion between participants with complete versus incomplete data. Participants with missing data on any of the variables included in a multivariable model were excluded.
Statistical analyses were conducted using Stata/SE 13.0 (College Station, TX).
Results
Any screening at year 1
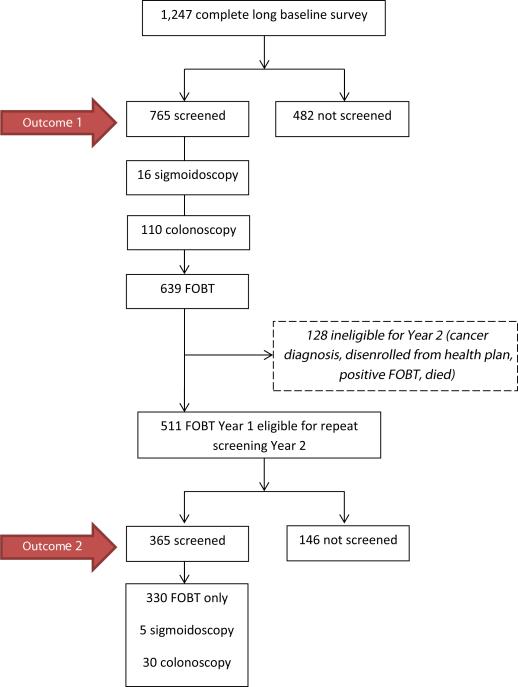
Of 1247 participants who completed the survey, 765 (61%) completed CRCS at the end of Year 1 (Fig. 1). The majority of the screened participants completed FOBT (84%) vs. endoscopy (17%).
Fig. 1.
Study flow diagram.
Univariable analysis (Table 1) showed that older age, race/ethnicity, more years of formal education, Medicare insurance, family history of CRC, no history of smoking, higher health rating, prior CRCS at baseline, physician recommendation for CRCS, test preference, higher self-efficacy, greater pros, fewer cons, more social influence, plans to be screened in the next year, importance of CRCS (very important/important), and intervention group were associated with CRCS at Year 1 (p < 0.25). Race/ethnicity, prior CRCS at baseline, and intervention group remained independent predictors of screening completion at Year 1 (Table 2). Having a family history of CRC, higher health rating, and higher self-efficacy scores trended towards significance.
Table 1.
Baseline characteristics by CRCS completion at Year 1 (n = 1247) and repeat screening at Year 2 among participants in the SOS Trial, August 2008 to November 2011 (n = 511).
| Variable | CRCS completion at Year 1 |
Repeat screening at Year 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screened |
Not screened |
p-value | Screened |
Not screened |
p-value | |||||
| (n = 765) |
(n = 482) |
(n = 365) |
(n = 146) |
|||||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||
| Sociodemographic | ||||||||||
| Age | 0.005 | 0.041 | ||||||||
| 50-64 | 641 | 59.8 | 431 | 40.2 | 298 | 69.6 | 130 | 30.4 | ||
| 65-73 | 124 | 70.9 | 51 | 29.1 | 67 | 80.7 | 16 | 19.3 | ||
| Sex | 0.670 | 0.537 | ||||||||
| Male | 335 | 60.7 | 217 | 39.3 | 171 | 72.2 | 64 | 27.2 | ||
| Female | 430 | 61.9 | 265 | 38.1 | 194 | 70.3 | 82 | 29.7 | ||
| Race/ethnicity | 0.028 | 0.142 | ||||||||
| Hispanic | 18 | 41.9 | 25 | 58.1 | 9 | 69.2 | 4 | 30.8 | ||
| Black | 27 | 50.0 | 27 | 50.0 | 20 | 90.9 | 2 | 9.1 | ||
| Asian | 32 | 60.4 | 21 | 39.6 | 13 | 61.9 | 8 | 38.1 | ||
| White | 639 | 62.9 | 377 | 37.1 | 304 | 71.7 | 120 | 28.3 | ||
| Other | 47 | 61.0 | 30 | 39.0 | 18 | 62.1 | 11 | 37.9 | ||
| Education | <0.001 | 0.499 | ||||||||
| High school grad/GED or less | 86 | 52.8 | 77 | 47.2 | 41 | 70.7 | 17 | 29.3 | ||
| Some college | 219 | 56.3 | 170 | 43.7 | 104 | 75.4 | 34 | 24.6 | ||
| Bachelor's degree or more | 458 | 66.1 | 235 | 33.9 | 219 | 70.0 | 94 | 30.0 | ||
| Marital status | 0.154 | 0.227 | ||||||||
| Not married | 191 | 58.1 | 138 | 42.0 | 89 | 67.4 | 43 | 32.6 | ||
| Married | 572 | 62.5 | 343 | 37.5 | 275 | 72.9 | 102 | 27.0 | ||
| Employment | 0.287 | 0.827 | ||||||||
| At least part time | 544 | 60.4 | 357 | 39.6 | 255 | 71.2 | 103 | 28.8 | ||
| Retired/other | 219 | 63.7 | 125 | 36.3 | 109 | 72.2 | 42 | 27.8 | ||
| Insurance | 0.006 | 0.202 | ||||||||
| Commercial/private pay | 661 | 60.0 | 441 | 40.0 | 312 | 70.4 | 131 | 29.6 | ||
| Medicare/basic health | 104 | 71.7 | 41 | 28.3 | 53 | 77.9 | 15 | 22.1 | ||
