Abstract
Cortical development is dependent on extrinsic stimulation. As such, sensory deprivation, as in congenital deafness, can dramatically alter functional connectivity and growth in the auditory system. Cochlear implants ameliorate deprivation-induced delays in maturation by directly stimulating the central nervous system, and thereby restoring auditory input. The scenario in which hearing is lost due to deafness and then reestablished via a cochlear implant provides a window into the development of the central auditory system. Converging evidence from electrophysiologic and brain imaging studies of deaf animals and children fitted with cochlear implants has allowed us to elucidate the details of the time course for auditory cortical maturation under conditions of deprivation. Here, we review how the P1 cortical auditory evoked potential (CAEP) provides useful insight into sensitive period cut-offs for development of the primary auditory cortex in deaf children fitted with cochlear implants. Additionally, we present new data on similar sensitive period dynamics in higher-order auditory cortices, as measured by the N1 CAEP in cochlear implant recipients. Furthermore, cortical re-organization, secondary to sensory deprivation, may take the form of compensatory cross-modal plasticity. We provide new case-study evidence that cross-modal re-organization, in which intact sensory modalities (i.e., vision and somatosensation) recruit cortical regions associated with deficient sensory modalities (i.e., auditory) in cochlear implanted children may influence their behavioral outcomes with the implant. Improvements in our understanding of developmental neuroplasticity in the auditory system should lead to harnessing central auditory plasticity for superior clinical technique.
Keywords: Plasticity, Cochlear implant, Auditory cortex, P1, N1, Development, Cortical auditory evoked potential, Sensitive period, Cross-modal re-organization, Visual evoked potentials, Somatosensory evoked potentials, Compensatory, Deafness, Auditory deprivation
1. Introduction
Appropriate development of the auditory cortex is largely dependent on sufficient and appropriate auditory input. In development, periods of increased neuroplasticity — termed sensitive periods — have been well described (Knudsen, 2004). Because of the increased ability of the cortex to be altered during sensitive periods, either auditory stimulation or deprivation can significantly impact the development of cortical infrastructure and associated behavioral abilities. Deprivation of auditory input during sensitive periods has adverse effects on many aspects of cortical maturation, leading to degraded behavioral performance. Thus, clinical management of children with hearing loss can be greatly augmented by considering the status of their central auditory maturation. For example, if a congenitally deaf child receives the necessary auditory stimulation within a sensitive period, appropriate cortical maturation occurs. That is, the connections and framework critical to age-appropriate auditory and spoken language skills are formed (Knudsen, 2004; Kral, 2013; Kral and Sharma, 2012). On the other hand, if for the same child auditory stimulation is withheld until after the close of this period of heightened neuroplasticity, the very organization of the auditory cortex can be severely altered. In many cases, these cortical modifications allow multisensory cortices to recruit available auditory regions for enhanced visual and/or somatosensory processing, at the expense of auditory processing abilities (Kral, 2013; Kral and Sharma, 2012).
Using cortical auditory evoked potentials (CAEPs), it is possible to non-invasively determine the maturational status of the auditory cortex in a given patient using various biomarkers. In deaf children, these biomarkers provide a tool for gauging whether auditory stimulation via hearing aids is appropriate or if cochlear implantation should be pursued. In addition, these biomarkers reflect various stages of sensitive periods during which auditory input must be provided. In this report we will review the P1 CAEP biomarker of primary auditory cortex development in deafness, as well as present new data describing the N1 CAEP as a biomarker of higher-order auditory cortical development. Cortical reorganization and functional outcomes related to auditory stimulation provided within and outside the sensitive period for central auditory development will also be discussed. Finally, we will explore the notion that cross-modal compensatory plasticity (from visual and somatosensory modalities) may underlie some of the variability in behavioral outcomes seen in cochlear implanted children.
2. Normal development of the central auditory pathways
Normal development of auditory cortex has been described in detail, with marked changes in thalamo-cortical and cortico-cortical pathways occurring well into the second decade of life (Ceponiene et al., 2002; Ponton et al., 2000b; Sharma et al., 1997). In initial stages, development relies on intrinsic factors, which are genetically controlled, such as synaptogenesis. However, extrinsic factors (i.e., auditory stimulation) drive the refinement of cortical connections, via pruning and stabilization of synaptic connections and myelination (Fields, 2005; Huttenlocher and Dabholkar, 1997; Kral and Sharma, 2012). For example, animal data have revealed that much of the feedforward neural connections in the central auditory system is genetically pre-determined and set in place at early stages of development (Hartmann et al., 1997; Kral and Eggermont, 2007). In contrast, cortical feedback projections, important to modulation of cortical and subcortical structures, are dependent primarily upon auditory input and experience (Kral, 2007; Kral and Eggermont, 2007; Kral et al., 2000, 2001, 2005). The heightened plasticity that operates during sensitive periods allows for rapid formation of synaptic connections, neural synchronization, and stabilization of cortical connections (Pallas, 2001). This plasticity results in intense adaptation to the surrounding environment and important refinement of the cortical pathways.
