Abstract
Background
Information is limited regarding utilization patterns and costs for chronic kidney disease mineral and bone disorder (CKD-MBD) medications in Medicare Part D-enrolled dialysis patients.
Study Design
Retrospective cohort study.
Setting & Participants
Annual cohorts of dialysis patients, 2007–2010.
Predictors
Cohort year, low-income subsidy status, and dialysis provider.
Outcomes
Utilization and costs of prescription phosphate binders, oral and intravenous vitamin D analogues, and cinacalcet.
Measurements
Using logistic regression, we calculated adjusted odds of medication use for low-income subsidy versus non-low-income subsidy patients and for patients from various dialysis organizations, and we report per-member-per-month costs and average out-of-pocket costs.
Results
Phosphate binders (∼83%) and intravenous vitamin D (77.5%−79.3%) were the most commonly used CKD-MBD medications from 2007 through 2010. The adjusted odds of prescription phosphate binder, intravenous vitamin D, and cinacalcet use were significantly higher for low-income subsidy than for non-low-income subsidy patients. Total Part D versus CKD-MBD Part D medication costs increased 22% versus 36% from 2007 to 2010. Among Part D-enrolled dialysis patients, CKD-MBD medications represented about 50% of overall net Part D costs in 2010.
Limitations
Inability to describe utilization and costs of calcium carbonate, an over-the-counter agent not covered under Medicare Part D; inability to reliably identify prescriptions filled through a non-Part D reimbursement or payment mechanism; findings may not apply to dialysis patients without Medicare Part D benefits or with Medicare Advantage plans, or to pediatric dialysis patients; could identify only prescription drugs dispensed in the outpatient setting; inability to adjust for MBD laboratory values.
Conclusions
Part D net costs for CKD-MBD medications increased at a faster rate than costs for all Part D medications in dialysis patients, despite relatively stable use within medication classes. In a bundled environment, there may be incentives to shift to generic phosphate binders and reduce use of cinacalcet.
Index words: Chronic kidney disease (CKD), Medicare Part D, medication costs, dialysis, mineral and bone disorder, phosphate binders, calcimimetics, vitamin D analogues
As kidney function declines, calcium/phosphorus homeostasis is progressively disrupted, and serum concentrations of these minerals and of circulating levels of vitamin D and parathyroid hormones are altered. Chronic kidney disease mineral and bone disorder (CKD-MBD) is not a single disease but a constellation of systemic disorders of mineral and bone metabolism that involve the kidneys, skeleton, parathyroid glands, and vasculature.1;2 It has been associated with increased risk of fractures, cardiovascular disease, and mortality.1
Successful management of CKD-MBD among dialysis patients relies on a combination of therapeutic agents, including phosphate binders, calcimimetics, and vitamin D analogues, that target MBD biochemical abnormalities associated with CKD. Clinical management of CKD-MBD is challenging and costly. We have previously shown that the combination of phosphate binders and cinacalcet represents almost half of Medicare Part D drug costs in Medicare Part D-enrolled dialysis patients.3 An understanding of the patterns of use and costs of these medications in the Medicare population will help guide clinical decision making and reimbursement policy, especially in the era of the end-stage renal disease (ESRD) prospective payment system (PPS). The primary objective of this study was to describe the medication utilization patterns and associated costs of CKD-MBD management in Medicare-insured populations of dialysis patients for 2007 through 2010.
Methods
Information on patient characteristics and comorbidity, dialysis providers, and medication utilization was obtained from the Centers for Medicare & Medicaid Services (CMS) ESRD database linked with Medicare Part D data. Yearly cohorts of patients for calendar years 2007 through 2010 were created. For each calendar year, we constructed a cohort of adult dialysis patients (aged ≥ 18 years) alive on December 31 of the previous year, with Medicare Parts A, B, and D coverage from January 1 to the earliest of death or December 31 of the year. We followed up patients from January 1 of each year to the earlier of death or December 31 of the year. In addition, we categorized patients as receiving low-income subsidy (LIS) assistance if they received it throughout the entire follow-up period. The LIS provides full or partial waivers for the cost-sharing components of the Medicare Part D benefit structure. The LIS eligibility requirements include dual eligibility for Medicare and Medicaid, receipt of supplemental security income, participation in Medicare savings programs, or having limited assets and income. Patient age was calculated as of January 1 of each year.
Patient demographic characteristics included age, sex, race, and ethnicity. For each yearly cohort, we identified users of three groups of CKD-MBD medications: phosphate binders (calcium acetate, lanthanum carbonate, sevelamer carbonate, sevelamer hydrochloride), vitamin D analogues (oral and intravenous [IV] calcitriol, doxercalciferol, paricalcitol), and cinacalcet (a calcimimetic). There are no data on sevelamer carbonate in 2007 because it only became available on the US market in 2008. We did not include calcium carbonate (an over-the-counter phosphate binding agent) because, by law, over-the-counter medications are not covered through Medicare Part D and plans do not routinely provide them. Information on IV vitamin D analogues was obtained from outpatient facility files from the CMS ESRD database.
Medication utilization was defined as one or more Part D prescription drug events for each medication (or dialysis administration claims for IV vitamin D analogues) during the follow-up period. We report use of these agents individually and within medication classes. Net Part D payment and associated out-of-pocket drug costs for each Part D-covered medication and medication class were calculated. Net Part D payment was defined as the sum of the Part D-covered plan payment and LIS cost-sharing amounts. Out-of-pocket costs for each prescription were payments made by or on behalf of the patient (not including the LIS amount). For cost calculations, we restricted the yearly cohorts to patients who survived the entire calendar year. Dialysis providers included large dialysis organizations (Fresenius, DaVita, Dialysis Clinics Inc [DCI]), small dialysis organizations, hospital-based units, and independent units.
We calculated tabular summaries of patient characteristics by cohort year. Descriptive statistics were reported for medication utilization and costs, with results stratified separately by LIS status and by dialysis organization. In these analyses, we weighted each patient by duration of follow-up, such that medication use can be interpreted as the proportion of patient-years with at least one prescription or record of administration. Using logistic regression models, we calculated odds of medication use for patients with versus without the LIS benefit, adjusted for age, sex, race, dialysis vintage, and dialysis organization. Similarly, we calculated odds of medication use among patients receiving treatment from Fresenius, DCI, small dialysis organizations, independent units, and hospital-based units compared with DaVita patients, adjusted for age, sex, race, and dialysis vintage. The logistic regression models were performed separately in each yearly cohort. All analyses were performed using SAS, version 9.1.2 (SAS Institute Inc, Cary, NC).
Results
Patient Characteristics
About 200,000 dialysis patients met inclusion criteria, having continuous Medicare Parts A, B, and D coverage during follow-up for each cohort year (2007–2010). About 45% of the study populations were aged ≥ 65 years, and mean age, sex, race and ethnicity, and LIS distributions were similar across yearly cohorts (Table 1).
Table 1.