| Medical/health history | ||||||||||
| Family history of CRC (1st degree relative) | 0.032 | 0.491 | ||||||||
| No | 710 | 60.7 | 459 | 39.3 | 339 | 71.1 | 138 | 28.9 | ||
| Yes | 42 | 75.0 | 14 | 25.0 | 19 | 79.2 | 5 | 20.8 | ||
| Smoker | 0.008 | 0.171 | ||||||||
| Never | 430 | 64.4 | 238 | 35.6 | 216 | 71.8 | 85 | 28.2 | ||
| Former | 261 | 60.0 | 174 | 40.0 | 121 | 74.2 | 42 | 25.8 | ||
| Current | 71 | 50.7 | 69 | 49.3 | 27 | 60.0 | 18 | 40.0 | ||
| Health rating, mean (SD) | 7.6 (1.3) | 7.3 (1.4) | 0.002 | 7.7 (1.2) | 7.6 (1.3) | 0.336 | ||||
| Prior CRCS (at baseline) | <0.001 | 0.003 | ||||||||
| No | 282 | 49.4 | 289 | 50.6 | 126 | 64.0 | 71 | 36.0 | ||
| Yes | 483 | 71.5 | 193 | 28.6 | 239 | 76.1 | 75 | 23.9 | ||
| Physician recommendation | 0.007 | 0.680 | ||||||||
| No/don't know | 183 | 55.5 | 147 | 44.6 | 96 | 70.6 | 40 | 29.4 | ||
| Yes | 538 | 64.0 | 303 | 36.0 | 250 | 72.5 | 95 | 27.5 | ||
| Test preference | 0.003 | 0.409 | ||||||||
| Colonoscopy/sigmoidoscopy/FOBT | 738 | 61.8 | 456 | 38.2 | 354 | 72.0 | 138 | 28.1 | ||
| No preference | 11 | 35.5 | 20 | 64.5 | 4 | 57.1 | 3 | 42.9 | ||
| Comorbidity score | 0.117 | 0.540 | ||||||||
| 1 | 244 | 58.5 | 173 | 41.5 | 122 | 69.7 | 53 | 30.3 | ||
| 2 | 376 | 64.4 | 208 | 35.6 | 182 | 73.7 | 65 | 26.3 | ||
| 3 | 145 | 58.6 | 101 | 41.1 | 61 | 68.5 | 28 | 31.5 | ||
| Continuity of care index | 0.845 | 0.220 | ||||||||
| <0.67 | 646 | 61.2 | 409 | 38.8 | 307 | 70.4 | 129 | 29.6 | ||
| ≥0.67 | 119 | 62.0 | 73 | 38.0 | 58 | 77.3 | 17 | 22.7 | ||
| Psychosocial | ||||||||||
| Self-efficacy, mean (SD) | 4.37 (0.74) | 4.17 (0.89) | <0.001 | 4.34 (0.74) | 4.20 (0.83) | 0.051 | ||||
| CRCS pros, mean (SD) | 4.54(0.51) | 4.44 (0.65) | 0.002 | 4.49 (0.56) | 4.46 (0.51) | 0.513 | ||||
| CRCS cons, mean (SD) | 1.70 (0.76) | 1.84 (0.76) | 0.002 | 1.76 (0.79) | 1.81 (0.79) | 0.459 | ||||
| Social influence, mean (SD) | 3.67 (0.96) | 3.53 (1.07) | 0.019 | 3.56 (0.98) | 3.64 (0.99) | 0.416 | ||||
| Cancer worry, mean (SD) | 2.25 ( 0.74) | 2.28 (0.77) | 0.521 | 2.21 (0.68) | 2.23 (0.77) | 0.732 | ||||
| Current plans to be screened | 0.006 | 0.967 | ||||||||
| Not thinking about it | 54 | 47.4 | 60 | 52.6 | 31 | 73.8 | 11 | 26.2 | ||
| Thinking...not in the next year | 78 | 57.8 | 57 | 42.2 | 44 | 69.8 | 19 | 30.2 | ||
| Thinking...within the next year | 256 | 61.2 | 162 | 38.8 | 125 | 71.0 | 51 | 29.0 | ||
| Want to get screened in the next year | 345 | 64.7 | 188 | 35.3 | 148 | 72.2 | 57 | 27.8 | ||
| Importance | 0.001 | 0.639 | ||||||||
| Very important/important | 639 | 63.5 | 367 | 36.5 | 295 | 71.8 | 116 | 28.2 | ||
| Not as important/not at all important | 124 | 52.1 | 114 | 47.9 | 68 | 69.4 | 30 | 30.6 | ||
| Intervention | ||||||||||
| Intervention group | <0.001 | <0.001 | ||||||||
| Usual care | 131 | 40.9 | 189 | 59.1 | 16 | 24.6 | 49 | 75.4 | ||
| Automated | 216 | 62.1 | 132 | 37.9 | 109 | 75.2 | 36 | 24.8 | ||
| Assisted | 198 | 69.7 | 86 | 30.3 | 117 | 78.5 | 32 | 21.5 | ||
| Navigated | 220 | 74.6 | 75 | 25.4 | 123 | 80.9 | 29 | 19.1 | ||
Participants with missing data were excluded from the analysis. Missing values ranged from 0 (age, sex, insurance, prior CRCS, comorbidity, continuity of care, intervention) to 76 (physician recommendation).
