Abstract
Purpose
To study temporal pattern of serum liver enzymes levels in newborns with hepatic injury associated with birth asphyxia (BA).
Methods
Singleton term newborns with BA and ≤72 hours of age admitted to neonatal intensive care unit were prospectively enrolled. Term newborns with physiological jaundice and without BA were studied as controls. Serum liver enzymes were measured at <24 hours, 24-72 hours, and at 6-12 days of age for cases and at 1-6 days of age for controls. BA was defined by 1 minute Apgar score <7 or delayed or absent cry with hypoxic ischemic encephalopathy. BA-associated liver injury was defined as serum alanine aminotransferase (ALT) elevation beyond +2 standard deviation (ALT > +2 SD) above the mean of control subjects at any of the three time points.
Results
Sixty controls and 62 cases were enrolled. Thirty-five cases (56%) developed BA-associated liver injury (ALT>81 IU/L). They had higher serum levels of ALT, aspartate aminotransferase, lactate dehydrogenase than the control infants, with peak at 24-72 hours. In controls, serum liver enzyme levels were significantly higher in appropriate-for-date (AFD) babies than small-for-date (SFD) babies. Serum enzyme pattern and extent of elevation were comparable between SFD and AFD babies. Degree of serum liver enzyme elevation had no relationship with severity of hypoxic encephalopathy.
Conclusion
Serum liver enzyme elevation is common in BA; it peaks at 24-72 hours followed by a sharp decline by 6-12 days of age. Pattern and extent of enzyme elevation are comparable between SFD and AFD babies.
Keywords: Asphyxia neonatorum, Hepatitis, Ischemia, Transaminases
INTRODUCTION
Birth asphyxia (BA), defined as Apgar score of below 7 at 1 minute after birth [1], is a common cause of neonatal morbidity and mortality. Recent estimates attribute 17.2% of neonatal deaths worldwide to BA or birth trauma [2]. BA has damaging effects on several body organ systems [3,4]. Extent of such organ injury depends on several factors such as gestational age (GA) of the baby, time of onset of BA (antepartum, intrapartum, or postpartum), and severity and duration of BA. Several studies are available on BA-related injury to brain [5,6,7], kidneys [8,9], and heart [10,11].
BA leads to a chain of adaptive responses to curtail the body's oxygen requirement, in an attempt to tide over the hypoxic crisis [12]. One such mechanism is the so called "diving reflex" or redistribution of left ventricular output in favor of more vital organs such as brain, heart, and adrenals at the expense of reduced blood flow to non-vital organs such as kidneys, lungs, gastrointestinal tract, and other abdominal viscera [13]. Liver receives nearly three-fourth of its blood supply from the portal vein and the rest through the hepatic artery. The "diving reflex" is associated with reduced hepatic blood flow through both the hepatic artery as well as the portal vein, since the latter is determined by the amount of mesenteric blood flow.
Hepatic injury is commonly assessed using serum levels of enzymes which are released from injured hepatocytes, namely alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH), which are commonly referred as the "liver enzymes". Attempts have been made previously to assess the hepatic damage in BA [14]. However, data on the temporal pattern of serum levels of liver enzymes after BA are very scanty. Such information may help to differentiate hepatic injury due to BA from that due to other causes, and to determine the baby's outcome. We therefore studied the temporal pattern of serum levels of liver enzymes in newborns with hypoxic hepatic injury (HHI) due to BA.
MATERIALS AND METHODS
This prospective case-control study was carried out in the Neonatal Intensive Care Unit of Department of Pediatrics, Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, India, between May 2007 and April 2008. It was approved by the institutional ethical committee of Vivekananda Polyclinic. Consecutive singleton newborns with BA, delivered at ≥37 weeks GA and admitted within 72 hours of birth, were enrolled after obtaining written informed consent from one of the parents. Babies were excluded if a major congenital anomaly, sepsis, inborn error of metabolism, congenital infection, hemolytic condition, hemorrhagic shock, or primary hepato-biliary disease was identified or suspected. Newborns delivered in our hospital and admitted with physiological jaundice, without BA, were enrolled as controls.