Characteristics of adult dialysis patients enrolled in Medicare Part D, 2007–2010
| Characteristic | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|
| No. of patients | 198,349 | 209,972 | 220,051 | 231,320 |
| Age (y) | 61.5 ±15.4 | 61.7 ±15.3 | 61.8 ±15.3 | 61.9 ±15.2 |
| Age category | ||||
| 18–44 y | 31,307(15.8) | 32,117 (15.3) | 32,826 (14.9) | 33,577 (14.5) |
| 45–64 y | 77,004 (38.8) | 81,487 (38.8) | 86,097 (39.1) | 91,561 (39.6) |
| 65–74 y | 47,952 (24.2) | 51,341 (24.5) | 54,012 (24.5) | 56,698 (24.5) |
| ≥ 75 y | 42,086 (21.2) | 45,027 (21.4) | 47,116 (21.4) | 49,484 (21.4) |
| Sex | ||||
| Male | 101,888 (51.4) | 108,723 (51.8) | 114,853 (52.2) | 121,163 (52.4) |
| Female | 96,461 (48.6) | 101,249 (48.2) | 105,198 (47.8) | 110,157 (47.6) |
| Race | ||||
| White | 103,381 (52.1) | 110,752 (52.7) | 116,927 (53.1) | 123,693 (53.5) |
| Black | 81,394 (41.0) | 85,099 (40.5) | 88,389 (40.2) | 92,008 (39.8) |
| Asian | 8965 (4.5) | 9661 (4.6) | 10,356 (4.7) | 11,291 (4.9) |
| Other | 4609 (2.3) | 4460 (2.1) | 4379 (2.0) | 4328 (1.9) |
| Ethnicity | ||||
| Hispanic | 31,511 (15.9) | 33,765 (16.1) | 36,322 (16.5) | 39,101 (16.9) |
| Non-Hispanic | 166,838 (84.1) | 176,207 (83.9) | 183,729 (83.5) | 192,219 (83.1) |
| Dialysis vintage | ||||
| Median [range], y | 2.98 [0.25–41.4] | 3.01 [0.25–44.8] | 3.07 [0.25–45.8] | 3.17 [0.25–46.8] |
| < 1.0 y | 33,498 (16.9) | 34,173 (16.3) | 35,043 (15.9) | 36,348 (15.7) |
| 1.0–1.9 y | 36,910 (18.6) | 39,654 (18.9) | 39,779 (18.1) | 40,815 (17.6) |
| 2.0–4.9 y | 70,015 (35.3) | 73,740 (35.1) | 78,203 (35.5) | 81,915 (35.4) |
| ≥ 5.0 y | 57,926 (29.2) | 62,405 (29.7) | 67,026 (30.5) | 72,242 (31.2) |
| LIS status | ||||
| Non-LIS | 55,134 (27.8) | 60,360 (28.7) | 64,335 (29.2) | 67,446 (29.2) |
| LIS | 143,215 (72.2) | 149,612 (71.3) | 155,716 (70.8) | 163,874 (70.8) |
| Dialysis organization | ||||
| Fresenius | 48,006 (26.4) | 66,568 (31.8) | 69,475 (31.7) | 72,410 (31.4) |
| DaVita | 52,935 (29.1) | 56,817 (27.2) | 59,924 (27.3) | 63,860 (27.7) |
| Dialysis Clinics Inc | 7458 (4.1) | 7680 (3.7) | 7767 (3.5) | 7839 (3.4) |
| Small dialysis organization | 13,331 (7.3) | 16,138 (7.7) | 18,843 (8.6) | 26,159 (11.4) |
| Independent | 35,746 (19.7) | 36,286 (17.3) | 36,021(16.4) | 32,972 (14.3) |
| Hospital-based | 24,292 (13.4) | 25,681 (12.3) | 27,156 (12.4) | 27,175 (11.8) |
LIS, low-income subsidy.
Note: Each annual cohort is a prevalent cohort of adult (age ≥ 18 years) dialysis patients alive on December 31 of the previous year, with Medicare Parts A, B, and D coverage from January 1 of the present year to the earlier of death or December 31 of the present year. Unless otherwise indicated, values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation.
Prescription and Utilization of MBD Medications
From 2007 through 2010, phosphate binders were the most commonly prescribed MBD medications, followed by IV vitamin D analogues, cinacalcet, and oral vitamin D analogues (Table 2). Sevelamer (both hydrochloride and carbonate) was the most common prescription phosphate binder, prescribed for 54.2% of patient-years in 2010. Paricalcitol constituted most IV vitamin D administered, and calcitriol predominated over the other oral vitamin D analogues.
Table 2.
Chronic kidney disease mineral and bone disorder medication use among dialysis patients enrolled in Medicare Part D, 2007–2010
| All | Without LIS | With LIS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | 2007 | 2008 | 2009 | 2010 | 2007 | 2008 | 2009 | 2010 | 2007 | 2008 | 2009 | 2010 |
| No. of patients | 198,349 | 209,972 | 220,051 | 231,320 | 55,134 | 60,360 | 64,335 | 67,446 | 143,215 | 149,612 | 155,716 | 163,874 |
| Phosphate binder | ||||||||||||
| Calcium acetate | 40.6 | 39.7 | 39.2 | 38.7 | 38.5 | 37.3 | 37.0 | 36.9 | 41.3 | 40.6 | 40.1 | 39.4 |
| Lanthanum carbonate | 13.0 | 12.0 | 11.3 | 9.5 | 10.1 | 8.5 | 7.3 | 6.0 | 14.1 | 13.4 | 12.9 | 10.9 |
| Sevelamer carbonate | 0.0 | 5.3 | 23.2 | 43.0 | 0.0 | 4.1 | 20.1 | 33.7 | 0.0 | 5.7 | 24.5 | 46.7 |
| Sevelamer hydrochloride | 52.2 | 51.3 | 40.3 | 19.9 | 42.4 | 41.0 | 28.8 | 11.4 | 55.9 | 55.4 | 44.9 | 23.3 |
| Sevelamer | 52.2 | 53.2 | 53.5 | 54.2 | 42.4 | 42.7 | 41.9 | 41.2 | 55.9 | 57.4 | 58.2 | 59.4 |
| Any phosphate binder | 83.0 | 83.2 | 83.2 | 82.6 | 74.4 | 73.7 | 72.5 | 71.4 | 86.2 | 87.0 | 87.5 | 87.1 |
| Oral vitamin D analogue | ||||||||||||
| Calcitriol | 5.5 | 5.8 | 6.0 | 6.3 | 5.6 | 6.2 | 6.2 | 6.5 | 5.4 | 5.7 | 5.9 | 6.3 |
| Doxercalciferol | 2.4 | 2.3 | 2.2 | 2.2 | 2.2 | 2.0 | 1.9 | 1.8 | 2.4 | 2.4 | 2.4 | 2.3 |
| Paricalcitol | 1.2 | 1.4 | 1.8 | 2.0 | 1.2 | 1.5 | 1.8 | 2.1 | 1.2 | 1.4 | 1.8 | 2.0 |
| Any oral vitamin D analogue | 8.5 | 9.1 | 9.5 | 9.9 | 8.5 | 9.2 | 9.3 | 9.8 | 8.6 | 9.0 | 9.5 | 10.0 |
| IV Vitamin D analogue | ||||||||||||
| Calcitriol | 2.4 | 1.7 | 1.6 | 1.8 | 1.5 | 1.1 | 1.0 | 1.1 | 2.7 | 1.9 | 1.8 | 2.0 |
| Doxercalciferol | 27.4 | 22.4 | 20.9 | 35.4 | 21.7 | 17.7 | 16.3 | 26.6 | 29.6 | 24.2 | 22.7 | 39.0 |
| Paricalcitol | 59.4 | 62.5 | 62.4 | 65.0 | 47.9 | 50.0 | 49.6 | 51.2 | 63.7 | 67.5 | 67.5 | 70.5 |
| Any IV vitamin analogue | 79.3 | 79.1 | 77.5 | 77.5 | 63.6 | 63.2 | 61.3 | 60.4 | 85.2 | 85.4 | 84.1 | 84.4 |
| Calcimimetic | ||||||||||||
| Cinacalcet | 31.8 | 33.3 | 31.9 | 31.0 | 21.2 | 22.3 | 21.0 | 20.8 | 35.8 | 37.7 | 36.3 | 35.1 |
Note: Values are given as percentages.
