Abstract
Background
Vestibular and ocular motor impairments and symptoms have been documented in patients with sport-related concussions. However, there is no current brief clinical screen to assess and monitor these issues.
Purpose
To describe and provide initial data for the internal consistency and validity of a brief clinical screening tool for vestibular and ocular motor impairments and symptoms after sport-related concussions.
Study Design
Cross-sectional study; Level of evidence, 2.
Methods
Sixty-four patients, aged 13.9 ± 2.5 years and seen approximately 5.5 ± 4.0 days after a sport-related concussion, and 78 controls were administered the Vestibular/Ocular Motor Screening (VOMS) assessment, which included 5 domains: (1) smooth pursuit, (2) horizontal and vertical saccades, (3) near point of convergence (NPC) distance, (4) horizontal vestibular ocular reflex (VOR), and (5) visual motion sensitivity (VMS). Participants were also administered the Post-Concussion Symptom Scale (PCSS).
Results
Sixty-one percent of patients reported symptom provocation after at least 1 VOMS item. All VOMS items were positively correlated to the PCSS total symptom score. The VOR (odds ratio [OR], 3.89; P <.001) and VMS (OR, 3.37; P <.01) components of the VOMS were most predictive of being in the concussed group. An NPC distance ≥5 cm and any VOMS item symptom score ≥2 resulted in an increase in the probability of correctly identifying concussed patients of 38% and 50%, respectively. Receiver operating characteristic curves supported a model including the VOR, VMS, NPC distance, and ln(age) that resulted in a high predicted probability (area under the curve = 0.89) for identifying concussed patients.
Conclusion
The VOMS demonstrated internal consistency as well as sensitivity in identifying patients with concussions. The current findings provide preliminary support for the utility of the VOMS as a brief vestibular/ocular motor screen after sport-related concussions. The VOMS may augment current assessment tools and may serve as a single component of a comprehensive approach to the assessment of concussions.
Keywords: concussion, vestibular, ocular motor, symptoms
A sport-related concussion is an individualized injury that presents with a myriad of cognitive, physical, emotional, somatic, and sleep-related symptoms and impairments that should require a multifaceted approach to assessment and management.14,26 Among the recommended assessments are physical examinations, clinical interviews, symptom reports, and neurocognitive and balance tests. Recently, researchers have reported that vestibular impairments are common after a concussion and may delay recovery from this injury.20,27 Dizziness, which may represent an underlying impairment of the vestibular and/or ocular motor systems, is reported by 50% of concussed athletes23 and is associated with a 6.4-times greater risk, relative to any other on-field symptom, in predicting protracted (>21 days) recovery.24 Despite the emerging evidence that vestibular-related impairments and symptoms are important to assess after concussions, there are currently no brief but comprehensive clinical tools to do so. Additional measures are needed to help clinicians identify vestibular impairments and symptoms after concussions.
The vestibular system is a complex network that includes small sensory organs of the inner ear (utricle, saccule, and semicircular canals) and connections to the brain stem, cerebellum, cerebral cortex, ocular system, and postural muscles. This system provides information regarding head movements and positions to maintain visual and balance control. The vestibular system is organized into 2 distinct functional units. The vestibulo-ocular system maintains visual stability during head movements, whereas the vestibulospinal system is responsible for postural control.12 Because of the organization and neurophysiology of the vestibular system, impairments in the vestibulo-ocular system commonly manifest as symptoms of dizziness and visual instability. Conversely, vestibulo-spinal system dysfunction commonly results in disrupted balance.21 Because these 2 functional vestibular networks do not share identical neuronal circuitry, it is possible to have impairments of the vestibulo-ocular system without impairments of the vestibulospinal system.1
It is known that vestibulospinal (ie, balance) impairments are common within the first few days after a concussion.11,17 Subjectively, nearly 40% of athletes report balance disruption in the first week after a sport-related concussion.23 However, the utility of balance impairments alone as a measure of a vestibular system injury may be limited because objective clinical balance impairments recover for the majority of athletes within 3 to 5 days after the injury.17,30 It is likely that balance impairments are distinct from other vestibular-related impairments and symptoms, as most athletes who experience dizziness after a concussion do not report concomitant balance impairments.24 In neuro-otology clinics, vestibulo-ocular and vestibulospinal functions are assessed separately, as their constructs are unique.33 Until recently, all vestibular impairments after concussions were commonly assessed using the Balance Error Scoring System (BESS)16 or the Sensory Organization Test (SOT).28 However, these measures are static assessments and only represent the vestibulospinal aspect of the vestibular system. These tests do not address dynamic aspects of the vestibular system or vestibulo-ocular control. Thus, dysfunction resulting from vestibulo-ocular impairments and symptoms may be overlooked when using only vestibulospinal assessments. As such, additional clinical vestibular assessments are warranted that go beyond the current vestibulospinal measures to include vestibulo-ocular and ocular motor aspects.