Cortical auditory evoked potentials (CAEPs) are non-invasive EEG measurements that can chart the maturation of the central auditory system via changes in latency and amplitude (see Steinschneider et al., 2011 for a review). For instance, the latency of the first component of the obligatory CAEP, or P1 — a positive-going peak reflecting the sum of the accumulated synaptic delays and neural conduction times as an auditory signal travels from the ear to the primary auditory cortex — decreases with age in normal hearing children (Eggermont, 1988; Eggermont et al., 1997; Liegeois-Chauvel et al., 1994; Sharma et al., 1997, 2002b). The distinction between the Pa, Pb, and P50 has more commonly been made in adults (e.g., Liegeois-Chauvel et al., 1994). However, in the literature regarding children, the classifier “P1” is used to denote the large broad positivity that is seen in the cortical auditory evoked potentials (CAEPs) of infants and young children (see, for example, Ceponiene et al., 2002; Liegeois-Chauvel et al., 1994; Ponton et al., 1996a,b, 2000b, 2002; Sharma et al., 1997). The P1 is typically first observed to occur in newborns and infants with normal hearing at a latency of around 300 ms post-auditory stimulation. The P1 undergoes a rapid decrease to approximately 100 ms at two years old, and then a more gradual decrease in latency to 50–70 ms in adulthood. This systematic drop in latency has been quantified with 95% confidence intervals, providing normative data for central auditory development (Sharma et al., 2002a,b,c). Gilley et al. (2008) demonstrated that the cortical generators of the P1 CAEP component in normal hearing children are comprised of the auditory cortex.
Another obligatory CAEP waveform component emerges with increasing age in childhood: the N1. In younger children, the N1 first emerges as a bifurcation in the P1 waveform. In older children, adolescents, and adults, the N1 is seen as a separate negative-going peak that follows the P1 component (Sussman et al., 2008). The appearance of the N1 as an invagination of the CAEP waveform is reliably observed after 6–7 years old (Sharma et al., 1997; Gilley et al., 2005; Sussman et al., 2008). However, if stimulus presentation is considerably slowed during CAEP recording, the N1 can be seen earlier in life (Ceponiene et al., 1998; Gilley et al., 2005). This phenomenon is due, in part, to the development of refractoriness of the neurons underlying the N1 response, suggesting that maturation of component 1 as described by Naatanen and Picton (1987) underlies the presence of the N1 in children (Ceponiene et al., 1998; Gilley et al., 2005; Sussman et al., 2008). Though many cortical auditory components contribute to the N1 CAEP peak, intracranial electrophysiological recordings in humans have determined that these all originate from secondary auditory cortical regions, with the result of scalp EEG recording the main N1 generator, planum temporale (Godey et al., 2001; Liegeois-Chauvel et al., 1994; Yvert et al., 2005). The N1 CAEP has also been shown to be a neurophysiological correlate of activation in supragranular layers and higher-order auditory cortex, including intra-hemispheric cortico-cortical connections (Eggermont and Ponton, 2003; Makela and Hari, 1992; Makela and McEvoy, 1996; see Naatanen and Picton, 1987 for a review).
The obligatory nature of the P1 and N1 CAEP waveform components means that no attention to auditory stimuli is required for elicitation of these cortical responses. Rather, they occur automatically in response to sound. Thus, these purely physiologic responses are optimal objective biomarkers of central auditory development. Additionally, due to their varying underlying generators and unique developmental time courses, the P1 and N1 CAEPs provide reliable biomarkers for maturation of both primary and higher-order auditory cortices, respectively. In practice, these biomarkers have become useful in chronicling central auditory development and plasticity when extrinsic auditory input is absent or degraded because of deafness and related conditions like Auditory Neuropathy Spectrum Disorder (ANSD), and then ameliorated via a cochlear implant (Cardon and Sharma, 2013; Eggermont and Ponton, 2003; Gordon et al., 2008; Kral and Sharma, 2012).