LIS, low-income subsidy; IV, intravenous.
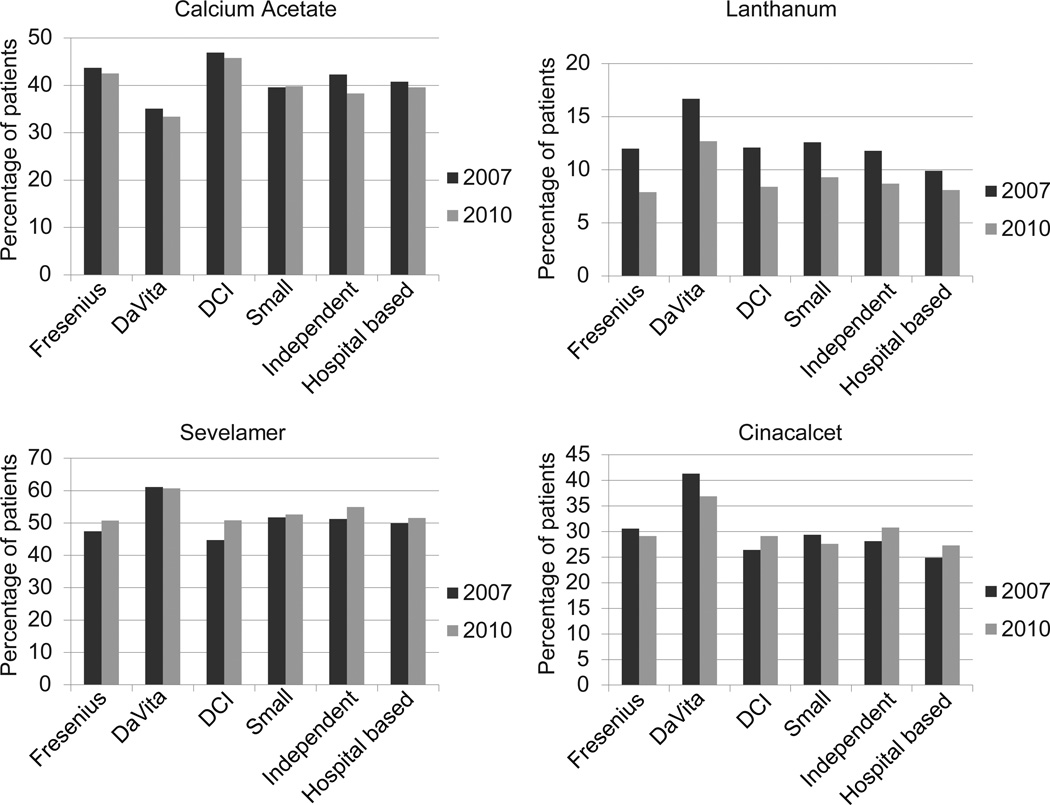
In the years 2007 through 2010, calcium acetate and lanthanum carbonate prescription decreased (40.6% to 38.7% and 13.0% to 9.5%, respectively), while sevelamer prescription increased. Sevelamer carbonate prescription increased sharply, from 5.3% in 2008 to 43.0% in 2010. Conversely, sevelamer hydrochloride prescription declined sizably, from 52.2% in 2007 to 19.9% in 2010. Despite substantial variation in use of individual phosphate binding agents, prescription of any agent was relatively constant from 2007 through 2010 at approximately 83%. Cinacalcet prescription increased slightly between 2007 and 2008 and decreased thereafter. For each of the 4 cohort years, the odds of prescription phosphate binding agent and cinacalcet use were significantly higher in dialysis patients with than without LIS status (Table 3).
Table 3.
Odds of chronic kidney disease mineral and bone disorder medication prescription in Medicare Part D-enrolled dialysis patients with versus without low-income subsidy
| 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|
| Medication | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Phosphate binder | ||||
| Calcium acetate | 1.17 (1.14–1.19) | 1.20 (1.17–1.23) | 1.20 (1.17–1.22) | 1.17 (1.14–1.19) |
| Lanthanum carbonate | 1.24 (1.20–1.29) | 1.44 (1.39–1.50) | 1.63 (1.57–1.69) | 1.61 (1.55–1.67) |
| Sevelamer carbonate | - | 1.21 (1.15–1.27) | 1.16 (1.13–1.19) | 1.48 (1.45–1.52) |
| Sevelamer hydrochloride | 1.50 (1.47–1.54) | 1.60 (1.57–1.64) | 1.89 (1.85–1.93) | 2.33 (2.26–2.40) |
| Any agent | 1.60 (1.56–1.64) | 1.75 (1.71–1.79) | 1.91 (1.86–1.95) | 1.91 (1.87–1.95) |
| Oral Vitamin D analogue | ||||
| Calcitriol | 0.72 (0.69–0.76) | 0.70 (0.67–0.73) | 0.73 (0.70–0.76) | 0.75 (0.72–0.78) |
| Doxercalciferol | 0.79 (0.73–0.86) | 0.85 (0.79–0.92) | 0.96 (0.89–1.03)b | 0.95 (0.88–1.03)b |
| Paricalcitol | 0.74 (0.66–0.82) | 0.74 (0.67–0.81) | 0.78 (0.71–0.83) | 0.72 (0.67–0.78) |
| Any agent | 0.74 (0.71–0.77) | 0.73 (0.71–0.76) | 0.78 (0.75–0.80) | 0.77 (0.75–0.80) |
| IV Vitamin D analogue | ||||
| Calcitriol | 1.37 (1.26–1.49) | 1.19 (1.09–1.31) | 1.24 (1.13–1.370 | 1.34 (1.22–1.46) |
| Doxercalciferol | 1.48 (1.44–1.52) | 1.44 (1.40–1.48) | 1.46 (1.42–1.50) | 1.64 (1.60–1.68) |
| Paricalcitol | 1.66 (1.63–1.70) | 1.73 (1.70–1.77) | 1.76 (1.72–1.80) | 1.86 (1.82–1.90) |
| Any agent | 2.21 (2.16–2.27) | 2.28 (2.23–2.33) | 2.26 (2.21–2.31) | 2.38 (2.33–2.43) |
| Calcimimetic | ||||
| Cinacalcet | 1.44 (1.41–1.48) | 1.51 (1.47–1.55) | 1.53 (1.49–1.57) | 1.44 (1.41–1.48) |
Note: P values were < 0.001 unless stated otherwise. Odd ratios are calculated from a model including age, sex, race, dialysis vintage, and dialysis organization.