Table 2.
Unadjusted and adjusted logistic regression of predictors of CRCS completion at Year 1 among participants in the SOS Trial, August 2008 to November 2011 (n = 1247).
| Variable | n | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Sociodemographic | |||||||
| Age | 0.006 | 0.837 | |||||
| 50-64 | 1072 | 1.00 | REF | 1.00 | REF | ||
| 65-73 | 175 | 1.63 | 1.15-2.32 | 0.94 | 0.50-1.75 | ||
| Race/ethnicity | 0.006 | 0.048 | |||||
| Hispanic | 43 | 1.00 | REF | 1.00 | REF | ||
| Black | 54 | 1.39 | 0.62-3.11 | 1.05 | 0.40-2.73 | ||
| Asian/white/other | 1146 | 2.33 | 1.26-4.32 | 1.92 | 0.92-4.03 | ||
| Education | <0.001 | 0.145 | |||||
| High school grad/GED or less | 163 | 1.00 | REF | 1.00 | REF | ||
| Some college | 389 | 1.15 | 0.80-1.66 | 0.86 | 0.55-1.35 | ||
| Bachelor's degree or more | 693 | 1.74 | 1.24-2.46 | 1.18 | 0.77-1.81 | ||
| Marital status | 0.155 | 0.701 | |||||
| Not married | 329 | 1.00 | REF | 1.00 | REF | ||
| Married | 915 | 1.20 | 0.93-1.56 | 1.06 | 0.77-1.47 | ||
| Insurance | 0.007 | 0.109 | |||||
| Commercial/private pay | 1102 | 1.00 | REF | 1.00 | REF | ||
| Medicare/basic health | 145 | 1.69 | 1.16 - 2.48 | 1.75 | 0.88-3.47 | ||
| Medical | |||||||
| Family history of CRC (1st degree relative) | 0.035 | 0.055 | |||||
| No | 1169 | 1.00 | REF | 1.00 | REF | ||
| Yes | 56 | 1.94 | 1.05-3.59 | 2.02 | 0.99-4.10 | ||
| Smoker | 0.009 | 0.303 | |||||
| Never | 668 | 1.00 | REF | 1.00 | REF | ||
| Former | 435 | 0.83 | 0.65-1.06 | 0.81 | 0.60-1.10 | ||
| Current | 140 | 0.57 | 0.39-0.82 | 0.77 | 0.48-1.22 | ||
| Health rating | 1244 | 1.14 | 1.05-1.25 | 0.002 | 1.10 | 0.99-1.22 | 0.090 |
| Prior CRCS (at baseline) | <0.001 | <0.001 | |||||
| No | 571 | 1.00 | REF | 1.00 | REF | ||
| Yes | 676 | 2.56 | 2.03-3.24 | 2.64 | 1.99-3.52 | ||
| Physician recommendation | 0.007 | 0.402 | |||||
| No/don't know | 330 | 1.00 | REF | 1.00 | REF | ||
| Yes | 841 | 1.43 | 1.10-1.85 | 1.14 | 0.83-1.57 | ||
| Test preference | 0.005 | 0.169 | |||||
| Any preference | 1194 | 1.00 | REF | 1.00 | REF | ||
| No preference | 31 | 0.34 | 0.16-0.72 | 0.51 | 0.20-1.25 | ||
| Comorbidity score | 0.117 | 0.505 | |||||
| 1 | 417 | 1.00 | REF | 1.00 | REF | ||
| 2 | 584 | 1.28 | 0.99-1.66 | 1.17 | 0.86-1.61 | ||
| 3 | 246 | 1.02 | 0.74-1.40 | 0.99 | 0.66-1.47 | ||
| Psychosocial | |||||||
| Self-efficacy | 1244 | 1.35 | 1.17-1.56 | <0.001 | 1.22 | 0.98-1.52 | 0.069 |
| Pros | 1245 | 1.37 | 1.13-1.67 | 0.002 | 1.05 | 0.76-1.45 | 0.777 |
| Cons | 1244 | 0.80 | 0.69-0.92 | 0.003 | 0.99 | 0.80-1.22 | 0.920 |
| Social influence | 1207 | 1.15 | 1.02-1.29 | 0.020 | 1.05 | 0.89-1.24 | 0.584 |
| Current plans to be screened | 0.003 | 0.437 | |||||
| Not thinking about it/thinking but not in next year | 249 | 1.00 | REF | 1.00 | REF | ||
| Thinking within the next year/want to be screened in the next year | 951 | 1.52 | 1.15-2.02 | 1.18 | 0.78-1.77 | ||
| Importance of CRCS | 0.001 | 0.151 | |||||
| Not as important/not at all important | 238 | 1.00 | REF | 1.00 | REF | ||
| Very important/important | 1006 | 1.60 | 1.20-2.13 | 1.35 | 0.90-2.03 | ||
| Intervention | |||||||
| Intervention group | <0.001 | <0.001 | |||||
| Usual care | 320 | 1.00 | REF | 1.00 | REF | ||
| Automated | 348 | 2.36 | 1.73-3.22 | 2.06 | 1.43-2.95 | ||
| Assisted | 384 | 3.32 | 2.37-4.65 | 4.03 | 2.69-6.03 | ||
| Navigated | 295 | 4.23 | 3.00-5.97 | 5.64 | 3.74-8.49 | ||