In addition to vestibular impairments, ocular motor impairments are also common after concussions. Nearly 30% of concussed athletes report visual problems during the first week after the injury.23 Ocular motor impairments and symptoms may manifest as blurred vision, diplopia, impaired eye movements, difficulty in reading, dizziness, headaches, ocular pain, and poor visual-based concentration.7 A recent study of rugby players illustrated the value of assessing saccadic eye movements to better identify concussions without reported signs/symptoms using the King-Devick test.22 However, the King-Devick test does not evaluate other areas of ocular motor function such as pursuit, convergence, or accommodation, all of which have been implicated in mild traumatic brain injury (mTBI) studies as important indicators of dysfunction.5,6 Current concussion evaluation tools such as the Sideline Assessment of Concussion (SAC), Sport Concussion Assessment Tool–3 (SCAT-3), BESS, and SOT do not include assessments of vestibulo-ocular and ocular motor function. The frequency of reported dizziness and visual problems in athletes with sport-related concussions suggests that a more comprehensive assessment of vestibular and ocular motor impairments and symptoms is needed. The identification of these vestibular and visual-related impairments and symptoms represents an emerging component of assessment that may positively augment current approaches to the evaluation and management of concussions.
The purpose of this article was to describe and provide initial data for the internal consistency of a new brief clinical screening tool of vestibular and ocular motor impairments and symptoms after sport-related concussions. We also examined the screening tool’s predictive validity in correctly identifying concussed athletes from healthy controls.
MATERIALS AND METHODS
Research Design
A cross-sectional research design was used to examine vestibular and ocular motor, balance, and symptom assessments of patients with a diagnosed sport-related concussion compared with healthy controls.
Participants
A total of 100 consecutive patients with a diagnosed sport-related concussion met study criteria and were enrolled in the study. Thirty-six of these patients were excluded because of ≥1 exclusion criteria (see below). Complete data were available for 64 of the concussed patients with time since injury of ≤21 days. A control group consisting of 78 healthy participants aged ≤18 years was selected from a total of 106 eligible athletes who participated in a baseline concussion testing and education program. Any concussed or control participant older than 18 years with a history of more than 1 concussion, brain surgery, neurological disorder, treatment for substance abuse, and/ or psychiatric disorder was excluded from the study.
Instrumentation
The Vestibular/Ocular Motor Screening (VOMS) Assessment
The VOMS was developed to assess vestibular and ocular motor impairments via patient-reported symptom provocation after each assessment. The VOMS employed in this study consisted of brief assessments in the following 5 domains: (1) smooth pursuit, (2) horizontal and vertical saccades, (3) convergence, (4) horizontal vestibular ocular reflex (VOR), and (5) visual motion sensitivity (VMS). A copy of the VOMS form and standardized instructions for each test are provided in Appendix 1 (available in the online version of this article at http://ajsm.sagepub.com/supplemental). A visual depiction representing each test is provided in Appendix 2 (available online). Patients verbally rate changes in headache, dizziness, nausea, and fogginess symptoms compared with their immediate preassessment state on a scale of 0 (none) to 10 (severe) after each VOMS assessment to determine if each assessment provokes symptoms. Convergence was assessed by both symptom report and objective measurement of the near point of convergence (NPC; see description in Appendix 1). The NPC values were averaged across 3 trials, and normal NPC values are within 5 cm.32 It is important to note that only horizontal VOR data are reported in this article; however, the VOMS has since been modified to incorporate the assessment of VOR in both the horizontal and vertical planes. The VOMS takes approximately 5 to 10 minutes to administer.
The Post-Concussion Symptom Scale (PCSS)
The PCSS was used to measure concussion-related symptoms. The scale consists of 22 self-reported symptom items (eg, dizziness, headache) rated on a scale from 0 (none) to 6 (severe). Total symptom scores on the PCSS range from 0 to 132. The PCSS takes approximately 5 minutes to complete.