CI, confidence interval; IV, intravenous; OR, odds ratio.
P values were > 0.05.
Intravenous (IV) paricalcitol use increased from 59.4% in 2007 to 65.0% in 2010, and IV doxercalciferol use from 27.4% to 35.4% in the same time period. Use of IV calcitriol was very low in each year. The odds of IV vitamin D use were higher among dialysis patients with than without LIS status (Table 3). Compared with IV vitamin D use, use of oral vitamin D analogues was small, but increasing (8.5% in 2007; 9.9% in 2010).
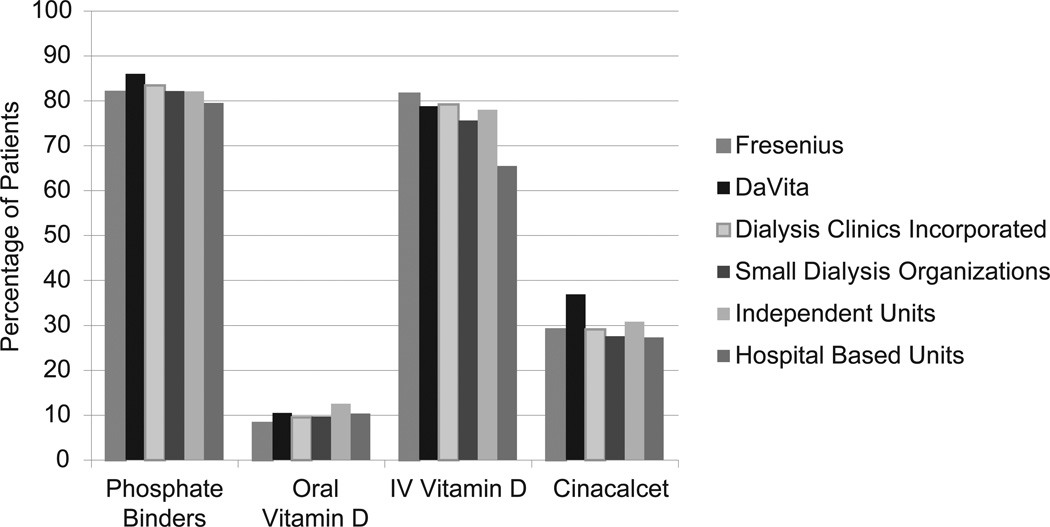
Prevalence of dialysis patients prescribed CKD-MBD medications across different dialysis organizations for 2010 is shown in Figure 1. Use of phosphate binders and cinacalcet was highest for patients receiving dialysis at DaVita facilities (86.0% and 36.9%, respectively), and use of IV vitamin D analogues was highest for patients at Fresenius (81.6%). Compared with patients from other dialysis organizations, DaVita patients were significantly more likely to be prescribed lanthanum carbonate, sevelamer, and cinacalcet, and less likely to be prescribed calcium acetate (Figure 2 and Table S1, available as online supplementary material). Patients in independent dialysis units were significantly more likely to receive oral vitamin D analogues compared with DaVita patients, and odds of receiving oral vitamin D analogues were significantly lower for patients receiving dialysis from Fresenius, DCI, and small dialysis organizations compared with odds for DaVita patients.
Figure 1.
Percentage of Medicare Part D-enrolled dialysis patients prescribed chronic kidney disease mineral bone disorder medications by dialysis organization type in 2010. IV, intravenous.
Figure 2.
Percentage of Medicare Part D-enrolled dialysis patients prescribed phosphate binders and cinacalcet by dialysis organization in 2007 and 2010. DCI, Dialysis Clinic Inc.
Costs
In 2010, overall per-member-per-month (PMPM) net Part D payments for all Part D medications were $555, $691, and $207 for all patients and for patients with and without LIS status, respectively (Table 4). Overall PMPM net Part D payments for all Part D medications increased steadily from 2007 through 2010; increases ranged from 13% for non-LIS patients to 20% for LIS patients (Table S2).
Table 4.
Per member per month net Medicare Part D payment for CKD-MBD medications by LIS status
| All | Without LIS | With LIS | ||||
|---|---|---|---|---|---|---|
| Medication | Total a | Percent b | Total a | Percent b | Total a | Percent b |
| 2007 | ||||||
| All Part D medications | 454.45 | 100.0 | 183.16 | 100.0 | 554.22 | 100.0 |
| All CKD-MBD medications | 204.22 | 44.94 | 73.47 | 40.11 | 252.31 | 45.52 |
| Phosphate binders | 122.11 | 26.87 | 48.47 | 26.46 | 149.19 | 26.92 |
| Calcium acetate | 11.71 | 2.58 | 6.21 | 3.39 | 13.73 | 2.48 |
| Lanthanum carbonate | 15.70 | 3.45 | 6.59 | 3.60 | 19.05 | 3.44 |
| Sevelamer carbonate | - | - | - | - | - | |
| Sevelamer hydrochloride | 94.70 | 20.84 | 35.66 | 19.47 | 116.42 | 21.01 |
| Sevelamer | 94.70 | 20.84 | 35.66 | 19.47 | 116.42 | 21.01 |
| Oral Vitamin D analogue | 4.45 | 0.98 | 2.17 | 1.18 | 5.29 | 0.95 |
| Calcitriol | 1.02 | 0.22 | 0.59 | 0.32 | 1.17 | 0.21 |
| Doxercalciferol | 2.32 | 0.51 | 1.04 | 0.57 | 2.80 | 0.51 |
| Paricalcitol | 1.11 | 0.24 | 0.55 | 0.30 | 1.31 | 0.24 |
| Calcimimetic | ||||||
| Cinacalcet | 77.66 | 17.09 | 22.83 | 12.46 | 97.83 | 17.65 |
| 2010 | ||||||
| All Part D medications | 555.22 | 100.0 | 207.22 | 100.0 | 691.15 | 100.0 |
| All CKD-MBD medications | 278.65 | 50.19 | 91.11 | 43.97 | 351.90 | 50.92 |
| Phosphate binders | 166.30 | 29.95 | 56.22 | 27.13 | 209.30 | 30.28 |
| Calcium acetate | 18.25 | 3.29 | 9.88 | 4.77 | 21.51 | 3.11 |
| Lanthanum carbonate | 23.92 | 4.31 | 6.67 | 3.22 | 30.66 | 4.44 |
| Sevelamer carbonate | 87.75 | 15.80 | 30.43 | 14.68 | 110.15 | 15.94 |
| Sevelamer hydrochloride | 36.38 | 6.55 | 9.24 | 4.46 | 46.98 | 6.80 |
| Sevelamer | 124.13 | 22.36 | 39.67 | 19.14 | 157.12 | 22.73 |
| Oral Vitamin D analogue | 9.23 | 1.66 | 3.78 | 1.82 | 11.36 | 1.64 |
| Calcitriol | 1.22 | 0.22 | 0.64 | 0.31 | 1.45 | 0.21 |
| Doxercalciferol | 4.99 | 0.90 | 1.82 | 0.88 | 6.23 | 0.90 |
| Paricalcitol | 3.01 | 0.54 | 1.32 | 0.64 | 3.68 | 0.53 |
| Calcimimetic | ||||||
| Cinacalcet | 103.12 | 18.57 | 31.11 | 15.01 | 131.24 | 18.99 |
CKD, chronic kidney disease; LIS, low-income subsidy; MBD, mineral and bone disorder.