OR, odds ratio; REF, referent group; 95% CI, 95% confidence interval.NOTE: Participants with missing data were excluded from the analysis. Missing values ranged from 0 (age, insurance, prior CRCS, intervention) to 76 (physician recommendation). The adjusted multivariable model consists of the 1050 participants with complete data. Multivariable model adjusted for all other variables included in the model.
Repeat screening at year 2
Of the 639 participants that completed a Year 1 FOBT, 128 were ineligible for Year 2 of the study for the following reasons: cancer diagnosis, disenrolled from the health plan, positive FOBT, or died (Fig. 1). Thus, 511 participants were eligible for repeat, on-schedule CRCS. Of these, 365 (71%) completed CRCS by the end of Year 2. The majority completed FOBT (90%).
Older age, race/ethnicity, marital status (married), Medicare insurance, no history of smoking, prior CRCS at baseline, continuity of care, higher self-efficacy, and intervention group were associated with repeat screening (p < 0.25) in univariable analysis (Table 1). CRCS prior to baseline, higher self-efficacy, and intervention group remained independent predictors of repeat screening (Table 3).
Table 3.
Unadjusted and adjusted logistic regression of predictors of repeat screening at Year 2 among participants in the SOS Trial, August 2008 to November 2011 (n = 511).
| Variable | n | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Sociodemographic | |||||||
| Age | 0.043 | 0.955 | |||||
| 50-64 | 428 | 1.00 | REF | 1.00 | REF | ||
| 65-73 | 83 | 1.83 | 1.02-3.27 | 1.03 | 0.40-2.63 | ||
| Race/ethnicity | 0.073 | 0.376 | |||||
| Hispanic | 13 | 1.00 | REF | 1.00 | REF | ||
| Black | 22 | 4.44 | 0.68-28.86 | 2.79 | 0.37-20.88 | ||
| Asian/white/other | 474 | 1.07 | 0.32-3.54 | 0.96 | 0.24-3.78 | ||
| Marital status | 0.227 | 0.413 | |||||
| Not married | 132 | 1.00 | REF | 1.00 | REF | ||
| Married | 377 | 1.30 | 0.85-2.00 | 1.23 | 0.75-2.03 | ||
| Insurance | 0.204 | 0.691 | |||||
| Commercial/private pay | 443 | 1.00 | REF | 1.00 | REF | ||
| Medicare/basic health | 68 | 1.48 | 0.81-2.73 | 1.22 | 0.46-3.23 | ||
| Medical/health history | |||||||
| Smoker | 0.187 | 0.838 | |||||
| Never | 301 | 1.00 | REF | 1.00 | REF | ||
| Former | 163 | 1.13 | 0.74-1.75 | 1.03 | 0.64-1.66 | ||
| Current | 45 | 0.59 | 0.31-1.13 | 0.81 | 0.37-1.74 | ||
| Prior CRCS (at baseline) | 0.003 | 0.004 | |||||
| No | 197 | 1.00 | REF | 1.00 | REF | ||
| Yes | 314 | 1.80 | 1.22-2.65 | 1.97 | 1.25-3.11 | ||
| Continuity of care index | 0.222 | 0.367 | |||||
| <0.67 | 436 | 1.00 | REF | 1.00 | REF | ||
| ≥0.67 | 75 | 1.43 | 0.80-2.56 | 1.35 | 0.70-2.61 | ||
| Psychosocial | |||||||
| Self-efficacy | 510 | 1.27 | 0.997-1.62 | 0.053 | 1.32 | 1.00-1.73 | 0.047 |
| Intervention | |||||||
| Intervention group | <0.001 | <0.001 | |||||
| Usual care | 65 | 1.00 | REF | 1.00 | REF | ||
| Automated | 145 | 9.28 | 4.70-18.28 | 9.27 | 4.56-18.82 | ||
| Assisted | 149 | 11.20 | 5.64-22.25 | 11.17 | 5.44-22.94 | ||
| Navigated | 152 | 12.99 | 6.49-26.01 | 13.10 | 6.33-27.08 | ||
OR, odds ratio; REF, referent group; 95% CI, 95% confidence interval.NOTE: Participants with missing data were excluded from the analysis. Missing values ranged from 0 (age, prior CRCS, intervention) to 1 (self-efficacy). The adjusted multivariable model consists of the 510 participants with complete data. Multivariable model adjusted for all other variables included in the model.