BA was diagnosed if a newborn had features of hypoxic ischemic encephalopathy (HIE) along with one of the following criteria (i) Apgar score <7 at 1 minute of life, or (ii) history of delayed cry (after 5 minutes of birth) or no cry after birth; this was as per the guidelines laid down by National-Neonatal Perinatal database network of India, which are widely accepted in the country [1]. HIE is a clinical syndrome due to acute or sub-acute hypoxic brain injury, and is defined clinically on the basis of a constellation of neurological findings that include any combination of altered muscle tone, altered sensorium, altered deep tendon reflexes, absent primitive neonatal reflexes, dilated pupils and seizures. Severity of HIE was assessed by using Sarnat & Sarnat staging system [7]. Babies were categorized, based on fetal growth, as small-for-date (SFD, birth weight <10th centile for GA), appropriate-for-date (AFD, birth weight between 10th and 90th centile for GA), and large-for-date (birth weight >90th centile for GA) [1].
Complete antenatal, perinatal and neonatal history was recorded in a predefined data collection form. In BA group, serum ALT, AST, LDH, alkaline phosphatase, total protein, albumin, bilirubin (total and conjugated), and prothrombin time (PT) were measured at enrollment (<24 hours) followed by ALT, AST, and LDH measurements at 24-72 hours, and at 6-12 days of life. Number of study samples obtained in an individual baby was decided by the age of the baby at hospitalization. In control babies, venous specimens were collected once at 1-6 days of life. All specimens were collected when blood sampling was being done for standard clinical care and were analyzed in central laboratory of the hospital.
HHI was defined by serum ALT elevation beyond +2 standard deviation (SD) above the mean value in control infants, at any time up to 12 days after birth. ALT levels were used to define HHI because it is the most specific serum marker of liver injury. By contrast, AST and LDH levels are widely present in other body organs, and may thus be elevated in infants with BA due to injury to red blood cells, or myocardial or skeletal muscle, and hence were not used to define the liver injury.
Statistical analysis
Numerical data were expressed as mean and SD and analyzed using t test. Categorical variables were expressed as proportions and compared using χ2 test. The relationship of variables with each other was studied using Pearson's coefficient. Statistical significance was considered at p-value<0.05. Data were analyzed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The study included 60 healthy newborns (controls). Eighty eight newborns were screened for BA; of those, 26 were excluded for various reasons (prematurity 15, sepsis 8, shock 2 and major congenital anomaly 1) and 62 were enrolled in the study (BA cases). The infants with BA were categorized into those with and those without HHI.
Findings in control babies
Clinical characteristics of controls are summarized in Table 1. Controls with ≤72 hours and 73-144 hours of age had comparable liver function test results (Table 2). Among the controls, AFD babies had significantly higher serum ALT (47±21 IU/L vs. 26±8 IU/L; p<0.001) and AST (64±18 IU/L vs. 50±19 IU/L; p=0.01) levels than the SFD babies; whereas LDH levels (858±215 IU/L vs. 739±269 IU/L; p=0.09) were similar in AFD and SFD babies. Male and female healthy babies had similar fetal growth category distribution (AFD/SFD: 24/12 vs. 16/8; p=1.00), birth weight (2,928±568 g vs. 2,755±488 g; p=0.21), serum levels of proteins (6.3±0.6 g/dL vs. 6.1±0.5 g/dL; p=0.09), total bilirubin (11.7±4.7 mg/dL vs. 10.6±3.9 mg/dL; p=0.32), ALT (38±20 IU/L vs. 42±22 IU/L; p=0.54), AST (58±17 IU/L vs. 61±22 IU/L; p=0.58) and LDH (798±278 IU/L vs. 848±165 IU/L; p=0.38). However, male babies were younger (age: 83±33 hours vs. 102±34 hours; p=0.04) and had higher albumin levels (3.4±0.4 g/dL vs.3.1±0.4 g/dL; p=0.005).
Table 1.