Procedures
This study was approved under an exempt medical records review protocol by the University of Pittsburgh Human Subjects Institutional Review Board. All concussed patients completed the VOMS and PCSS assessments during their initial clinical visit after a sport-related concussion. Physical therapists trained in screening vestibular and ocular motor function administered the 3 measures in private examination rooms. The order of administration of these measures was (1) the PCSS, (2) a computerized neurocognitive test whose data were not analyzed for the purposes of this study, and (3) the VOMS. All healthy controls completed the VOMS and PCSS as part of a standard baseline testing and education program. The VOMS was administered individually in a clinic setting to the control group by vestibular physical therapists and athletic trainers educated in vestibular and ocular motor screening. The PCSS was administered to the controls in small groups (with ≤3 participants) in supervised examination rooms.
Data Analysis
Patient and control differences on group demographic characteristics and VOMS domain measures were tested using a nonparametric Mann-Whitney U test for continuous variables and contingency table analyses, with the χ2 test for categorical variables. Age was tested against the hypothesis of a normal distribution with the Kolmogorov-Smirnov test. Transformations were evaluated for use as covariates in multivariate analyses. A significance level of P <.05 was set for the preceding analyses.
To examine the internal consistency of the VOMS, a Cronbach α analysis was conducted to assess internal consistency. A series of Spearman rank-order correlations between VOMS and PCSS scores among the concussed patients were conducted to examine the convergent validity of the VOMS.
Logistic regression sensitivity and specificity analyses were performed to examine the predictive validity of the VOMS to discriminate between concussed patients and controls. Univariate associations with odds ratios (ORs) between the likelihood of concussions and all demographic and VOMS test outcomes were first assessed. Variables demonstrating a significant association at a P < .10 threshold were then retained for the multivariate estimation of the best subset of predictors of the likelihood of concussions. A step forward likelihood ratio process was used with a P < .05 criterion to select predictors for a final multivariate model. Receiver operating characteristic (ROC) curves with area under the curve (AUC) analyses, cutoff scores, and likelihood ratios (LRs) were used to describe the accuracy of individual VOMS item scores and the predictive probabilities from the final best subset model to identify concussed patients.
RESULTS
Demographic Data
The sample of concussed patients consisted of 64 patients (36 male, 28 female) aged 13.9 ± 2.5 years (range, 9–18 years) who were seen approximately 5.5 ± 4.0 days (range, 1–21 days) after the injury. The majority of the sample (93.8%; n = 60) was enrolled in the study within 14 days of the injury. The control sample consisted of 78 participants (57 male, 21 female) aged 12.9 ± 1.6 years (range, 10–17 years). Patients in the concussed group were significantly (P <.01) older, and this group had a greater proportion of female patients (44%; P = .04) than the control group (27%). With regard to previous concussions, the patients and controls were not significantly different (P = .10). There was a history of concussions in 14 (22%) of the patients and 9 (12%) of the controls. The mean NPC distance was obtained from 62 of the concussed patients. The data for age demonstrated a nonnormal distribution. This variable demonstrated a normal distribution after natural logarithmic transformation.
Internal Consistency of the VOMS
The internal consistency of the VOMS total symptom score and the NPC distance was high, with Cronbach α = .92. All of the items contributed positively to the overall internal consistency. The lowest interitem correlations were seen between the NPC distance and the VOMS symptom scores, ranging from 0.44 (vertical saccade) to 0.53 (smooth pursuit) (Table 1).
TABLE 1.
Interitem Correlations for VOMS Assessment Domain Scores and Convergence Distance in Concussed Patientsa
| VOMS Domain | Smooth Pursuit | Horizontal Saccade | Vertical Saccade | Convergence | Horizontal Vestibular Ocular Reflex | Visual Motion Sensitivity |
|---|---|---|---|---|---|---|
| Smooth pursuit | — | — | — | — | — | — |
| Horizontal saccade | 0.88 | — | — | — | — | — |
| Vertical saccade | 0.85 | 0.85 | — | — | — | — |
| Convergence | 0.83 | 0.82 | 0.81 | — | — | — |
| Horizontal vestibular ocular reflex | 0.62 | 0.72 | 0.63 | 0.71 | — | — |
| Visual motion sensitivity | 0.82 | 0.84 | 0.82 | 0.77 | 0.71 | — |
| Near point of convergence distance, cm | 0.53 | 0.52 | 0.44 | 0.49 | 0.52 | 0.50 |
VOMS, Vestibular/Ocular Motor Screening.