3. Central auditory development in deafness
3.1. The P1 CAEP biomarker
Cortical auditory evoked potentials recorded in congenitally deaf children describe a brief period in early childhood when developmental plasticity of the central auditory system is at its peak. Studies by Ponton, Eggermont, and colleagues compared waveform morphologies and latencies of CAEP responses from deaf children to those from age-matched normal hearing persons and showed that deafness essentially halts central auditory development, resulting in an immature auditory cortex (Eggermont, 1988; Eggermont and Ponton, 2002, 2003; Ponton and Eggermont, 2001; Ponton et al., 1993, 1996a,b, 1999a,b, 2000a,b, 2002). This immaturity is a direct result of a lack of extrinsic driving factors spurring on the generation and stabilization of neural connections. Eggermont et al. (1997) hypothesized that their results suggest that the auditory brain is ‘frozen in time’ as a result of sensory deprivation. However, after cochlear implantation, maturation proceeds at a normal rate, i.e., a rate roughly equivalent to that of a normal hearing newborn. As a consequence, they concluded that P1 latencies reflect the ‘time in sound’ experienced by the implanted child. These studies provided the first critical evidence that the potential for normal development of the auditory system is maintained in deaf children during their years of sensory deprivation.
Over the last decade, Sharma and colleagues have conducted large-scale studies examining cortical development in congenitally deaf children fitted with a cochlear implant at different ages (e.g., Sharma et al., 2002a,b,c, 2007, 2009). Sharma and colleagues (Sharma and Dorman, 2006; Sharma et al., 2002b) examined P1 latencies in 245 congenitally deaf children fit with a cochlear implant and reported that children who received stimulation via an implant early in childhood (<age 3.5 years) showed normal P1 morphology and latency, while children who received cochlear implant stimulation late in childhood (>age 7 years) had abnormal cortical response latency and morphology. Children receiving implants between 3.5 and 7 years revealed normal P1 latencies only 50% of the time, regardless of age of implantation within that 3.5–7 year age range. In another study, Sharma and colleagues examined individual developmental trajectories for the P1 response after cochlear implantation in 231 children (Sharma et al., 2007). Although all children showed delayed P1 latencies prior to implantation, children implanted under age 3.5 years showed normal P1 response latencies within 6–8 months after implantation. Though children implanted after age 7 also showed latency decreases over time, their developmental trajectories were abnormal, with P1 latencies never reaching normal limits even after years of implant usage. Based on these studies, Sharma and colleagues concluded that there is a sensitive period of 3.5 years in childhood during which sensory stimulation must be introduced if the central auditory system is to develop normally. In all likelihood, the sensitive period ends by age 7 years, resulting in a re-organized auditory cortex that is unable to effectively process the stimulation provided by the cochlear implant.
3.2. The N1 CAEP biomarker
Thus far, most of the data describing sensitive periods for cortical development in children with cochlear implants is based on the P1 CAEP (Ponton and Eggermont, 2001; see Sharma et al., 2011 for a review). However, as development proceeds in childhood, the CAEP undergoes extensive changes in morphology. As the P1 component decreases in latency and amplitude, the N1 CAEP, which emerges at about 6–7 years old as an invagination in the P1 response, begins to dominate the waveform in the second decade of life and in adulthood. The development of the N1 CAEP component coincides with structural refinements in auditory cortical maturation such as increased cortico-cortical coupling and enhanced auditory processing and language abilities (Eggermont and Ponton, 2003; Moore and Guan, 2001). Given that the N1 is a dominant CAEP component in typically hearing older children and adults, and has primary input from higher order auditory cortex, it would be useful to examine longer-term development of the central auditory pathways in cochlear implanted children using the N1 CAEP. In this section, we will report on new data where we recorded and analyzed the N1 CAEP in children with normal hearing and deaf children who received cochlear implants at different ages in childhood.
Participants included 41 typically developing children with normal hearing, aged newborn to 15 years (mean age = 6.15 years, S.D. = 4.49 years) and 80 children with cochlear implants, aged 2.27 to 16.48 years (mean age = 8.02 years, S.D. = 4.14 years). CAEPs were collected using a Compumedics Neuroscan® EEG system from the Cz vertex electrode, elicited by a speech syllable/ba/, and presented at an interstimulus interval of 610 ms (Sharma et al., 1997, 2002a,b,c). At least two runs of 300 sweeps each were recorded for each participant and all data recording and analysis procedures, including the removal of the scalp-recorded artifact from the cochlear implant, were identical to those reported in our previous studies (Gilley et al., 2006; Sharma et al., 2002a,b,c). Finally, subjects’ grand averaged waveforms were band pass filtered at 4 to 30 Hz to enhance the presence of the N1 peak component (Ceponiene et al., 2002; Gilley et al., 2005; King et al., 2008).