Clinical Characteristics of Control Infants
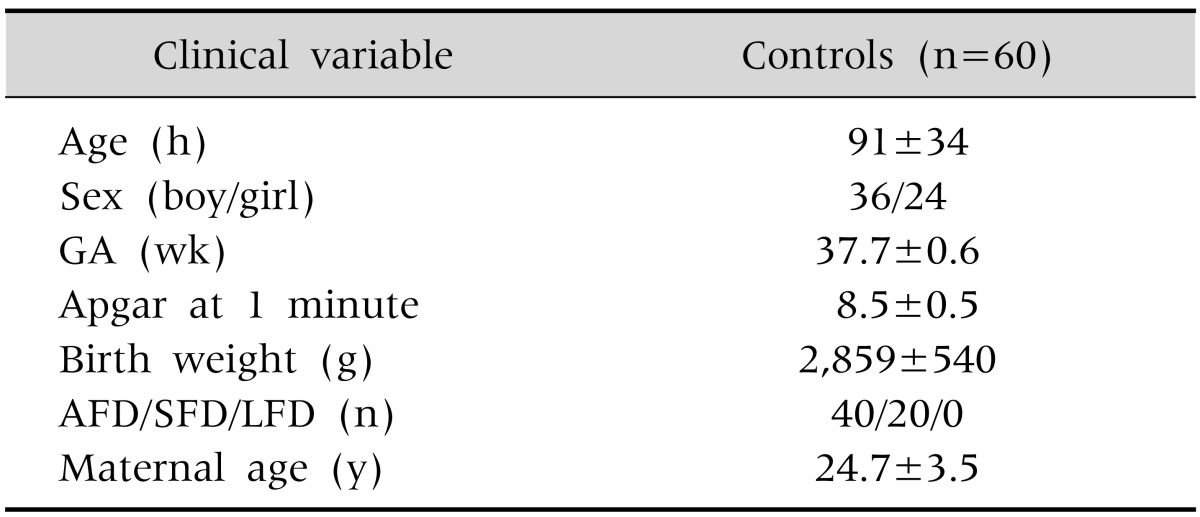
Values are presented as mean±standard deviation or number.
GA: gestational age, AFD: appropriate-for-date (newborn with birth weight between 10th and 90th centile for GA), SFD: small-for-date (newborn with birth weight <10th centile for GA), LFD: large-for-date (newborn with birth weight >90th centile for GA).
Table 2.
Liver Function Tests in Control Infants
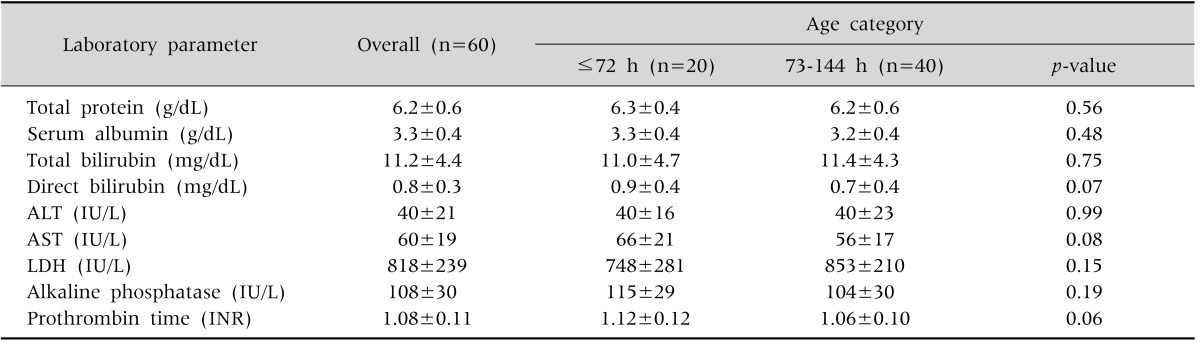
Values are presented as mean±standard deviation.
ALT: alanine aminotransferase, AST: aspartate aminotransferase, LDH: lactate dehydrogenase, INR: international normalized ratio.
Controls versus infants with birth asphyxia
Based on the serum ALT levels in control group of 39.6±20.6 IU/L, ALT values of ≥81 IU/L (mean+ 2SD) were used to define HHI in the BA group.
Among the 62 babies with BA, 35 (56%) had HHI whereas 27 (44%) did not. The babies with BA were comparable to the controls in sex ratio (45/62 [73%] and 36/60 [60%] boys respectively; p=0.18), fetal growth categories (AFD: 40/62 [64.5%] and 40/60 [66.7%] respectively; p=0.85) and birth weight (2,700±394 g and 2,859±540 g respectively; p=0.07), but differed in having been born at a lower GA (37.3±0.8 wk and 37.7±0.6 wk respectively; p=0.003) and having older mothers (26.6±4.0 y and 24.7±3.5 y respectively; p=0.006).