Symptom Provocation After VOMS Assessments
The VOR item was associated with the highest percentage of concussed patients reporting symptom provocation after administration (61%; n = 39) and the highest mean total symptom score (3.7 ± 5.1). The smooth pursuit and vertical saccade items evoked symptoms in the minimum percentage of concussed patients (33%; n = 21), with mean total symptom scores of 2.1 ± 4.8 and 2.1 ± 4.6, respectively. The maximum percentage of controls reporting symptom provocation on any VOMS test item was 9% (n = 7) and was found for the VOR, horizontal saccade, and smooth pursuit items. No controls reported a total symptom score greater than 2 after any VOMS individual item test. The mean total symptom scores for all VOMS tests were significantly (all P <.001) greater in the concussed patients compared with controls (Table 2).
TABLE 2.
VOMS Assessment Domains for Symptom Provocation and Total Symptom Scores in Concussed Patients and Healthy Controlsa
| VOMS Domain | Concussed Patients (n = 64b) | Controls (n = 78) | P Value, Group Differencec | Correlation to PCSSd |
|---|---|---|---|---|
| Smooth pursuit | 2.1 ± 4.8 (0–31) | 0.1 ± 0.3 (0–2) | <.001 | 0.38 |
| Horizontal saccade | 2.5 ± 4.8 (0–29) | 0.1 ± 0.3 (0–2) | <.001 | 0.59 |
| Vertical saccade | 2.1 ± 4.6 (0–29) | 0.1 ± 0.3 (0–2) | <.001 | 0.47 |
| Convergence | 2.2 ± 4.0 (0–20) | 0.1 ± 0.3 (0–2) | <.001 | 0.65 |
| Horizontal vestibular ocular reflex | 3.7 ± 5.1 (0–22) | 0.1 ± 0.3 (0–2) | <.001 | 0.54 |
| Visual motion sensitivity | 3.1 ± 5.7 (0–35) | 0.1 ± 0.3 (0–2) | <.001 | 0.44 |
| Near point of convergence distance, cm | 5.9 ± 7.7 (0–41.3) | 1.9 ± 3.2 (0–15.3) | <.001 | 0.28 |
Values are expressed as mean ± SD (range). PCSS, Post-Concussion Symptom Scale; VOMS, Vestibular/Ocular Motor Screening.
n = 62 concussed patients for near point of convergence distance.
Mann-Whitney U nonparametric test.
All P <.01 except near point of convergence distance (P <.03, Spearman nonparametric correlation).
NPC Distance
The mean NPC distance was significantly greater in the concussed group compared with the control group (P < .001), with a mean difference between groups of 4.0 cm (95% CI, 1.9–6.1 cm). The mean NPC distance across the 3 trials for the concussed patient sample was 5.9 ± 7.7 cm (range, 0–41.3 cm), whereas the NPC distance for the control group averaged 1.9 ± 3.2 cm (Table 2).
Relationship Between the VOMS and PCSS Among Concussed Patients
In the concussed group, results from Spearman rank-order correlations yielded several significant relationships between the VOMS items and PCSS scores (Table 2). The VOMS total symptom scores were moderately positively correlated (all P < .05) to the PCSS, ranging from 0.28 (NPC distance) to 0.65 (convergence symptom score).
Predicting Concussions and Healthy Controls
Age (ln transformed) (OR, 17.65; P = .01) and male sex (OR, 0.49; P = .05) were independently associated with the likelihood of concussions and were included as potential confounding variables in the assessment of each VOMS item. All VOMS symptom scores and the NPC distance demonstrated a significant relationship with the likelihood of concussions. Age, and not sex, was a significant covariate with each VOMS item in the association with the likelihood of concussions. With an adjustment for ln(age), individual VOMS scores predicted between 23% (NPC distance) and 53% (VOR) of the variance in the likelihood of concussions. The strongest individual score associations were supported for VOR (OR, 3.89; P < .001), VMS (OR, 3.37; P < .01), and NPC distance (OR, 1.21 for each 1-cm increase; P <.001) (Table 3).
TABLE 3.
