Dear Editor
Cushing syndrome (CS) is a rare disease in children associated with weight gain and stunting of their linear growth. (Magiakou, et al. 1994b; McArthur, et al. 1980; Stratakis 2012) In the present study, we assessed growth in pediatric patients with CS after cure, caused by either, ACTH-dependent (CD) or a form of ACTH-independent CS, patients with micronodular adrenal hyperplasia (MAH).
We reviewed medical records of patients who had successful transsphenoidal surgery or adrenalectomy at the NIH between the years of 2002 and 2012. 18 children with CD (9F, mean age 12.2±3.0) and 19 children with MAH (15 F, mean age 9.8±4.4 yr), were included. All patients were evaluated under the clinical protocol 97CH0076, and 95CH0059 that were approved by the NICHD Institutional Review Board. Informed consent was signed from the patients’ parents. The diagnosis of CS was established as previously reported (Batista, et al. 2007). Patients that were Tanner Stage 5 at the time of surgery were excluded from the analysis of annual growth velocity and IGF1 z scores.
The mean follow up after surgery was 402±27 days for the CD and 365±87 days for the MAH patients (p=0.09). No significant difference was found in terms of mean age (although CD patients were 2.4 years older), gender distribution, duration of symptoms, midnight cortisol levels, IGF1 z score and mean urinary free cortisol at the time of surgery. Demographics are presented in Table 1. The baseline Ht z scores and BMI z scores were not significantly different between the two groups (p=0.85 and p=0.66 respectively) (Figure1A).
Table 1. Clinical characteristics of CS patients at time of surgery, delta Height z scores and delta BMI z scores before and after surgery, growth velocities and mutations in PRKARA1A.
| Diagnosis | Age | Tanner | Gender | Dx Height | Dx BMI | Growth velocity (cm/y) | PRKAR1A Mutation |
|---|---|---|---|---|---|---|---|
| MAH1 | 9.9 | Tanner 3 | Female | 1.166 | -1.138 | 12 | YES |
| MAH2 | 3.3 | Tanner 1 | Male | 2.485 | -2.488 | 5.5 | NO |
| MAH3 | 13.8 | Tanner 3 | Male | 0.243 | 0.604 | 9.1 | YES |
| MAH4 | 9.2 | Female | 1.006 | -1.298 | 12.2 | NO | |
| MAH5 | 6.4 | Tanner 1 | Female | 0.792 | -0.744 | 10.9 | NO |
| MAH6 | 4.5 | Tanner 1 | Female | 0.842 | -2.965 | 7.6 | NO |
| MAH7 | 16.4 | Tanner 5 | Female | 0.055 | 0.117 | ||
| MAH8 | 8.5 | Tanner 1 | Male | 0.964 | -1.646 | 12 | NO |
| MAH9 | 8.6 | Tanner 2 | Female | 1.372 | -0.338 | 13.2 | NO |
| MAH10 | 17.6 | Tanner 5 | Female | 0.288 | -0.167 | ||
| MAH11 | 3.8 | Tanner 1 | Female | 1.551 | -2.519 | 12.5 | NO |
| MAH12 | 12 | Tanner 3 | Female | 0.527 | -0.875 | 10.8 | NO |