Moderation analysis
For CRCS completion at Year 1, the intervention moderated the association between smoking status and prior CRCS at baseline on screening completion (Table 4). In the combined intervention groups, current smokers were less likely to complete CRCS (53.9%) compared with non-smokers (70.2%). There was no association between smoking status and screening completion in the usual care group; approximately 40% of smokers and non-smokers completed screening. Prior CRCS at baseline was a significant predictor in the combined intervention and usual care groups, but the association was more pronounced in the combined intervention group. Participants in the intervention groups with prior CRCS had over three times the odds of completing screening at Year 1 compared with those with no prior screening (79.5% vs. 55.2%, OR 3.25). In the usual care group, 48% of those with prior CRCS completed CRCS compared with 32.7% with no prior screening (OR 1.90).
Table 4.
Analysis of the intervention as a moderator between prior CRCS, smoking status, and CRCS completion at Year 1 among participants in the SOS Trial, August 2008 to November 2011 (n = 1247).
| Usual care (n = 320) |
Intervention (n = 927) |
||||
|---|---|---|---|---|---|
| %Screened | ORa (95% CI) | % Screened | ORa (95% CI) | p-valueb | |
| Current smoker | 0.052 | ||||
| No (former, never) | 40.8 | 1.00 | 70.2 | 1.00 | |
| Yes | 42.1 | 1.06 (0.53-2.10) | 53.9 | 0.47 (0.31-0.71) | |
| Prior CRCS | 0.048 | ||||
| No | 32.7 | 1.00 | 55.2 | 1.00 | |
| Yes | 48.0 | 1.90 (1.21-3.00) | 79.5 | 3.25 (2.43-4.36) | |
OR, odds ratio; 95% CI, 95% confidence interval.
Odds ratio refers to the odds of screening related to levels of the predictors and stratified by intervention group.
p-value refers to the significance of interaction between the predictor and the intervention group.
The intervention did not significantly moderate the association of any predictors with repeat screening at Year 2. Although the interaction term was only marginally significant (p = 0.07), and the confidence intervals were wide, older age was a stronger predictor of CRCS in the usual care group (OR 7.83, 95% CI 1.28–48.0) than in the intervention group (OR 1.31, 95% CI 0.70–2.50). Similarly, although the interaction term was not significant (p = 0.07), self-efficacy was associated with screening in the usual care group (OR 4.44, 95% CI 1.14–17.32) but not the intervention group (OR 1.24, 95% CI 0.94–1.63).
Discussion
This study extends the findings of our intervention trial (Green et al., 2013) by examining longitudinal predictors of CRCS in the context of the intervention. Although the outcomes of the trial supported the effectiveness of the interventions, our results provide an assessment of the role of psychosocial predictors of screening behavior and of repeat CRCS. The moderation analyses shed some additional light on how the intervention affected the pattern and magnitude of the associations between predictors and CRCS.
Consistent with other studies, our results indicate that prior CRCS experience predicts screening completion among those who were overdue at Year 1, as well as repeat, on-schedule screening at Year 2 (Vernon et al., 2011; Sifri et al., 2010; Manne et al., 2009; Myers et al., 2007). In the moderation analysis, prior CRCS had a stronger association with screening in the combined intervention groups compared with usual care, suggesting the intervention amplified the effect of prior screening experience. Increased self-efficacy was also associated with both screening outcomes in multivariable analysis, although the adjusted point estimate at Year 1 was not significant. The association between self-efficacy and CRCS has been consistently reported in both cross-sectional and longitudinal studies (DeVellis et al., 1990; Hoogewerf et al., 1990; Kremers et al., 2000; McQueen et al., 2007; Menon et al., 2003; Myers et al., 1994; Vernon and McQueen, 2010), underscoring the importance of targeting self-efficacy in CRCS interventions to increase initiation, adherence among those overdue, and maintenance. A recent population-based study of repeat FOBT in Australia similarly found that self-efficacy was the only variable able to distinguish between participants who engaged in consistent, on-schedule screening and those who were never screened (Duncan et al., 2014). Although our intervention did not moderate the effects of self-efficacy at Year 1, at Year 2 self-efficacy showed a stronger association with repeat screening in the usual care group compared with the combined intervention groups. Collectively, these findings suggest that increased self-efficacy is an important predictor of screening in usual care settings but may be less important when screening is made easy through intervention. Further, repeat behaviors such as completion of annual FOBT may be more dependent on high self-efficacy in the absence of support for screening.