VOMS Assessment Domain Scores: Individual Item Associations With the Likelihood of Concussionsa
| VOMS Domain | β | Wald χ2 | P Value | Odds Ratio | R2 b |
|---|---|---|---|---|---|
| Smooth pursuit | .83 | 7.89 | <.01 | 2.29 | 0.28 |
| Horizontal saccade | 1.01 | 10.31 | <.01 | 2.75 | 0.34 |
| Vertical saccade | .98 | 8.96 | <.01 | 2.65 | 0.31 |
| Convergence | .78 | 7.98 | <.01 | 2.18 | 0.30 |
| Horizontal vestibular ocular reflex | 1.36 | 16.97 | <.001 | 3.89 | 0.53 |
| Visual motion sensitivity | 1.21 | 10.35 | <.01 | 3.37 | 0.40 |
| Near point of convergence distance, cm | .19 | 13.33 | <.001 | 1.21 | 0.23 |
Logistic regression (maximum likelihood estimation), adjusted for ln(age). VOMS, Vestibular/Ocular Motor Screening.
Nagelkerke R2.
The ROC AUC analyses demonstrated that all unadjusted VOMS scores accurately identified patients with concussions, with a maximum AUC of 0.78 (VOR) (Table 4). A cutoff of ≥2 total symptoms on any VOMS item demonstrated positive LRs between 23.9 (smooth pursuit, vertical saccade) and 42.8 (VOR). An NPC distance of ≥5 cm demonstrated a positive LR of 5.8 (Table 4). These results implied a minimum increase in the posttest probability of correctly identifying a concussed patient of approximately 50% for any VOMS symptom score of ≥2 and 38% for an NPC distance of ≥5 cm based on a pretest probability of 44% in the study sample.
TABLE 4.
AUC Analysis, Cutoff Score, and LR of Positive Results for VOMS Domain Scoresa
| VOMS Domain | AUC | P Value | Cutoff Score for Positive Test Result (≥) | LR for Positive Test Result |
|---|---|---|---|---|
| Smooth pursuit | 0.64 | <.01 | 2 | 23.9 |
| Horizontal saccade | 0.68 | <.001 | 2 | 28.9 |
| Vertical saccade | 0.65 | <.01 | 2 | 23.9 |
| Convergence | 0.64 | <.01 | 2 | 26.4 |
| Horizontal vestibular ocular reflex | 0.78 | <.001 | 2 | 42.8 |
| Visual motion sensitivity | 0.73 | <.001 | 2 | 32.7 |
| Near point of convergence distance, cm | 0.73 | <.001 | 5 | 5.8 |
AUC, area under the curve; LR, likelihood ratio; VOMS, Vestibular/Ocular Motor Screening.
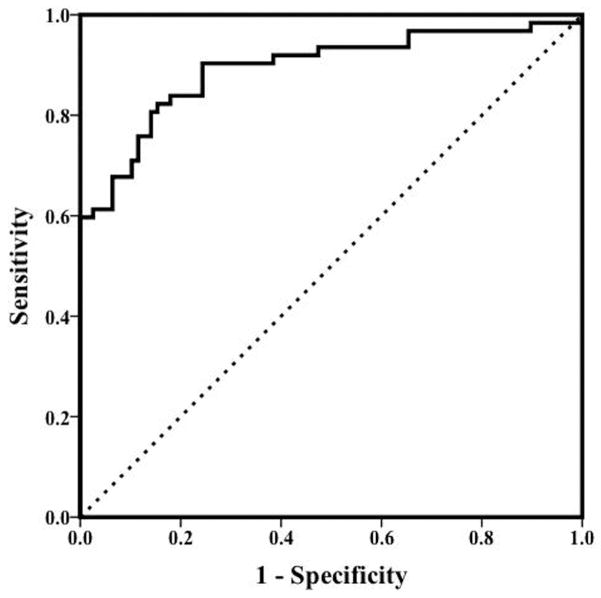
Multivariate logistic regression using a forward entry method identified the best subset of independent predictors of concussions as VMS (OR, 2.84; P < .02), VOR (OR, 2.80; P < .01), and convergence distance (OR, 1.15; P < .05), with ln(age) as a significant covariate (P = .03). This 4-factor model predicted 61% of the variance in the likelihood of concussions. The ROC analysis for the accuracy of the predicted probability from this model to identify patients with concussions demonstrated an AUC of 0.89 (95% CI, 0.84–0.95; P <.001) (Figure 1).
Figure 1.
Receiver operating characteristic curve describing the area under the curve (AUC) for identifying patients with concussions versus healthy controls using vestibular ocular reflex and visual motion sensitivity symptom scores and near point of convergence distance. *Adjusted for ln(age): AUC = 0.89. Dotted line indicates AUC = 0.50 (P <.001).