As can be seen in Fig. 1A, the N1 began to emerge in the normal hearing children around 3–6 years old. In children ages 0–3 years, 18% demonstrated the presence of the N1 component in this age range. Detectability of the N1 increased to 60% in the 3–6 year old group, 71% in the 6–9 year old group, and 100% in the 9–12 and 12–15 year old age groups. This pattern of detectability of the N1 as a function of age in typically developing children is consistent with previous studies (Ceponiene et al., 2002; Gilley et al., 2005; King et al., 2008).
Fig. 1.
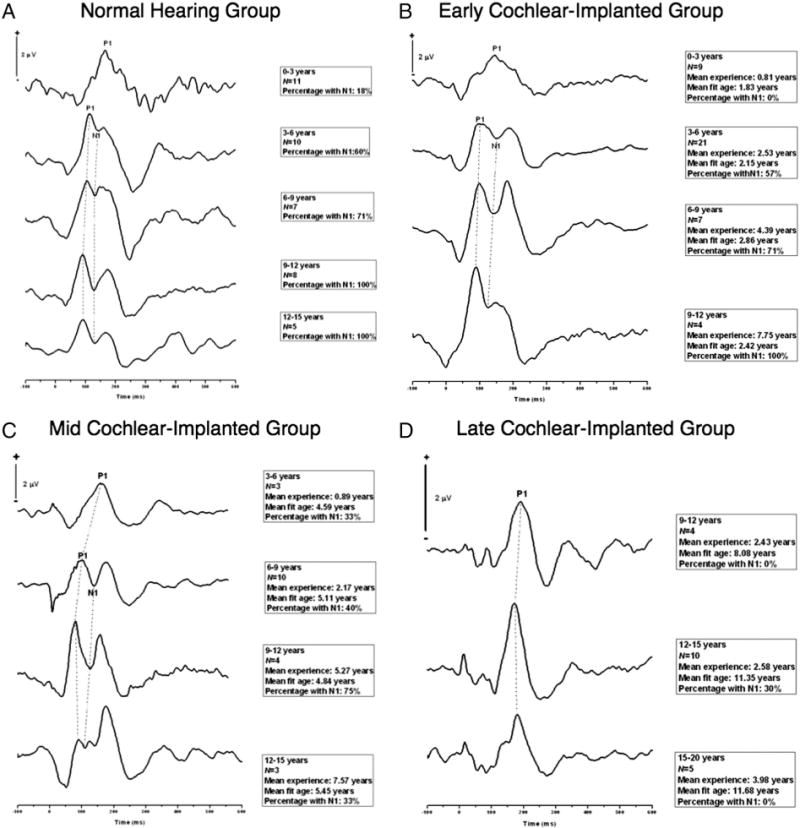
N1 CAEP development in cochlear implanted children. A. Cortical auditory evoked potentials (CAEPs) recorded in normal hearing children ages 0 to 15 years. Time in milliseconds is shown on the horizontal axis, and amplitude in microvolts on the vertical axis. The grand average waveform per age group, number of subjects included in the grand average, and percentage of children showing the N1 for that age group are described in the panels to the right of the waveforms. Developmental changes in P1 and N1 latencies are depicted by the dashed lines. B. CAEP grand average waveforms in early cochlear implanted children (implanted under 3.5 years). C. CAEP grand average waveforms in the mid cochlear implanted group (implanted between 3.5 and 7 years). D. CAEP grand average waveforms in the late cochlear implanted group (implanted after 7 years). For panels B, C and D, the grand average waveform per age group, the number of subjects included in the grand average, mean implant experience, fit age, and percentage of children showing the N1 for the specific age group are described for each age group.
We then divided 80 children with cochlear implants into 3 groups to examine the trajectory of N1 development in cochlear implanted children as a function of age. These groups consisted of early implanted children, mid implanted children and late implanted children. The early implanted group was implanted under the age of 3.5 years (n = 41, age of implantation: mean = 2.23 years, S.D. = 0.57 years, implant experience: mean = 2.98 years, S.D. = 2.17 years) and ranged in age from 0 to 12 years (mean = 5.21 years, S.D. = 2.35 years) at the time of testing. The mid implanted group received a cochlear implant between the ages of 3.5 to 7 years (n = 20, age of implantation: mean = 5.03 years, S.D. = 0.83 years, implant experience: mean = 3.41 years, S.D. = 2.62 years) and ranged in age from 3 to 15 years (mean = 8.44 years, S.D. = 2.58 years) at the time of testing. The late implanted group received a cochlear implant after the age of 7 years (n = 19, age of implantation: mean = 10.75 years, S.D. = 2.19 years, implant experience: mean = 2.91 years, S.D. =2.03 years) and ranged in age from 9 to 20 years (mean = 13.67 years, S.D. = 2.02 years) at the time of testing.