Total Part D net payment, $.
Percent of all Part D net payment.
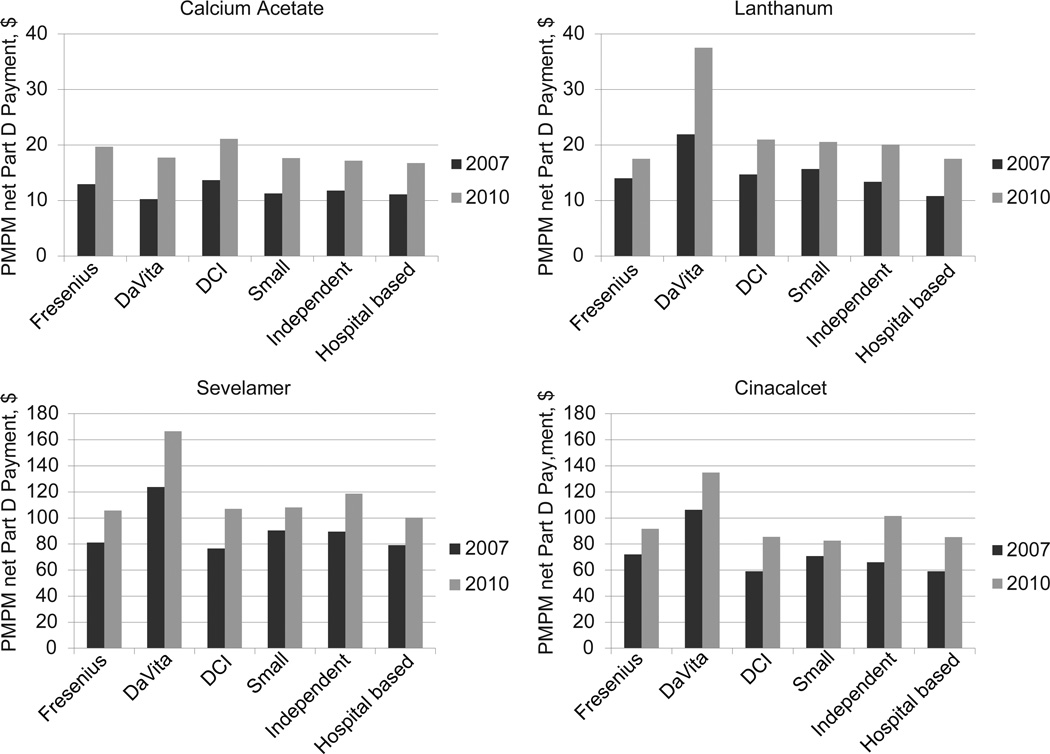
In 2010, PMPM net Part D payments for calcium acetate, lanthanum carbonate, and sevelamer (hydrochloride and carbonate) were about $18, $24, and $124, respectively, representing increases of 36%, 52%, and 31% , respectively, from 2007 (Figure S1). Phosphate binders accounted for 27% to 30% of all PMPM net Part D spending during the study period. Expenditures for oral vitamin D analogues increased from about 1% in 2007 to 1.7% of all PMPM net Part D payments in 2010 (Table 4). Among all patients, PMPM net Part D payments for cinacalcet rose from $78 in 2007 to $103 in 2010, accounting for about 19% of net Part D payments in 2010. The PMPM net Part D expenditures for cinacalcet, sevelamer, and lanthanum carbonate during the study period were highest among DaVita patients compared with patients receiving dialysis from other providers (Figure 3, Table S3).
Figure 3.
Per member per month net Part D payment for chronic kidney disease mineral bone disorder medications in dialysis patients by dialysis organization in 2007 and 2010. DCI, Dialysis Clinic Inc.; PMPM, per member per month.
Overall, the per-user-per-month (PUPM) out-of-pocket costs for phosphate binders increased from 2007 to 2008 and decreased from 2008 to 2009 (Table 5). For calcium acetate, the PUPM out-of-pocket costs increased by 22% from 2007 to 2008, then decreased by 42% from 2008 to 2009 (Table 5). The PUPM out-of-pocket costs for lanthanum carbonate increased from 2007 to 2008 and remained approximately unchanged thereafter. Increases in PUPM out-of-pocket costs for sevelamer carbonate were sustained year to year (Table 5), but PUPM out-of-pocket costs for sevelamer (both hydrochloride and carbonate) have declined since 2008. The PUPM out-of-pocket costs for phosphate binding agents were much higher for non-LIS than for LIS patients.
Table 5.