DISCUSSION
The results of this initial study suggest that the VOMS, a brief (5–10 minute) screen for vestibular and ocular motor impairments and symptoms, possesses internal consistency and demonstrates basic validity compared with the PCSS and may serve to augment current assessments used after sport-related concussions. Our findings also provide preliminary evidence for the use of the VOMS to identify patients with sport-related concussions from healthy controls.
The VOMS demonstrated excellent internal consistency (α = .92) in the current sample. The highest interitem correlations were between the individual symptom scores, with lower correlations between the symptom scores and NPC distance measures. This finding suggests that the VOMS items measure related, but not identical, components of the vestibular and ocular motor systems. The VOMS was able to distinguish concussed from noncon-cussed athletes. Patients in the concussed group scored significantly higher on all of the VOMS items than did the controls. In fact, it was clear from the data that the controls exhibited very few symptoms after each VOMS component. In addition, the mean NPC distance for the concussed group was more than 3 times greater than that for the control group. Moreover, the variability in symptoms and NPC distance was very low for the controls. Together, these findings indicate that the VOMS provides a measure that may be useful in differentiating concussed patients from controls.
To examine the concurrent validity of the VOMS, we compared it to an established measure of concussions, namely, the PCSS total score. Each of the VOMS items was positively correlated with the PCSS total score. These correlations were moderate and provide partial initial support for the concurrent validity of the VOMS but suggest that the VOMS and PCSS may not measure the same construct. In addition, the NPC distance was correlated at a lower level (r = 0.28). Ideally, 2 measures should be moderately (r = 0.30–0.60) to highly (r > 0.70) correlated to indicate concurrent validity.
The findings indicate that the VOR, VMS, and NPC distance components of the VOMS in combination are clinically useful in identifying concussions. The current study’s results also provide clinically practical cutoff values for the VOMS item symptom scores and the NPC distance to accurately identify patients with concussions. Assuming an initial 50% probability (ie, chance) of a concussion, any individual VOMS item with a total symptom score of ≥2 increases the probability of being concussed by at least 46%. Similarly, an NPC distance of ≥5 cm increases the probability of a concussion by at least 34%. The nature of these cutoff values is both intuitive and useful to clinicians for identifying patients with concussions.
The current study’s findings highlight the importance of the ocular motor components of the VOMS, particularly NPC distance. Clinically, convergence insufficiency can mimic many of the signs/symptoms attributed to concussions such as headache, difficulty in reading, difficulty in focusing, and blurred vision.32 Although ocular motor impairments after an mTBI have been reported by researchers,5,6 this study is the first to examine ocular motor impairments and symptoms after sport-related concussions. Ocular motor components (smooth pursuit, vertical/ horizontal saccades, convergence) of the VOMS provoked symptoms in 33% to 42% of patients in the current sample. Additionally, NPC distance measures were, on average, 4.0 cm greater in concussed patients than in controls. According to the literature, NPC values up to 5 cm are considered normal in the general population.32 Our findings also support using a cutoff value of ≥5 cm for the NPC distance after sport-related concussions, which resulted in a 34% increase in accurately diagnosing a concussion.
Common concussion assessment tools such as the SAC25 and BESS, which are components of the SCAT-3,3,26 do not include measures of vestibular or ocular motor function. The King-Devick test,29 a test that includes saccadic eye movements, has recently been used for assessments after concussions.13,22 According to the present study’s results, pursuit eye movements and NPC distance, in addition to saccades, should be included in any ocular motor assessment of concussions.
Clinical Implications
The VOMS demonstrated high sensitivity, indicating that a positive test result was highly accurate in identifying athletes who experienced a sport-related concussion. As such, it may have additional utility in providing information to guide clinical management. A concussion has typically been conceptualized as a uniform condition, which has limited the assessment and management approach to this injury. However, researchers and clinicians have begun to conceptualize concussions using more individualized methods in which each injury has a predominant clinical presentation and trajectory that should inform both the assessment and treatment.10 The current findings suggest that through the VOMS, patients with impairments and symptoms in vestibular and ocular motor function after sport-related concussions can be identified. As such, the VOMS may assist in prompting referrals for more targeted vestibular and vision assessments and rehabilitation when any item is positive.