| MAH13 | 10.7 | Tanner 2 | Female | 1.098 | -0.957 | 14.3 | YES |
| MAH14 | 5.8 | Tanner 1 | Female | 0.477 | -2.249 | 9.2 | NO |
| MAH15 | 10.8 | Tanner 3 | Female | 0.625 | -1.311 | 11 | YES |
| MAH16 | 4.4 | Tanner 1 | Male | 0.126 | 0.341 | 5.1 | YES |
| MAH17 | 15.9 | Tanner 5 | Female | -0.110 | -0.013 | ||
| MAH18 | 13.9 | Tanner 5 | Female | 0.019 | 0.216 | ||
| MAH19 | 10.6 | Tanner 2 | Female | 0.808 | -1.399 | 11.6 | NO |
| CD1 | 15.1 | Tanner 1 | Male | 0.550 | -1.721 | 8.5 | |
| CD2 | 9.2 | Tanner 1 | Male | 0.500 | -1.548 | 8.8 | |
| CD3 | 16.4 | Tanner 4 | Female | 0.113 | -0.645 | 0.9 | |
| CD4 | 13.2 | Tanner 1 | Male | 0.972 | -1.035 | 14.9 | |
| CD5 | 14.3 | Tanner 3 | Male | 0.272 | -1.646 | 7.8 | |
| CD6 | 10.9 | Tanner 2 | Male | 0.965 | -0.409 | 12.5 | |
| CD7 | 15.7 | Tanner 3 | Male | -0.251 | 0.041 | 0.5 | |
| CD8 | 13.4 | Tanner 3 | Male | 0.315 | -1.947 | 8.6 | |
| CD9 | 16.7 | Tanner 5 | Female | -0.239 | -0.565 | ||
| CD10 | 11.1 | Tanner 3 | Female | -0.241 | -0.514 | 5.3 | |
| CD11 | 6.8 | Tanner 1 | Female | 0.773 | -1.243 | 9.2 | |
| CD12 | 12.2 | Tanner 2 | Female | -1.060 | -0.216 | 0 | |
| CD13 | 10.3 | Male | 0.416 | -0.497 | 7.1 | ||
| CD14 | 10.8 | Tanner 3 | Female | 0.556 | -0.472 | 10.5 | |
| CD15 | 10.4 | Tanner 4 | Female | 0.349 | -0.758 | 9.2 | |
| CD16 | 10.8 | Tanner 1 | Male | -0.043 | -1.900 | 4.5 | |
| CD17 | 15.3 | Female | 1.562 | -0.483 | 9.3 | ||
| CD18 | 7.1 | Tanner 1 | Female | 0.337 | -1.446 | 7.4 |
Figure 1.
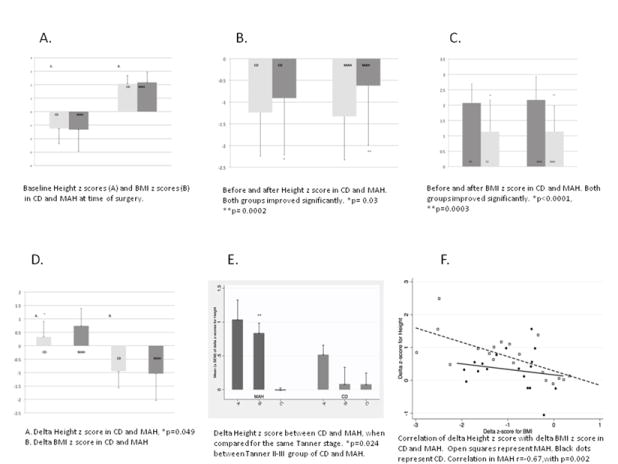
The mean Ht z score in CD before and after surgery was -1.24±1.14 and -0.91 ± 1.30 respectively (p=0.03). The mean Ht z score in MAH before and after surgery was -1.33 ± 1.64 and -0.62 ± 1.37 respectively (p=0.0002) (Figure1B). The mean BMI z score in CD before and after surgery was 2.07 ± 0.62 and 1.13 ± 1.03 respectively (p<0.0001). The mean BMI z score in MAH before and after surgery was 2.17 ± 0.76 and 1.14± 0.84 respectively (p= 0.0003). (Figure1C). The mean delta height z score was 0.32 ±0.58 in the CDs vs. 0.74 ±0.65 in the MAH group, which was significantly better in MAH (p=0.049). The mean delta BMI z score was -0.94 ±0.62 in the CD group and -1.04 ±1.0 in the MAH group (p=0.74) (Figure1D).
We divided each group of patients into three categories depending on their pubertal status: Tanner 1, Tanner 2-3 and Tanner 4-5. The mean delta Ht z scores in CD versus MAH were: 0.51±0.35 versus 1.03±0.78 for Tanner 1 (p=0.16); 0.08±0.66 versus 0.83±0.40 for Tanner 2/3 (p=0.024); and 0.07±0.30 versus -0.01±0.07 for Tanner 4/5 (p=0.59) (Figure1E).