Babies with and without hypoxic hepatic injury
Babies with and without HHI were comparable in various clinical characteristics (Table 3) except that the former had higher grades of HIE. However, a favorable final outcome was equally frequent in the babies with and without HHI.
Table 3.
Infants with Birth Asphyxia (BA), with and without Hypoxic Hepatic Injury (HHI)
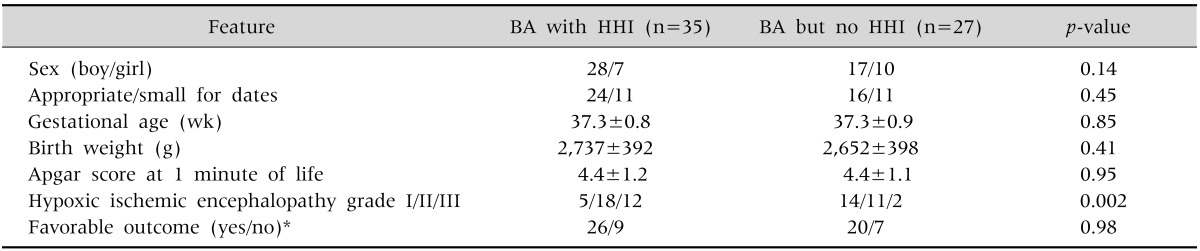
Values are presented as number or mean±standard deviation.
*Unfavorable outcome denotes neonatal death or discharge against medical advice.
Appropriate-for-dates: birth weight between 10th and 90th centile for gestational age. Small-for-dates: birth weight less than 10th centile for gestational age.
Babies with birth asphyxia and hypoxic hepatic injury
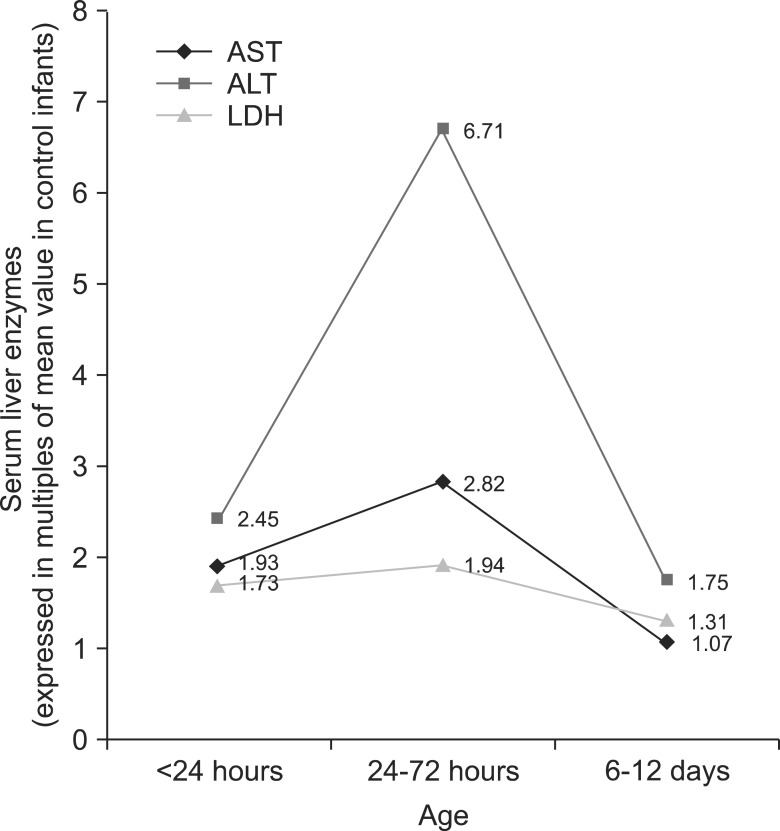
Serum liver enzymes measurements at all the three time points (<24 hours, 24-72 hours, and at 6-12 days) were available in 26 of the 35 babies (74%) with HHI. Temporal pattern of liver enzyme levels in these babies showed that ALT and LDH levels were significantly higher than controls at all three time points, whereas the AST levels though initially high, returned to normal at 6-12 days (Table 4). All three serum liver enzymes showed the maximum elevation at 24-72 hours of age, followed by a sharp decline by the age of 6-12 days (Fig. 1). Levels of none of the serum liver enzymes levels at any time point showed a correlation with the highest grade of HIE (range of correlation coefficients for ALT [-0.08-0.32], AST [-0.09-0.07] and LDH [-0.22-0.16]).