The concept of rehabilitation in concussion management is evolving. Vestibular rehabilitation is known to be effective in the management of specific conditions such as vestibular hypofunction, benign paroxysmal positional vertigo, migraine-related dizziness, and central vestibular disorders.4,18 The emerging literature also supports vestibular rehabilitation for dizziness, balance, and vestibulo-ocular impairments after concussions.2,19,27 Many ocular motor problems can also be managed with vision training or a modification to lenses.8 Research has shown that convergence insufficiency, in particular, is responsive to targeted vision therapy.31 Additionally, there is evidence to support the use of vision therapy for accommodative deficits, impaired version movements, and minor ocular misalignments.8 The value of incorporating vestibular and visual rehabilitation into the management of post-concussive patients with vestibular and ocular motor impairments, as identified by the VOMS, warrants further study.
Future Directions and Research
To our knowledge, there are no clinical tools that provide a brief but comprehensive assessment of vestibular and ocular motor functioning and symptoms after concussions. The results of the current study suggest that the VOMS has the potential to fill this void in the clinical assessment of this injury. Our preliminary study provides initial evidence for the use of the VOMS to assess vestibular and ocular motor screening as part of a comprehensive approach that also includes clinical examination, symptom evaluation, neuro-cognitive testing, and balance assessment components.
Researchers have indicated that the utility of many tools used for the identification of deficits after a concussion is limited to the acute stage of the injury.9,15,16,30 As such, researchers should examine the ability of the VOMS to detect impairments after concussions across time with serial administration in the acute (sideline), subacute, and chronic phases as an adjunct to other concussion management tools. Additional research on whether the VOMS can help predict recovery time from this injury is also warranted. Moreover, the use of the VOMS as a screening tool to trigger immediate referral for vestibular and ocular motor therapy and its effect on recovery time is warranted. Such a study would allow researchers to determine the clinical utility of the VOMS for identifying patients for early intervention.
Limitations
The data from the current study are cross-sectional, and complete data were not available for all participants. The VOMS was not administered in a standardized order to all participants. The use of subjective patient reporting of symptoms after VOMS testing may lead to recall bias. The lack of baseline measures in this study precludes us from knowing whether scores on the VOMS are representative of the effects of concussions per se. The concussed patients may have had pre-existing vestibular and ocular motor symptoms before their injuries. However, the very low VOMS symptom and NPC distance scores for the healthy controls in the current study suggest that this a priori group difference was unlikely. Participants in the control group were significantly younger than those in the concussed group. However, age differences between the groups were controlled for using statistical procedures. The sample represents only patients presenting to a concussion clinic, which may have biased the sample toward a selection effect for a specific type of patient with pronounced impairments and symptoms after a concussion. Finally, it is important to note that the VOMS is a screening tool that is primarily symptom based and is not intended to serve as a comprehensive measure of vestibular and ocular motor impairments. The VOMS is designed to elicit symptoms that can be used to identify and refer patients with possible vestibular and ocular motor involvement after concussions for additional evaluation.
CONCLUSION
The current findings indicate that the VOMS possessed internal consistency and was able to differentiate between concussed athletes and healthy unmatched controls. The results supported moderate correlations between the VOMS items and total concussion symptom scores, providing initial evidence for the concurrent validity of the measure. Cutoff scores of ≥2 total symptoms after any VOMS item or an NPC distance of ≥5 cm resulted in high rates (96% and 84%, respectively) of identifying concussions. Moreover, a combination of VOR, VMS, and NPC distance scores (controlling for age) resulted in a positive prediction rate of 0.89 for identifying this injury. The VOMS appears to assess distinct vestibular and ocular motor symptoms, which are unrelated to current clinical balance measures. The VOMS may help clinicians to identify patients for vestibular and ocular referrals and more targeted treatment, thereby enhancing recovery from this injury.
Supplementary Material
Acknowledgments
Source of funding: This research was supported in part by a grant to the University of Pittsburgh from the National Institute on Deafness and Other Communication Disorders (1K01DC012332-01A1).
The authors thank Dr Patrick Sparto and Dr Susan Whitney from the University of Pittsburgh for their assistance in the development of the VOMS and physical therapists Heather Christain and Kirsten Hogg from the UPMC Centers for Rehabilitation Services for their assistance with data collection.
Footnotes
One or more of the authors has declared the following potential conflict of interest: M.W.C. is a cofounder and 10% shareholder of ImPACT Applications Inc.