There was a strong positive correlation between BMI z score at surgery and the delta Ht z score, (r=0.69,p=0.0012) in the MAH group but not in the CD group (r=0.19,p=0.44).The correlation of the delta BMI z score with delta Ht z score was also strongly correlated in the MAH group ( r=-0.67 ,p=0.002) but not in the CD group(r=-0.18,p=0.48). (Figure1F) Bone age z score was 0.8±2 and 0.6±1.4 in the MAH and CD group respectively (p=0.64), presented previously. (Lodish, et al. 2014)
For patients in Tanner categories 1 to 4 at surgery, the mean annual growth velocity was 10.47±2.69cm in the MAH group versus 7.35±4.06 cm in the CD group (p=0.017). The mean IGF1 z score after surgery was 0.40±1.90 versus -0.77 ±1.33 for the MAH and CD patients, respectively (p=0.050). When we excluded from the analysis the five MAH patients who are positive for a PRKAR1A mutation (patients that could have Carney complex, and thus increased IGF1 levels) we found a significant difference in the post surgery IGF1 z score, which was 0.98±2.03 in the MAH group versus -0.77±1.33 in the CD group (p=0.012). None of the patients in this analysis received growth or thyroid hormone hormone treatment.
This is the first study to compare, after a curative surgical procedure, the growth of pediatric patients with two forms of CS: CD and MAH. Our findings show that generally patients with MAH demonstrate better post-operative growth. There are several mechanisms that could explain the above observation.
First, the surgery can have a direct effect on the pituitary somatotrophs. In cases where the ACTH-secreting pituitary adenoma requires extensive surgical exploration, it is likely that the remaining pituitary cells will lose some of their function. Also, it is likely that the ACTH-producing tumor has a pressure effect on the somatotrophs that indirectly impairs the ability of the cells to produce GH.
Another reason is related to the direct adverse effects that the glucocorticoids have on the skeleton. It is possible that CD patients, who tend to be older, are exposed for a longer period of time to glucocorticoids, suffer vertebral fractures more frequently, and thus their skeleton and, hence their overall growth potential, is more affected. Moreover, patients with MAH can have cyclical forms of CS (Batista et al. 2007; Stratakis 2012), with hypercortisolism only intermittently present and an overall milder form of CS.
It is also possible that patients with CD are exposed to higher doses of glucocorticoids than what they need during their first 12 months of recovery. Although our group has shown that 75% of patients with CD should not require any hydrocortisone replacement by 14 months after surgery, and their glucocorticoids should be tapered starting after the first 6 months (and sometimes even sooner) (Lodish, et al. 2012), several patients are maintained on routine replacement for much longer, although the patients in the present study were all followed under our routine replacement doses, as described by Lodish et al.
We have recently shown that patients with CD may, in some cases , have advanced bone age due to their ACTH-stimulated adrenal androgen production and the latter’s increased aromatization (Lodish et al. 2014), as well as their increased obesity and adiposity (London, et al. 2014) and tendency to have higher insulin resistance (London et al. 2014) than those with MAH, especially those with PRKAR1A mutations (London et al. 2014). Thus, it is also possible that patients with CD would have decreased postoperative growth rate as a function of their advanced skeletal maturation compared to that of patients with MAH. Although this may be true in general, in the subgroup of patients studied here, the degree of skeletal maturity at surgery was not most likely a factor, since the bone age z scores were 0.8±2 and 0.6±1.4 in the MAH and CD groups respectively (p=0.64).
In addition, the patients with the greatest improvement in their BMI had better growth after cure. This is probably because the patients with severe obesity represent the most affected patients with CS, who also get the most benefit after cure. Whether the final adult height compared to their target height is also compromised remains to be seen.
Another possible mechanism to explain our findings may be the related to the fact that CS is associated with GH suppression (Frantz and Rabkin 1964; Krieger and Glick 1972; Magiakou, et al. 1994a). Previous studies have shown that longer duration and higher degree of hypercortisolism leads to greater GH suppression (Frantz and Rabkin 1964; Krieger and Glick 1972). The GH secretion profile was abnormal even one year after cure of CS in a study done previously at NIH (Magiakou et al. 1994a). Some of our patients with MAH also have Carney complex and germline mutations in PRKAR1A gene. (Stratakis, et al. 2001). Carney complex can be associated with GH excess (Bertherat et al. 2009; Boikos and Stratakis 2006; Stratakis et al. 2001). Clinically apparent acromegaly in Carney complex occurs mostly in young adulthood (Bertherat et al. 2009; Boikos and Stratakis 2006; Stratakis et al. 2001) , but it is possible abnormal GH secretion exists in early childhood (Boikos and Stratakis 2006) and this is manifested in our patients with MAH as an earlier recovery of GH secretion than one would expect. However, even when we excluded the subset of patients with PRKAR1A mutations, the MAH patients still had higher IGF1 z scores after cure when compared to CD patients.