Table 4.
Pattern of Serum Liver Enzyme Levels at Three Time Points in Babies with Birth Asphyxia and Hypoxic Hepatitis Injury (n=26)
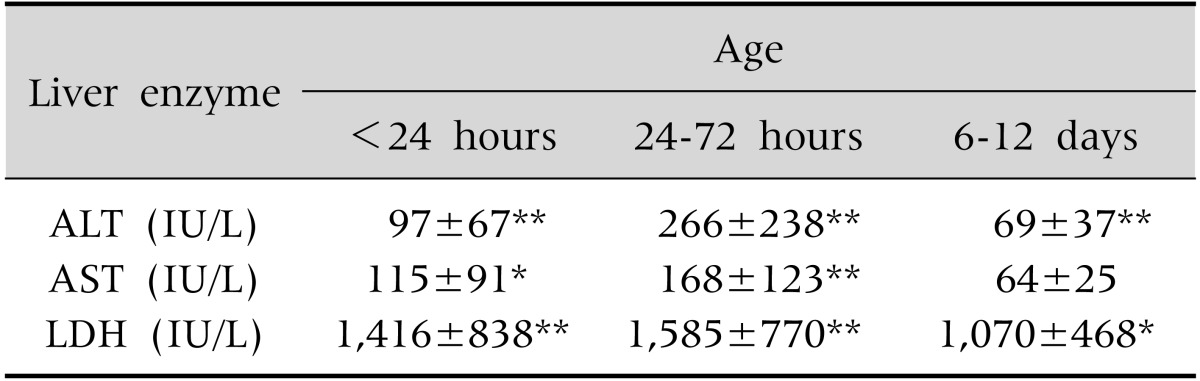
Values are presented as mean±standard deviation.
ALT: alanine aminotransferase, AST: aspartate aminotransferase, LDH: lactate dehydrogenase.
*p<0.05, **p≤0.001, p-values are as compared to control infants.
Fig. 1.
Temporal pattern of serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) in newborns with birth asphyxia and hypoxic hepatic injury.
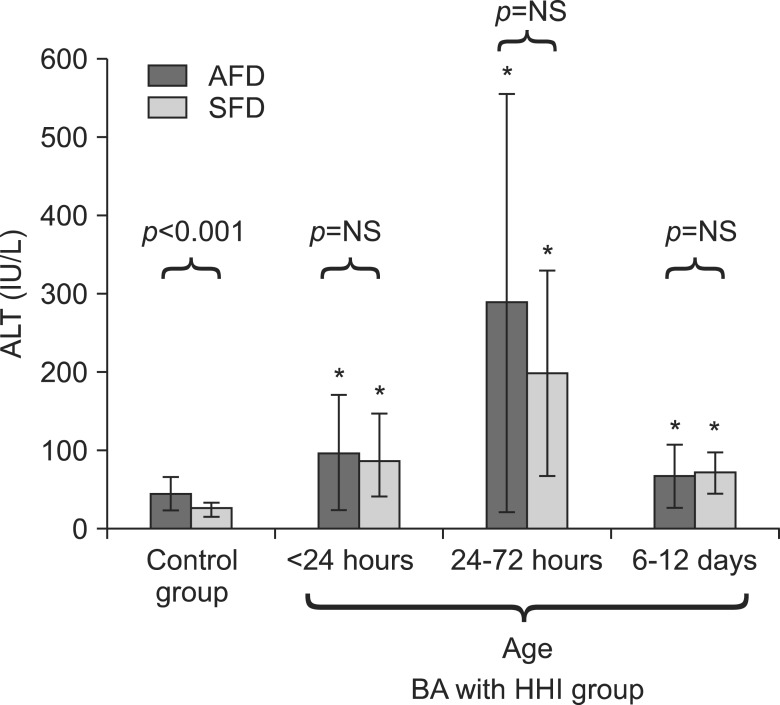
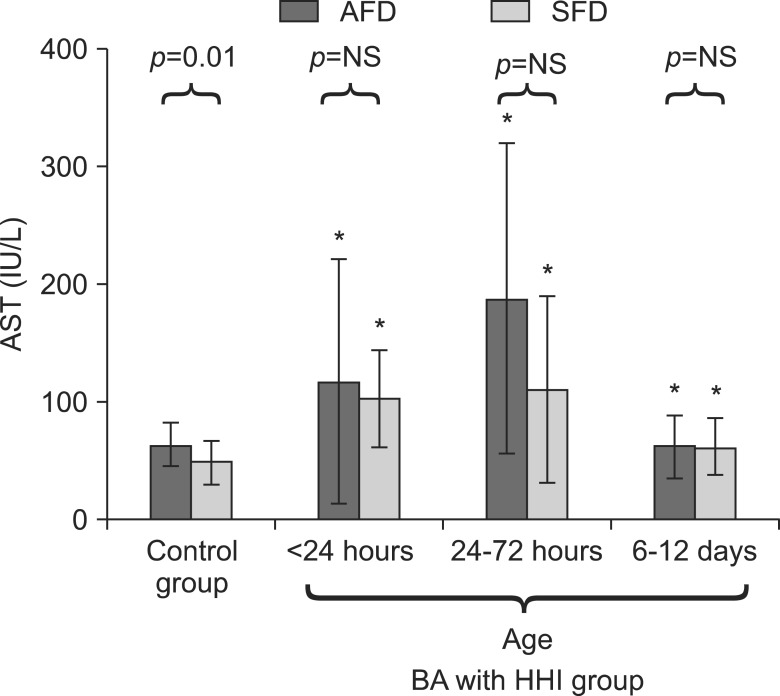
AFD and SFD babies in HHI group, when compared to corresponding AFD and SFD controls, had similar pattern of changes in serum ALT and AST levels. Serum ALT levels were significantly elevated till last estimation (Fig. 2) whereas AST levels were normalized at 6-12 days (Fig. 3). Serum liver enzyme levels were comparable between SFD and AFD babies at all three time points in HHI group (Fig. 2 and 3).
Fig. 2.
Comparison of serum levels of alanine aminotransferase (ALT) in small-for-date (SFD) and appropriate-for-date (AFD) infants. Bars represent means and error bars represents standard deviation. *p<0.05 when ALT levels are compared with corresponding SFD or AFD control infants. BA: birth asphyxia, HHI: hypoxic hepatic injury, NS: not significant.
Fig. 3.
Comparison of serum levels of aspartate aminotransferase (AST) in small-for-date (SFD) and appropriate-for-date (AFD) infants. Bars represent means and error bars represent standard deviation. *p<0.05 when AST levels are compared with corresponding SFD or AFD control infants. BA: birth asphyxia, HHI: hypoxic hepatic injury, NS: not significant.
DISCUSSION