Out-of-pocket costs per user per month for chronic kidney disease mineral and bone disorder
| All | Without LIS | With LIS | All | |||||
|---|---|---|---|---|---|---|---|---|
| Medication | No. of users | Costs ($) | No. of users |
Costs ($) | No. of users |
Costs ($) | No. of users |
Costs ($) |
| 2007 | ||||||||
| All Part D medications | 156,940 | 36.16 | 41,796 | 113.34 | 115,144 | 8.15 | 156,940 | 36.16 |
| Phosphate binders | 127,351 | 9.05 | 30,481 | 33.04 | 96,870 | 1.50 | 127,351 | 9.05 |
| Calcium acetate | 62,435 | 4.42 | 15,880 | 14.09 | 46,555 | 1.12 | 62,435 | 4.42 |
| Lanthanum carbonate | 20,587 | 6.62 | 4247 | 28.63 | 16,340 | 0.90 | 20,587 | 6.62 |
| Sevelamer carbonate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sevelamer hydrochloride | 80,873 | 9.15 | 17,586 | 37.63 | 63,287 | 1.24 | 80,873 | 9.15 |
| Sevelamer (any) | 80,873 | 9.15 | 17,586 | 37.63 | 63,287 | 1.24 | 80,873 | 9.15 |
| Oral Vitamin D analogue | 13,644 | 3.71 | 3633 | 12.16 | 10,011 | 0.65 | 13,644 | 3.71 |
| Calcitriol | 8753 | 1.44 | 2408 | 4.39 | 6345 | 0.32 | 8753 | 1.44 |
| Doxercalciferol | 3787 | 6.32 | 948 | 21.93 | 2839 | 1.11 | 3787 | 6.32 |
| Paricalcitol | 1874 | 7.54 | 501 | 25.55 | 1373 | 0.97 | 1874 | 7.54 |
| Calcimimetic | ||||||||
| Cinacalcet | 50,679 | 10.82 | 9038 | 54.29 | 41,641 | 1.38 | 50,679 | 10.82 |
| 2008 | ||||||||
| All Part D medications | 167,651 | 37.54 | 46,055 | 115.44 | 121,596 | 8.03 | 167,651 | 37.54 |
| Phosphate binders | 136,464 | 10.66 | 33,237 | 38.96 | 103,227 | 1.54 | 136,464 | 10.66 |
| Calcium acetate | 65,517 | 5.40 | 16,975 | 17.66 | 48,542 | 1.11 | 65,517 | 5.40 |
| Lanthanum carbonate | 20,280 | 7.96 | 3935 | 37.11 | 16,345 | 0.95 | 20,280 | 7.96 |
| Sevelamer carbonate | 9601 | 5.11 | 2083 | 21.97 | 7518 | 0.43 | 9601 | 5.11 |
| Sevelamer hydrochloride | 84,539 | 10.53 | 18,552 | 43.31 | 65,987 | 1.32 | 84,539 | 10.53 |
| Sevelamer (any) | 87,964 | 10.68 | 19,403 | 43.77 | 68,561 | 1.31 | 87,964 | 10.68 |
| Oral Vitamin D analogue | 15,293 | 4.52 | 4253 | 14.47 | 11,040 | 0.68 | 15,293 | 4.52 |
| Calcitriol | 9868 | 1.55 | 2854 | 4.54 | 7014 | 0.33 | 9868 | 1.55 |
| Doxercalciferol | 3832 | 8.12 | 950 | 29.24 | 2882 | 1.17 | 3832 | 8.12 |
| Paricalcitol | 2441 | 9.29 | 686 | 30.33 | 1755 | 1.07 | 2441 | 9.29 |
| Calcimimetic | ||||||||
| Cinacalcet | 56,368 | 12.46 | 10,368 | 61.29 | 46,000 | 1.46 | 56,368 | 12.46 |
| 2009 | ||||||||
| All Part D medications | 176,374 | 37.37 | 49,277 | 112.46 | 127,097 | 8.26 | 176,374 | 37.37 |
| Phosphate binders | 143,040 | 9.01 | 34,924 | 32.68 | 108,116 | 1.36 | 143,040 | 9.01 |
| Calcium acetate | 67,737 | 3.15 | 17,920 | 10.23 | 49,817 | 0.60 | 67,737 | 3.15 |
| Lanthanum carbonate | 19,917 | 7.70 | 3579 | 38.44 | 16,338 | 0.97 | 19,917 | 7.70 |
| Sevelamer carbonate | 42,153 | 8.07 | 10,261 | 30.15 | 31,892 | 0.97 | 42,153 | 8.07 |
| Sevelamer hydrochloride | 69,178 | 8.40 | 13,782 | 37.08 | 55,396 | 1.27 | 69,178 | 8.40 |
| Sevelamer (any) | 92,857 | 9.92 | 20,388 | 40.24 | 72,469 | 1.39 | 92,857 | 9.92 |
| Oral Vitamin D analogue | 16,737 | 5.07 | 4600 | 16.50 | 12,137 | 0.75 | 16,737 | 5.07 |
| Calcitriol | 10,496 | 1.63 | 3044 | 4.72 | 7452 | 0.37 | 10,496 | 1.63 |
| Doxercalciferol | 3989 | 9.18 | 927 | 35.44 | 3062 | 1.23 | 3989 | 9.18 |
| Paricalcitol | 3135 | 9.95 | 871 | 32.89 | 2264 | 1.12 | 3135 | 9.95 |
| Calcimimetic | ||||||||
| Cinacalcet | 56,599 | 13.52 | 10,539 | 65.93 | 46,060 | 1.53 | 56,599 | 13.52 |
| 2010 | ||||||||
| All Part D medications | 187,163 | 37.39 | 52,179 | 111.51 | 134,984 | 8.74 | 187,163 | 37.39 |
| Phosphate binders | 151,309 | 8.60 | 36,553 | 31.18 | 114,756 | 1.40 | 151,309 | 8.60 |
| Calcium acetate | 71,171 | 3.18 | 18,941 | 10.41 | 52,230 | 0.56 | 71,171 | 3.18 |
| Lanthanum carbonate | 17,860 | 7.89 | 3155 | 39.73 | 14,705 | 1.06 | 17,860 | 7.89 |
| Sevelamer carbonate | 80,452 | 9.00 | 17,708 | 36.09 | 62,744 | 1.36 | 80,452 | 9.00 |
| Sevelamer hydrochloride | 35,901 | 5.83 | 5648 | 31.57 | 30,253 | 1.02 | 35,901 | 5.83 |
| Sevelamer (any) | 100,079 | 9.33 | 21,322 | 38.33 | 78,757 | 1.47 | 100,079 | 9.33 |
| Oral Vitamin D analogue | 18,584 | 5.63 | 5130 | 18.21 | 13,454 | 0.84 | 18,584 | 5.63 |
| Calcitriol | 11,813 | 1.63 | 3383 | 4.72 | 8430 | 0.39 | 11,813 | 1.63 |
| Doxercalciferol | 4135 | 11.32 | 977 | 43.43 | 3158 | 1.39 | 4135 | 11.32 |
| Paricalcitol | 3813 | 10.12 | 1083 | 32.34 | 2730 | 1.30 | 3813 | 10.12 |
| Calcimimetic | ||||||||
| Cinacalcet | 58,206 | 15.06 | 10,954 | 72.73 | 47,252 | 1.69 | 58,206 | 15.06 |
LIS, low-income subsidy.
Among non-LIS patients, the PUPM out-of-pocket costs for oral doxercalciferol and cinacalcet increased steadily between 2007 and 2010 (Figure S2). For each year from 2007 through 2010, the PUPM out-of-pocket costs for cinacalcet in non-LIS patients were about 40 times the costs for that in LIS patients (e.g., about $73 for non-LIS patients and $2 for LIS patients in 2010).
Discussion
To our knowledge, we present the first longitudinal, comprehensive evaluation of CKD-MBD medication prescription, utilization, and cost trends in dialysis patients since the Medicare Part D prescription drug program began in 2006. Most (over 70%) dialysis patients were enrolled in Medicare Part D in 2010.3 Most dialysis patients are prescribed phosphate binders, with sevelamer products most highly prescribed. Over the study timeframe, oral vitamin D use increased slightly and IV vitamin D use decreased slightly; IV paricalcitol use increased and IV doxercalciferol use decreased and then increased. Cinacalcet use remained fairly stable from 2007 to 2010. After adjustment for age, race, sex, dialysis vintage, and dialysis organization, odds of receiving a phosphate binder were higher for LIS patients. Additionally, LIS patients were more likely than non-LIS patients to receive prescriptions for more expensive brand-name phosphate binders and cinacalcet. Patients dialyzing at DaVita units were significantly more likely to receive a phosphate binder and cinacalcet than patients at other dialysis providers. DaVita patients were significantly more likely to receive sevelamer or lanthanum carbonate and less likely to receive calcium acetate than patients at other providers. Overall, MBD medication costs increased relatively faster than all Part D medication costs in dialysis patients from 2007 to 2010.