Finally, this study showed that patients with MAH grow significantly better after surgery, even when compared to a group of CD patients of the same Tanner stage at the time of surgery. (Dupuis, et al. 2007) A limitation of our study is that we do not have GH secretion profiles after surgery and adult final Ht in these patients.
In conclusion, there are important messages from these clinical observations: patients with MAH are less likely to need GH therapy after surgery for their CS. On the other hand, physicians should monitor patients with CD very closely and have a low threshold for considering treatment with GH to improve their final adult height. Finally, every effort for improvement in BMI with lifestyle modification could potentially improve growth of children with CS after they are cured.
Acknowledgments
Funding
This study was supported by the NIH Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
This work was supported by the Intramural program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development
Footnotes
Author contribution statement
Evgenia Gourgari performed most of the data collection described in the manuscript and prepared the manuscript. Maya Lodish is currently the principal investigator for the clinical protocols. Meg Keil, Charalampos Lyssikatos and Elena Belyavskaya participated in the clinical care of the patients. Robert Wesley did the statistical analysis. Suvimol Hill was responsible for the reading and interpretation of bone age films of the patients. Sierra Maria De La Luz and Paraskevi Xekouki participated in the genetic analysis of the patients. Constantine A Stratakis was the principal investigator of the clinical protocol until recently, saw all the patients, and his NIH Intramural Grant provided allof the funding for this project; he also supervised the presentation of results, design of figures, and assisted in writing this manuscript.
Declaration of interest
Disclosure summary: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 2.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. The Journal of clinical endocrinology and metabolism. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney Complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006;9:203–209. doi: 10.1007/s11102-006-0265-2. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis CC, Storr HL, Perry LA, Ho JT, Ahmed L, Ong KK, Dunger DB, Monson JP, Grossman AB, Besser GM, et al. Abnormal puberty in paediatric Cushing’s disease: relationship with adrenal androgen sex hormone binding globulin and gonadotrophin concentrations. Clin Endocrinol (Oxf) 2007;66:838–843. doi: 10.1111/j.1365-2265.2007.02822.x. [DOI] [PubMed] [Google Scholar]
- 5.Frantz AG, Rabkin MT. Human Growth Hormone. Clinical Measurement Response to Hypoglycemia and Suppression by Corticosteroids. N Engl J Med. 1964;271:1375–1381. doi: 10.1056/NEJM196412312712701. [DOI] [PubMed] [Google Scholar]
- 6.Krieger DT, Glick SM. Growth hormone and cortisol responsiveness in Cushing’s syndrome. Relation to a possible central nervous system etiology. Am J Med. 1972;52:25–40. doi: 10.1016/0002-9343(72)90005-8. [DOI] [PubMed] [Google Scholar]
- 7.Lodish M, Dunn SV, Sinaii N, Keil MF, Stratakis CA. Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing’s disease. The Journal of clinical endocrinology and metabolism. 2012;97:1483–1491. doi: 10.1210/jc.2011-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodish MB, Gourgari E, Sinaii N, Hill S, Libuit L, Mastroyannis S, Keil M, Batista DL, Stratakis CA. Skeletal Maturation in Children with Cushing Syndrome Is Not Consistently Delayed: The Role of Corticotropin Obesity, and Steroid Hormones and the Effect of Surgical Cure. J Pediatr. 2014 doi: 10.1016/j.jpeds.2013.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.London E, Rothenbuhler A, Lodish M, Gourgari E, Keil M, Lyssikatos C, de la Luz Sierra M, Patronas N, Nesterova M, Stratakis CA. Differences in adiposity in Cushing syndrome caused by PRKAR1A mutations: clues for the role of cyclic AMP signaling in obesity and diagnostic implications. The Journal of clinical endocrinology and metabolism. 2014;99:E303–310. doi: 10.1210/jc.2013-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magiakou MA, Mastorakos G, Gomez MT, Rose SR, Chrousos GP. Suppressed spontaneous and stimulated growth hormone secretion in patients with Cushing’s disease before and after surgical cure. The Journal of clinical endocrinology and metabolism. 1994a;78:131–137. doi: 10.1210/jcem.78.1.7507118. [DOI] [PubMed] [Google Scholar]
- 11.Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr, Nieman LK, Chrousos GP. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994b;331:629–636. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 12.McArthur RG, Hayles AB, Salassa RM. Growth retardation in Cushing disease. J Pediatr. 1980;96:783–784. doi: 10.1016/s0022-3476(80)80782-7. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41:793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. The Journal of clinical endocrinology and metabolism. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]