HHI denotes injury caused to the hepatocytes by hypoxia. It is characterized by a sudden rise in serum levels of hepatic enzymes, starting soon after hypoxic insult, reaching a peak after 1-3 days of injury and returning to normal after 7-10 days [15]. These enzymes are present in cytosol (ALT, AST, and LDH) and mitochondria (AST) of hepatocytes. Serum level of liver enzymes may increase either due to hepatocyte death or to an increase in cell membrane permeability which allows efflux of enzymes into the blood [16].
Our observation of 56% of newborns with BA developing HHI lies well within the range of previous observations of 39% to 85% prevalence of this condition [3,4,14,17,18]. This wide range could be due to variations in criteria used in different studies to diagnose BA as well as HHI, severity of BA, time-points used for serum transaminase estimation, cut-off used for liver enzyme elevation, GA of the babies, and other obstetrical or neonatal factors. A previous prospective study had shown HHI in 65% of babies with BA, similar to our observation [17]. All these observations, including our study, suggest that HHI is common in babies with BA.
Mean serum level of ALT (39.6±20.6 IU/L) in our control infants was higher than the reference values for healthy adults [19]. This could be due to differences in hepatocyte characteristics such as metabolic rate, metabolic requirements, or cell membrane permeability due to immaturity. Moreover, reference levels of liver enzymes in adults are based on specimens collected in the morning after an overnight fast, which was not possible in our group. Hence, diurnal variations in liver enzyme levels or absence of fasting state may have had a bearing on our observations [20].