The intervention also moderated the association between smoking status and CRCS completion at Year 1. There was no difference in CRCS completion among current smokers and non-smokers in usual care (40.8% vs. 42.1%, respectively), but in the intervention group, current smokers were less likely to complete CRCS compared to non-smokers (53.9% vs. 70.2%, respectively). Although some smokers in the intervention group may have been motivated to be screened, the magnitude of the intervention effect was much larger among non-smokers. Prevalence data from the Behavioral Risk Factor Surveillance System (BRFSS) also suggest that screening rates differ by smoking status. CRCS in current smokers only slightly increased from 45% in 2006 to 50% in 2010 compared to an overall increase from 59% to 66% during the same period (Oluyemi et al., 2014). Although uptake of CRCS is increasing in other high risk groups (e.g., minority, obese, or diabetic populations), screening remains relatively low among smokers. Very little is known about the reasons that smokers are less likely to be screened or about interventions to encourage screening in this population (Rakowski et al., 1999).
For both Year 1 and Year 2 screening outcomes, the intervention was the strongest predictor of CRCS completion. Many of the other variables were statistically significant in univariable analysis, but few remained significant in multivariable analysis. This finding is consistent with the hypothesis that when major access barriers are removed through intervention (e.g., mailed FOBT kits), the importance of sociodemographic and psychosocial variables diminishes. Similar to other intervention studies that included mailed FOBT kits (Church et al., 2004; Coronado et al., 2011; Gupta et al., 2013; Myers et al., 2013; Sequist et al., 2009; Walsh et al., 2010), adherence to CRCS recommendations increased when screening was convenient and accessible, regardless of patient factors or preferences (Daskalakis et al., 2014; Inadomi et al., 2012; Myers et al., 2013). Studies of mammography screening have also found that psychosocial and other demographic factors did not predict screening receipt when barriers to mammography were removed (Glenn et al., 2006; Taplin et al., 2000). The lack of statistical significance of most of the psychosocial constructs in multivariable analysis also may indicate that the intervention successfully targeted key constructs.
Strengths of our study include the ability to examine association between baseline predictors and prospectively measured CRCS in a well-defined sample of patients who completed a survey of psychosocial constructs associated with CRCS. The richness of the survey data allowed us to study potentially important factors influencing screening uptake. Another strength of our study was the inclusion of two screening outcomes: completion of any CRCS among patients overdue for screening, as well as repeat, on-schedule screening—an outcome that has not been extensively studied. In addition, we used previously validated measures of psychosocial constructs of CRCS (McQueen et al., 2008; Murphy et al., 2013; Rawl et al., 2001; Ritvo et al., 2008; Tiro et al., 2005) and validated measures of patient comorbidity and continuity of care (Breslau and Reeb, 1975; Starfield et al., 1991; Weiner et al., 1991).
Although we were able to examine predictors of CRCS, a limitation of the study was our inability to examine mediation or change in predictors over time due to the timing of survey administration; the follow-up survey was administered 2 years after the baseline survey. As a next step, researchers should consider repeating survey measures at closer intervals in order to examine mediators and to test models of behavior change that explain CRCS completion (e.g., competitive hypothesis testing). Behavioral theory models have been studied in mammography screening (Murphy et al., 2014), and CRCS research has started to move in this direction (McQueen et al., 2010). Testing competing hypotheses of health behavior theories may help researchers better understand the underlying mechanisms and design interventions to further optimize CRCS. Meanwhile, there are a number of effective, evidence-based intervention strategies to increase CRCS (Sabatino et al., 2012), including those in this study, that could be implemented in a variety of healthcare and community settings while factors associated with adherence continue to be explored.
Other limitations of the study include the generalizability of the findings to other healthcare settings or population subgroups. Study participants had health insurance, access to follow-up testing, and no or low copays for screening, and provided verbal consent to participate in the study. In systems with different cost-share measures, there may be different facilitators and barriers to screening completion. The sample was also largely white, and predictors of screening may differ in other racial/ ethnic groups. In addition, participants with missing predictor data were excluded from the analysis. Those with missing data were older, retired, and were more likely to have had prior CRCS compared with those included in the analysis, all characteristics associated with higher likelihood of screening. However, there were no differences in screening completion; thus, it is unlikely that we overestimated associations.
Longitudinal and moderation analyses are rarely done in health promotion trials of CRCS and are useful for identifying factors to target in interventions. This is one of few studies to use longitudinal data to examine prospective predictors of CRCS. Our results suggest that self-efficacy and prior experience with CRCS are important predictors of screening behavior and may be used to refine intervention strategies.
Acknowledgments
This study was supported, in part, by the National Cancer Institute at the National Institutes of Health (R01 CA121125, “Systems of Support to Increase Colon Cancer Screening and Follow-Up”).