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Allum JH. Recovery of vestibular ocular reflex function and balance control after a unilateral peripheral vestibular deficit. Front Neurol. 2012;3:83. doi: 10.3389/fneur.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- 3.Baillargeon A, Lassonde M, Leclerc S, Ellemberg D. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj. 2012;26(3):211–220. doi: 10.3109/02699052.2012.654590. [DOI] [PubMed] [Google Scholar]
- 4.Brown KE, Whitney SL, Marchetti GF, Wrisley DM, Furman JM. Physical therapy for central vestibular dysfunction. Arch Phys Med Rehabil. 2006;87(1):76–81. doi: 10.1016/j.apmr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Capo-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177(7):804–813. doi: 10.7205/milmed-d-12-00061. [DOI] [PubMed] [Google Scholar]
- 6.Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2007;78(4):155–161. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Ciuffreda KJ, Ludlam D, Thiagarajan P. Oculomotor diagnostic protocol for the mTBI population. Optometry. 2011;82(2):61–63. doi: 10.1016/j.optm.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Ciuffreda KJ, Rutner D, Kapoor N, Suchoff IB, Craig S, Han ME. Vision therapy for oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2008;79(1):18–22. doi: 10.1016/j.optm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Coldren RL, Kelly MP, Parish RV, Dretsch M, Russell ML. Evaluation of the Military Acute Concussion Evaluation for use in combat operations more than 12 hours after injury. Mil Med. 2010;175(7):477–481. doi: 10.7205/milmed-d-09-00258. [DOI] [PubMed] [Google Scholar]
- 10.Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235–246. doi: 10.1007/s00167-013-2791-6. [DOI] [PubMed] [Google Scholar]
- 11.Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 12.Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35(3):185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galetta KM, Brandes LE, Maki K, et al. The King-Devick test and sports-related concussion: study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci. 2011;309(1–2):34–39. doi: 10.1016/j.jns.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubenhoff JA, Kirkwood M, Gao D, Deakyne S, Wathen J. Evaluation of the standardized assessment of concussion in a pediatric emergency department. Pediatrics. 2010;126(4):688–695. doi: 10.1542/peds.2009-2804. [DOI] [PubMed] [Google Scholar]
- 16.Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182–189. doi: 10.1097/00042752-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuro-psychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]
- 18.Hillier SL, Hollohan V. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2007;(4):CD005397. doi: 10.1002/14651858.CD005397.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Hoffer ME, Balaban C, Gottshall K, Balough BJ, Maddox MR, Penta JR. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol. 2010;31(2):232–236. doi: 10.1097/MAO.0b013e3181c993c3. [DOI] [PubMed] [Google Scholar]
- 20.Hoffer ME, Gottshall KR, Moore R, Balough BJ, Wester D. Characterizing and treating dizziness after mild head trauma. Otol Neurotol. 2004;25(2):135–138. doi: 10.1097/00129492-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Chang R. Anatomy of the vestibular system: a review. Neuro-Rehabilitation. 2013;32(3):437–443. doi: 10.3233/NRE-130866. [DOI] [PubMed] [Google Scholar]
- 22.King D, Brughelli M, Hume P, Gissane C. Concussions in amateur rugby union identified with the use of a rapid visual screening tool. J Neurol Sci. 2013;326(1–2):59–63. doi: 10.1016/j.jns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the Post-Concussion Symptom Scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375–2384. doi: 10.1177/0363546512455400. [DOI] [PubMed] [Google Scholar]
- 24.Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–2318. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- 25.McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48(3):586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- 26.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 27.Naguib MB, Madian Y, Refaat M, Mohsen O, El Tabakh M, Abo-Setta A. Characterisation and objective monitoring of balance disorders following head trauma, using videonystagmography. J Laryngol Otol. 2012;126(1):26–33. doi: 10.1017/S002221511100291X. [DOI] [PubMed] [Google Scholar]
- 28.Nashner LM, Black FO, Wall C., 3rd Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2(5):536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oride MK, Marutani JK, Rouse MW, DeLand PN. Reliability study of the Pierce and King-Devick saccade tests. Am J Optom Physiol Opt. 1986;63(6):419–424. doi: 10.1097/00006324-198606000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 31.Scheiman M, Cotter S, Kulp MT, et al. Treatment of accommodative dysfunction in children: results from a randomized clinical trial. Optom Vis Sci. 2011;88(11):1343–1352. doi: 10.1097/OPX.0b013e31822f4d7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheiman M, Gallaway M, Frantz KA, et al. Nearpoint of convergence: test procedure, target selection, and normative data. Optom Vis Sci. 2003;80(3):214–225. doi: 10.1097/00006324-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Slattery EL, Sinks BC, Goebel JA. Vestibular tests for rehabilitation: applications and interpretation. NeuroRehabilitation. 2011;29(2):143–151. doi: 10.3233/NRE-2011-0688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.