Serum liver enzymes levels were not influenced by age (≤72 hours and 73-144 hours) of control infants. This indicates that serum liver enzymes levels do not change in the first week of life. As previously shown by other workers [21], in contrast to adults [19], gender had little effect on serum ALT levels in newborn in our study. One recent study, however, showed that ALT levels were different between adolescent boys and girls [22]. This may be because male and female infants have similar levels of sex hormones, which are believed to determine ALT levels. We also found higher AST level than ALT level in healthy newborns; this could be due to widespread presence of AST in non-hepatic tissues, such as cardiac muscle, skeletal muscle, kidneys, brain, pancreas, lungs, leukocytes, erythrocytes and placenta, which may be affected by the stress of delivery.
In infants with BA and HHI, serum levels of liver enzymes (ALT, AST, and LDH) showed a significant rise within 24 hours after birth, peaked at 24-72 hours, and then declined sharply at 6-12 days of age. This time-trend was comparable to previous observations in adults [23] and neonates [17]. It is important to note that the degree of rise in serum levels of all three liver enzymes was much lower than that reported in adults with hypoxic hepatitis. This could be due to the relatively smaller size or immaturity of infant liver, or a relatively milder degree of hepatic injury in infants with BA than that observed in adults with hypoxic hepatitis which is usually caused by systemic hypotension.
Babies with BA and HHI had significantly higher grades of HIE but did not have an unfavorable outcome than those with BA but no HHI. Further, levels of serum liver enzymes had no correlation with highest grades of HIE. Our result suggests that usually babies with more severe BA, as indicated by higher grades of HIE, develop HHI, but that their outcome is determined by HIE and not by HHI. Serum transaminases are known to not correlate even with severity of primary hepatic injury such as acute viral hepatitis [16]. Previous studies have reported contradictory results [3,14]. One previous study showed significant correlation of HHI with HIE [17]. On the other hand, Shah et al. [3] failed to find any association between HIE and hepatic dysfunction. There may be a place for further studies in this issue.
One of our findings was that SFD babies had significantly lower ALT and AST levels at birth than AFD babies. This could be explained in several ways such as lower body mass, lower metabolic rate, relative deficiency of cofactors required for transaminase biosynthesis, or more mature hepatocyte cell membrane that prevents seepage of cytosolic enzymes into the circulation. A previous study also showed that babies with intrauterine growth retardation had lower ALT levels than controls but normal AST levels [24]. Interestingly, among babies with HHI, pattern of serum enzyme (ALT, AST, LDH) change was similar in SFD and AFD babies. Furthermore, levels of all three serum liver enzymes were comparable between SFD and AFD babies with HHI (Fig. 2 and 3). In brief, the control SFD babies have lower serum liver enzyme levels than AFD babies at birth, but the extent and temporal pattern of serum liver enzyme elevation in HHI was similar for SFD and AFD babies.
Several reasons restrained us from studying total or conjugated serum bilirubin levels between different groups. These include: (i) bilirubin level may be high because of physiological jaundice, (ii) serum bilirubin levels may not always rise following hepatic injury, (iii) serum bilirubin elevation could persist beyond our planned follow up of up to 6-12 days of life, (iv) relative non-specificity of serum bilirubin as a marker for liver injury, and (v) relatively normal or minor bilirubin elevation in ischemic hepatitis [25]. Similarly, PT could not be used as a HHI marker as it may be abnormal in disseminated intravascular coagulation. Likewise, hypoalbuminemia could not be used as a marker for acute liver involvement because its levels do not change rapidly with hepatic involvement but may change as a result of capillary leakage.
Our study had strengths of being a prospective study, inclusion of a comparable control group and a good sample size. However, it had several limitations, including: not having healthy controls as the comparator group; inclusion of out-born babies making the study group non-homogeneous; failure to measure neonatal blood pressure, and umbilical cord blood pH, PaO2, PaCO2; single estimation of serum liver enzymes in controls; incomplete liver enzyme data in some babies with HHI; widely separated time points for serum liver enzyme measurements; and, failure to follow serum liver enzymes till normalization in all babies. However, many of these were related to ethical considerations that limited blood sampling and procedure in infants.
In conclusion, serum liver enzymes in babies with BA and HHI peak after 24 hours of age followed by sharp decline in 6-12 days of age. Furthermore, though term SFD babies have lower ALT and AST levels than term AFD babies at birth, the extent and temporal pattern of serum liver enzyme elevation on development of HHI is similar in these two types of infants.