Medications for CKD-MBD constituted 50.2% of Medicare net expenditure for Part D-covered medications for adult Part D-enrolled patients in 2010; the PMPM net Part D costs were greatest for cinacalcet ($103), sevelamer products ($124), and lanthanum carbonate ($24) compared with other CKD-MBD medications.
In 2010, non-LIS patients using CKD-MBD medications bore the highest monthly out-of-pocket costs, ranging from about $5 per month for oral calcitriol to $10 for calcium acetate, $32-$44 for brand-name non-calcium-containing phosphate binders or other vitamin D analogues, to $73 for cinacalcet. This contrasts sharply with costs for LIS patients, who paid less than $9 per month on average for all Part D medications combined.
Phosphate binding agents were the most commonly used CKD-MBD medications in dialysis patients. Use was relatively constant (approximately 83% of patients) in the post-Part D era. This is not surprising, as elevated serum phosphorus is an unavoidable consequence in patients receiving dialysis on the typical three-times-weekly schedule.1 Additionally, extensive use of vitamin D analogues to manage elevated parathyroid hormone has resulted in heightened problems with hyperphosphatemia.4
Sevelamer products were the most commonly used phosphate binding agents, followed by calcium acetate and lanthanum carbonate. Since its market introduction in early 2008, sevelamer carbonate has substantially replaced sevelamer hydrochloride as the sevelamer product of choice for hyperphosphatemia treatment in dialysis patients. While both drugs appear equivalent in their phosphorus reducing abilities, sevelamer carbonate does not worsen metabolic acidosis.5
Most dialysis patients were treated with IV vitamin D analogues, but oral vitamin D use is increasing, particularly in independent dialysis units. Although substantive comparative data do not exist for reduced incidence of hypercalcemia with calcitriol versus paricalcitol or doxercalciferol, most patients were using one of these two analogues; calcitriol was used in a minority of patients. The choice of vitamin D agent was probably influenced by purchasing policies that dialysis providers have in place for individual agents. For instance, in 2010, the percentages of DaVita and DCI patients receiving doxercalciferol substantially increased, primarily due to switching from paricalcitol to doxercalciferol during that year (Table S4 and Table S5). However, as these contracts expire, US providers will most likely shift to using available oral and parenteral generic versions of doxercalciferol and paricalcitol, which will reduce provider costs.
Overall, use of CKD-MBD medications was higher among dialysis patients with than without LIS status. This finding is similar to earlier reports on the use of other medication classes among dialysis patients and among Medicare Part D beneficiaries in the general Medicare population.6;7 Patients without LIS represented 30% of the adult dialysis population enrolled in Part D in 2010. Compared with non-LIS patients, LIS patients are older and more predominantly of minority races, have longer dialysis vintage and lower cost sharing, and do not experience a coverage gap (doughnut hole).6;8 Black hemodialysis patients have more severe secondary hyperparathyroidism and are more likely than white patients to be prescribed cinacalcet and vitamin D.9–12 However, after adjustment for age, race, dialysis vintage, and dialysis provider type, LIS patients remained significantly more likely than non-LIS patients to receive any phosphate binder, any IV vitamin D analog, and cinacalcet, and less likely to receive any oral vitamin D product. In addition, LIS patients were 1.5 to 2 times more likely than non-LIS patients to receive brand-name only phosphate binders in 2010. Possibly, clinicians treat LIS patients more aggressively with CKD-MBD and brand-name agents because the out-of-pocket costs for these patients are low. We did not have access to laboratory data; thus, possibly even after adjustment for important variables affecting secondary hyperparathyroidism severity, LIS patients may have higher prevalence of hypercalcemia or more severe hyperparathyroidism requiring higher use of non-calcium-containing phosphate binders and cinacalcet. However, the high out-of-pocket cost that non-LIS patients experience with these trade-name products is likely a predominant factor affecting product choice, particularly for those who reach the coverage gap.
Out-of-pocket costs for CKD-MBD medications among dialysis patients with LIS status were much lower than for non-LIS patients. Monthly out-of-pocket costs for non-LIS patients can be substantive if they are prescribed a brand name phosphate binder (sevelamer or lanthanum carbonate), cinacalcet, and/or a brand-name oral vitamin D product (paricalcitol or doxercalciferol). Thus, it is not surprising that non-LIS patients are less likely to be prescribed sevelamer, lanthanum carbonate, or cinacalcet. Fortunately, non- LIS patients will experience lower out-of-pocket expenses each year until the coverage gap is completely phased out by CMS in 2020.
An interesting finding was the higher use of CKD-MBD medications among DaVita patients compared with patients receiving dialysis from other providers. After accounting for several factors that may impact prescribing, patients in all other dialysis provider groups had significantly lower odds of being prescribed sevelamer, lanthanum carbonate, or cinacalcet, but significantly higher odds of being prescribed calcium acetate, after adjustment, compared with DaVita patients. Results of a recently published observational study showed that risk of mortality and hospitalization was lower in DaVita dual-eligible hemodialysis patients enrolled in an integrated pharmacy services program than in propensity-score matched non-enrolled patients; interestingly, odds of using phosphate binders, cinacalcet, and some antihypertensive agents were higher for enrollees.13 This study was not designed to evaluate the impact of single factors on outcomes, but one could hypothesize that more aggressive CKD-MBD therapy or better adherence to therapies may impact outcome. Conversely, substantial differences in comorbidity profiles among patients across dialysis organizations may be driving the differential utilization we observed. This issue warrants further exploration.
Odds of using oral vitamin D products were higher for patients of independent providers than for patients of other providers. The odds of IV calcitriol use in DCI, independent, and hospital-based units were about twice the odds in DaVita units. One possible explanation is the existence of preferred product contractual agreements between dialysis providers and IV vitamin D product manufacturers.