Footnotes
Funding: National Cancer Institute Grant R01 CA121125 (“Systems of Support to Increase Colon Cancer Screening and Follow-Up”)
Clinical Trial Information: Clinicaltrials.gov registration number NCT00697047 (registration date: June 11, 2008); funded by National Cancer Institute Grant R01 CA121125 (“Systems of Support to Increase Colon Cancer Screening and Follow-Up”); approved by the IRB at Group Health Research Institute on April 17, 2007
Conflict of interest
The authors declare there is no conflict of interest.
References
- Bastani R, Maxwell AE, Bradford C. Cross-section versus prospective predictors of screening mammography. Cancer Epidemiol. Biomarkers Prev. 1996;5(10):845–848. [PubMed] [Google Scholar]
- Breslau N, Reeb KG. Continuity of care in a university-based practice. J. Med. Educ. 1975;50(10):965–969. doi: 10.1097/00001888-197510000-00006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control, Prevention (CDC) Cancer screening—United States, 2010. MMWR Morb. Mortal. Wkly Rep. 2012;61(3):41–45. [PubMed] [Google Scholar]
- Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J. Natl. Cancer Inst. 2004;96(10):770–780. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- Daskalakis C, Vernon SW, Sifri R, Dicarlo M, Cocroft J, Andrel Sendecki J, et al. The effects of test preference, test access, and navigation on colorectal cancer screening. Cancer Epidemiol. Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-13-1176. http://dx.doi.org/10.1158/1055-9965.EPI-13-1176 (Epub ahead of print 2014 May 9) [DOI] [PMC free article] [PubMed]
- DeVellis BM, Blalock SJ, Sandler RS. Predicting participation in cancer screening: the role of perceived behavioral control. J. Appl. Soc. Psychol. 1990;20:639–660. [Google Scholar]
- Duncan A, Turnbull D, Wilson C, Osborne JM, Cole SR, Flight I, et al. Behavioural and demographic predictors of adherence to three consecutive faecal occult blood test screening opportunities: a population study. BMC Public Health. 2014;14:238. doi: 10.1186/1471-2458-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann. Fam. Med. 2010;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellad ZF, Stechuchak KM, Fisher DA, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am. J. Gastroenterol. 2011;106(6):1125–1134. doi: 10.1038/ajg.2011.11. [DOI] [PubMed] [Google Scholar]
- Glenn B, Bastani R, Reuben D. How important are psychosocial predictors of mammography receipt among older women when immediate access is provided via on-site service? Am. J. Health Promot. 2006;20(4):237–246. doi: 10.4278/0890-1171-20.4.237. [DOI] [PubMed] [Google Scholar]
- Green BB, Wang CY, Horner K, Catz S, Meenan RT, Vernon SW, et al. Systems of support to increase colorectal cancer screening and follow-up rates (SOS): design, challenges, and baseline characteristics of trial participants. Contemp. Clin. Trials. 2010;31(6):589–603. doi: 10.1016/j.cct.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Bogart A, Chubak J, Vernon SW, Morales LS, Meenan RT, et al. Nonparticipation in a population-based trial to increase colorectal cancer screening. Am. J. Prev. Med. 2012;42(4):390–397. doi: 10.1016/j.amepre.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann. Intern. Med. 2013;158(5 Pt 1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern. Med. 2013;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle JD, Armitage NC, Chamberlain J, Amar SS, James PD, Balfour TW. Fecal occult blood screening for colorectal cancer in the general population. Results of a controlled trial. Cancer. 1986;58(2):397–403. doi: 10.1002/1097-0142(19860715)58:2<397::aid-cncr2820580235>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hardcastle JD, Thomas WM, Chamberlain J, Pye G, Sheffiled J, James PD, et al. Randomised, controlled trial of faecal occult blood screening for colorectal cancer. Results for first 107,349 subjects. Lancet. 1989;1(8648):1160–1164. doi: 10.1016/s0140-6736(89)92750-5. [DOI] [PubMed] [Google Scholar]
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- Hoogewerf PE, Hislop TG, Morrison BJ, Burns SD, Sizto R. Health belief and compliance with screening for fecal occult blood. Soc. Sci. Med. 1990;30:721–726. doi: 10.1016/0277-9536(88)90257-2. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Wiley; Hoboken, NJ: 2013. [Google Scholar]
- Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers SP, Mesters I, Pladdet IE, van den Borne B, Stockbrugger RW. Participation in a sigmoidoscopic colorectal cancer screening program: a pilot study. Cancer Epidemiol. Biomarkers Prev. 2000;9(10):1127–1130. [PubMed] [Google Scholar]
- Liss DT, Petit-Homme A, Feinglass J, Buchanan DR, Baker DW. Adherence to repeat fecal occult blood testing in an urban community health center network. J. Community Health. 2013;38(5):829–833. doi: 10.1007/s10900-013-9685-x. [DOI] [PubMed] [Google Scholar]
- Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J. Natl. Cancer Inst. 1999;91(5):434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann. Behav. Med. 2009;37(2):207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automootive workers. Cancer Epidemiol. Biomarkers Prev. 2007;16(3):500–509. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiol. Biomarkers Prev. 2008;17(9):2231–2237. doi: 10.1158/1055-9965.EPI-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Rothman AJ, Norman GJ, Myers RE, Tilley BC. Examining the role of perceived susceptibility on colorectal cancer screening intention and behavior. Ann. Behav. Med. 2010;40(2):205–217. doi: 10.1007/s12160-010-9215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon U, Champion VL, Larkin GN, Zollinger TW, Gerde PM, Vernon SW. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. J. Occup. Environ. Med. 2003;45(8):891–898. doi: 10.1097/01.jom.0000083038.56116.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, McQueen A, Bartholomew LK, Del Junco DJ, Coan SP, Vernon SW. Factorial validity and invariance of four psychosocial constructs of colorectal cancer screening: does screening experience matter? Cancer Epidemiol. Biomarkers Prev. 2013;22(22):2295–2302. doi: 10.1158/1055-9965.EPI-13-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Vernon SW, Diamond PM, Tiro JA. Competitive testing of health behavior theories: how do benefits, barriers, subjective norm, and intention influence mammography behavior? Ann. Behav. Med. 2014;47(1):120–129. doi: 10.1007/s12160-013-9528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Adherence to continuous screening for colorectal neoplasia. Med. Care. 1993;31(6):508–519. doi: 10.1097/00005650-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Myers RE, Ross E, Jepson C, Wolf T, Balshem A, Millner L, et al. Modeling adherence to colorectal cancer screening. Prev. Med. 1994;23(2):142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- Myers RE, Bittner-Fagan H, Daskalakis C, Sifri R, Vernon SW, Cocroft J, et al. A randomized controlled trail of a tailored navigation and standard intervention in colorectal cancer screening. Cancer Epidemiol. Biomarkers Prev. 2013;22(1):109–117. doi: 10.1158/1055-9965.EPI-12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluyemi AO, Welch AR, Yoo LJ, Lehman EB, McGarrity TJ, Chuang CH. Colorectal cancer screening in high-risk groups is increasing, although current smokers fall behind. Cancer. 2014 doi: 10.1002/cncr.28707. http://dx.doi.org/10.1002/cncr.28707 (Epub ahead of print 2014 Apr 15) [DOI] [PubMed]
- Rakowski W, Clark MA, Ehrich B. Smoking and cancer screening for women ages 42–75: associations in the 1990–1994 National Health Interview Surveys. Prev. Med. 1999;29(6 Pt 1):487–495. doi: 10.1006/pmed.1999.0578. [DOI] [PubMed] [Google Scholar]
- Rawl S, Champion V, Menon U, Loehrer PJ, Vance GH, Skinner CS. Validation of scales to measure benefits of and barriers to colorectal cancer screening. J. Psychosoc. Oncol. 2001;19(3/4):47–63. [Google Scholar]
- Ritvo P, Myers R, Del Giudice ML, Pazsat L, Campbell PT, Howlett RI, et al. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening in Ontario, Canada—a replication study. Cancer Epidemiol. Biomarkers Prev. 2008;17(11):3279–3283. doi: 10.1158/1055-9965.EPI-08-0241. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am. J. Prev. Med. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Sequist TD, Zaslavsky AM, Marshal R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch. Intern. Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sifri R, Rosenthal M, Hyslop T, Andrel J, Wender R, Vernon SW, et al. Factors associated with colorectal cancer screening decision stage. Prev. Med. 2010;51(3–4):329–331. doi: 10.1016/j.ypmed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv. Res. 1991;26(1):53–74. [PMC free article] [PubMed] [Google Scholar]
- Taplin SH, Barlow WE, Ludman E, MacLehos R, Meyer DM, Seger D, et al. Testing reminder and motivational telephone calls to increase screening mammography: a randomized study. J. Natl. Cancer Inst. 2000;92(3):233–242. doi: 10.1093/jnci/92.3.233. [DOI] [PubMed] [Google Scholar]
- Tilley BC, Vernon SW, Myers R, Glanz K, Lu M, Hirst K, et al. The Next Step Trial: impact of a worksite colorectal cancer screening promotion program. Prev. Med. 1999;28(3):276–283. doi: 10.1006/pmed.1998.0427. [DOI] [PubMed] [Google Scholar]
- Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol. Biomarkers Prev. 2005;14(12):2855–2861. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- Vernon SW, McQueen A. Colorectal Cancer Screening. In: Holland JC, Breitbart WS, Jacobsen PB, Lederberg MS, Loscalzo MJ, McCorkle R, editors. Psycho-Oncology. 2nd edition. Oxford University Press; New York: 2010. pp. 71–83. [Google Scholar]
- Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann. Behav. Med. 2011;41(3):284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JM, Salazar R, Nguyen TT, Kaplan C, Nguyen LK, Hwang J, et al. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am. J. Prev. Med. 2010;39(1):1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med. Care. 1991;29(5):452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Lansdorp-Bogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Juntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]