References
- 1.NNPD Network, Indian Council of Medical Research, National Neonatology Forum (India) National neonatal-perinatal database: report 2002-2003. New Delhi: Nodal Centre, AIIMS; 2005. [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–F155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankins GD, Koen S, Gei AF, Lopez SM, Van Hook JW, Anderson GD. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol. 2002;99:688–691. doi: 10.1016/s0029-7844(02)01959-2. [DOI] [PubMed] [Google Scholar]
- 5.Al-Macki N, Miller SP, Hall N, Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr Neurol. 2009;41:399–405. doi: 10.1016/j.pediatrneurol.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Volpe J. Neurology of the newborn. 4th ed. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 7.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 8.Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr. 2005;42:928–934. [PubMed] [Google Scholar]
- 9.Jayashree G, Dutta AK, Sarna MS, Saili A. Acute renal failure in asphyxiated newborns. Indian Pediatr. 1991;28:19–23. [PubMed] [Google Scholar]
- 10.Shastri AT, Samarasekara S, Muniraman H, Clarke P. Cardiac troponin I concentrations in neonates with hypoxic-ischaemic encephalopathy. Acta Paediatr. 2012;101:26–29. doi: 10.1111/j.1651-2227.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanik E, Ozer EA, Bakiler AR, Aydinlioglu H, Dorak C, Dogrusoz B, et al. Assessment of myocardial dysfunction in neonates with hypoxic-ischemic encephalopathy: is it a significant predictor of mortality? J Matern Fetal Neonatal Med. 2009;22:239–242. doi: 10.1080/14767050802430834. [DOI] [PubMed] [Google Scholar]
- 12.Rennie JM, Roberton NRC. Textbook of neonatology. 3rd ed. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 13.Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur J Obstet Gynecol Reprod Biol. 1999;84:155–172. doi: 10.1016/s0301-2115(98)00325-x. [DOI] [PubMed] [Google Scholar]
- 14.Tarcan A, Tiker F, Güvenir H, Gürakan B. Hepatic involvement in perinatal asphyxia. J Matern Fetal Neonatal Med. 2007;20:407–410. doi: 10.1080/14767050701287459. [DOI] [PubMed] [Google Scholar]
- 15.Feldman M, Lawrence SF, Lawrence JB. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 9th ed. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 16.Schiff ER, Sorrell MF, Maddrey WC. Schiff's diseases of the liver. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 17.Karlsson M, Blennow M, Nemeth A, Winbladh B. Dynamics of hepatic enzyme activity following birth asphyxia. Acta Paediatr. 2006;95:1405–1411. doi: 10.1080/08035250600693488. [DOI] [PubMed] [Google Scholar]
- 18.Godambe SV, Udani RH, Malik S, Kandalkar BM. Hepatic profile in asphyxia neonatorum. Indian Pediatr. 1997;34:927–930. [PubMed] [Google Scholar]
- 19.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 20.Córdoba J, O'Riordan K, Dupuis J, Borensztajin J, Blei AT. Diurnal variation of serum alanine transaminase activity in chronic liver disease. Hepatology. 1998;28:1724–1725. doi: 10.1002/hep.510280640. [DOI] [PubMed] [Google Scholar]
- 21.Ramakumar L, Dogra KN, Sood SC. Serum transaminases in the newborn. Indian Pediatr. 1965;2:43–48. [PubMed] [Google Scholar]
- 22.Park SH, Park HY, Kang JW, Park J, Shin KJ. Aminotransferase upper reference limits and the prevalence of elevated aminotransferases in the Korean adolescent population. J Pediatr Gastroenterol Nutr. 2012;55:668–672. doi: 10.1097/MPG.0b013e3182660669. [DOI] [PubMed] [Google Scholar]
- 23.Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039–1052. doi: 10.1111/j.1478-3231.2011.02655.x. [DOI] [PubMed] [Google Scholar]
- 24.Kocylowski R, Dubiel M, Gudmundsson S, Fritzer E, Kiserud T, von Kaisenberg C. Hepatic aminotransferases of normal and IUGR fetuses in cord blood at birth. Early Hum Dev. 2012;88:461–465. doi: 10.1016/j.earlhumdev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070. doi: 10.2169/internalmedicine.46.0059. [DOI] [PubMed] [Google Scholar]