Oral CKD-MBD medications accounted for half of all PMPM net Part D spending in dialysis patients in 2010 compared with 45% in 2007; total Part D versus CKD-MBD Part D medication costs increased 22% versus 36% from 2007 to 2010. Although use of each class of CKD-MBD medications remained fairly stable, use of various agents within each class shifted over the study timeframe. In addition, manufacturer prices of trade-name CKD-MBD medications generally increased during the study timeframe.14 Phosphate binders account for almost two-thirds and cinacalcet one-third of CKD-MBD Part D spending in 2010. Generic sevelamer carbonate should be available in 2014, which will greatly affect Part D spending in this therapeutic area. We also found that the share of net Part D payments attributed to cinacalcet increased (17% in 2007, 19% in 2010) despite a decreasing percentage of patients prescribed cinacalcet from 2008 to 2010; this may be partially explained by increasing manufacturer prices since 2007.14
This study has several strengths. To our knowledge, it is the first comprehensive, longitudinal analysis of CKD-MBD prescription and cost trends in adult US dialysis patients. We used Medicare Part D data linked to the CMS ESRD database to provide data on utilization and costs of current CKD-MBD pharmacotherapy. These data are nationally representative and generalizable to most adult US dialysis patients, as most of these patients are enrolled in Medicare Part D. This study provides valuable information on the out-of-pocket costs of CKD-MBD medications that may aid clinicians in decision-making. In addition, our study included Medicare net payments for these medications, which will be useful to dialysis providers as they assess the potential cost impact of cinacalcet and phosphate binder inclusion in the ESRD PPS bundled payment in 2016. In our assessment of odds of prescription of various CKD-MBD medications in patients with and without LIS status and among dialysis providers, we controlled for several key patient factors that could affect prescription choice.15
The most serious limitation of this study is our inability to describe the utilization and costs of calcium carbonate, an over-the-counter agent not covered under Medicare Part D. When non-LIS Medicare Part D beneficiaries encounter the coverage gap, they may possibly fill some of their prescriptions through a non-Part D reimbursement or payment mechanism, and we were unable to reliably identify such prescriptions. Furthermore, our findings may not apply to dialysis patients without Medicare Part D benefits, to dialysis patients with Medicare Advantage plans, or to pediatric dialysis patients. The Part D data allow us to identify only prescription drugs dispensed in the outpatient setting. There is no way to ascertain whether patients take these medications. Although we were able to evaluate the odds of patients receiving specific CKD-MBD agents after adjustment for important patient factors that affect product selection and CKD-MBD disease severity, we were unable to adjust for MBD laboratory values as they are not available in the Medicare data.
This study of Medicare Part D-insured dialysis patients provides estimates of utilization and costs of medications for CKD-MBD management since implementation of the Medicare Part D program. We also report temporal trends in the use and costs of these agents and showed significant variation in use among dialysis organizations and in patients with and without LIS status. The inclusion of cinacalcet and phosphate binding agents in the ESRD bundled payment in 2016 will likely pose unique challenges to dialysis providers in simply providing these oral CKD-MBD medications to Medicare patients, and in bearing the costs. In a bundled payment environment, it is important to closely monitor changes in CKD-MBD management strategies implemented by providers and the impact of such strategies on achieving therapy goals. The prescription and cost information revealed in this study can help providers understand the impact of providing these medications to Medicare-covered dialysis patients given current usage patterns. Further investigations examining the broader parameters of CKD-MBD medication use, such as adherence, persistence, and discontinuation, on health care outcomes is also warranted.
Supplementary Material
Acknowledgements
The authors thank US Renal Data System colleagues Beth Forrest for regulatory assistance, Delaney Berrini, BS, for manuscript preparation, and Nan Booth, MSW, MPH, ELS, for manuscript editing.
Support: This study was performed as a deliverable under contract no. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Contributions
Research idea and study design: AAY, WLStP, BLH, CAP; data acquisition: WLStP; data analysis/interpretation: AAY, WLStP; statistical analysis: AAY; supervision or mentorship: WLStP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. WLStP takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Odds of CKD-MBD medication prescription in Part D-enrolled dialysis patients by organization, 2010.
Table S2: PMPM net Part D payment for CKD-MBD medications by low-income subsidy status; 2007–2010.
Table S3: PMPM net Part D payment for CKD-MBD medications by dialysis organization type; 2007–2010.
Table S4: Percentage of patients prescribed CKD-MBD medications by dialysis organization type; 2007–2010.
Table S5: Percentage of Part D-enrolled dialysis patients using both IV doxercalciferol and paricalcitol in each year.
Figure S1: PMPM net Part D payment for selected CKD-MBD medications in Part D-enrolled dialysis patients, 2007-2010.
Figure S2: PUPM out-of-pocket costs for CKD-MBD medications in Part D-enrolled dialysis patients without low-income subsidy.
Note: The supplementary material accompanying this article (doi: ________) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF)
Odds of CKD-MBD medication prescription in Part D-enrolled dialysis patients by organization, 2010.
Supplementary Table S2 (PDF)
PMPM net Part D payment for CKD-MBD medications by low-income subsidy status; 2007–2010.
Supplementary Table S3 (PDF)
PMPM net Part D payment for CKD-MBD medications by dialysis organization type; 2007–2010.
Supplementary Table S4 (PDF)
Percentage of patients prescribed CKD-MBD medications by dialysis organization type; 2007–2010.
Supplementary Table S5 (PDF)
Percentage of Part D-enrolled dialysis patients using both IV doxercalciferol and paricalcitol in each year.
Supplementary Figure S1 (PDF)
PMPM net Part D payment for selected CKD-MBD medications in Part D-enrolled dialysis patients, 2007–2010.
Supplementary Figure S2 (PDF)
PUPM out-of-pocket costs for CKD-MBD medications in Part D-enrolled dialysis patients without low-income subsidy.
References
- 1.KDIGO CKD-MBD Workgroup. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76(suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14(1):3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 4.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int. 2010;78(suppl 117):S10–S21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pai AB, Shepler BM. Comparison of sevelamer hydrochloride and sevelamer carbonate: risk of metabolic acidosis and clinical implications. Pharmacotherapy. 2009;29(5):554–561. doi: 10.1592/phco.29.5.554. [DOI] [PubMed] [Google Scholar]
- 6.Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, St.Peter WL. Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis. 2012;59(5):670–681. doi: 10.1053/j.ajkd.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Sacks NC, Burgess JF, Jr, Cabral HJ, Pizer SD, McDonnell ME. Cost sharing and decreased branded oral anti-diabetic medication adherence among elderly part d medicare beneficiaries. J Gen Intern Med. 2013;28(7):876–885. doi: 10.1007/s11606-013-2342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell BL, Powers CA, Weinhandl ED, St Peter WL, Frankenfield DL. Sources of drug coverage among Medicare beneficiaries with ESRD. J Am Soc Nephrol. 2012;23(5):959–965. doi: 10.1681/ASN.2011070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf M, Betancourt J, Chang Y, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19(7):1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25(12):2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Kallenbach LR, Zasuwa G, Divine GW. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol. 2000;11(2):330–334. doi: 10.1681/ASN.V112330. [DOI] [PubMed] [Google Scholar]
- 12.Newsome BB, Kilpatrick RD, Liu J, et al. Racial differences in clinical use of cinacalcet in a large population of hemodialysis patients. Am J Nephrol. 2013;38(2):104–114. doi: 10.1159/000353298. [DOI] [PubMed] [Google Scholar]
- 13.Weinhandl ED, Arneson TJ, Peter WL. Clinical outcomes associated with receipt of integrated pharmacy services by hemodialysis patients: a quality improvement report. Am J Kidney Dis. 2013;62(3):557–567. doi: 10.1053/j.ajkd.2013.02.360. [DOI] [PubMed] [Google Scholar]
- 14.MediSpan. PriceChek PC (R): A drug database by MediSpan (Indianapolis, IN), a division of Wolters Kluwer Health, Inc. Indianapolis, IN: Wolters Kluwer Health, Inc; 2013. [Google Scholar]
- 15.Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. doi: 10.1371/journal.pmed.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.