As can be seen in Fig. 1B, for early implanted children, 57% of subjects in the 3–6 year-old group presented with the N1 peak, followed by 71% of subjects in the 6–9 year old group. Finally, 100% of subjects in the 9–12 year old group showed the N1 component. In general, these percentages for detectability of the N1 are similar to those for age-matched normal hearing children described above.
Within the mid cochlear implanted group (Fig. 1C), 33% of subjects in the 0–3 year old group presented with the N1, increasing to 40% of subjects in the 6–9 year old group. This jumped to 75% in the 9–12 year old group, while only 33% in the 12–15 year old group showed the N1. Compared to 100% of normal hearing and early-implanted children who show an N1 in the 12–15 year age range, the percentage of mid-implanted children showing an N1 is clearly lower.
In contrast to the normal hearing, early implanted and mid implanted children, very few children in the late implanted group showed an N1 response. Zero percent of late implanted children aged 9–12 years had an N1, 30% of late implanted children in the 12–15 year group demonstrated the presence of the N1 and none of the children in the 15–20 year age group had an N1 (Fig. 1D).
In a separate analysis, age 6 years was used as a criterion for reasonable detectability of the N1 based on studies of normal hearing children (Ceponiene et al., 2002; Gilley et al., 2005; King et al., 2008). We examined data from a group of 47 cochlear implanted children aged 6 years and older at the time of testing. A cluster analysis using Schwarz’s Bayesian Criterion and a log-likelihood distance measure revealed two separate groups (silhouette measure of cohesion and separation = 0.7). The first cluster included 28 children, of whom 17 showed the N1 component, while the second group included 19 children, of whom 16 showed no evidence of the N1 component. The clusters depended on age of implantation, such that the first cluster consisted of children implanted between 2.02 and 6.58 years, and the second group of children implanted between 7.61 and 14.63 years (Fig. 2). Fig. 2 shows the two clusters and indicates the approximate age of implantation which separated the two clusters. Note: in Fig. 2, we further sub-divided the implanted children into one-year intervals based on age of implantation for ease of viewing.
Fig. 2.
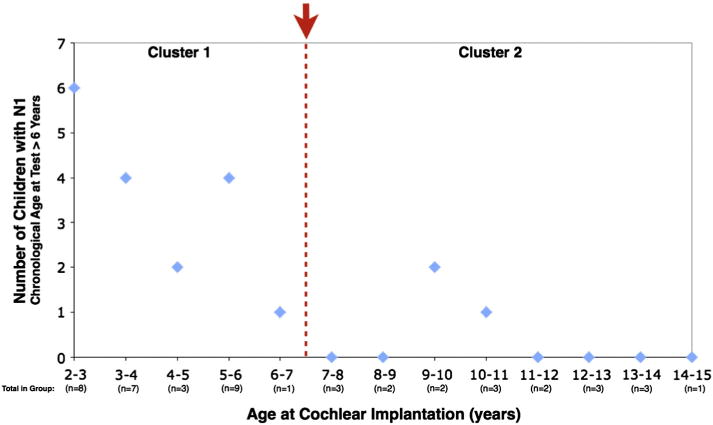
N1 CAEP occurrence as a function of age of implantation. A subset of children ages 6 years and older was analyzed. Age 6 years was used as a criterion for reasonable detectability of the N1 based on studies of normal hearing children. The red arrow indicates the age of cochlear implantation at which cluster analysis determined two separate age clusters (ages 2.02–6.58 years; ages 7.61–14.63 years) of subjects based on their implantation age and the presence of the N1. The vertical axis shows the number of children presenting with the N1 and the horizontal axis shows the age of implantation, divided into one-year increments.
As Fig. 2 shows, the age of implantation at which the two clusters reflecting N1 development in cochlear implanted children separate is approximately 7 years. Thus, the implantation age of approximately 7 years as a cut-off for the development of the N1 component is consistent with the end of the sensitive period of auditory cortical development as documented in previous studies using the P1 CAEP response (Kral and Sharma, 2012; Sharma et al., 2002b). Taken together, these data provide new evidence to further validate age 7 years as the age after which auditory cortical plasticity is significantly reduced.
The developmental trajectory of the N1 CAEP in cochlear implanted children appears to be similar to that of the P1, in that early implantation has the best results for normal development of the N1 response (Fig. 2). Of the late implanted subjects showing an N1, one of the subjects had progressive hearing loss and another showed mild-moderate aided auditory thresholds, likely providing adequate auditory stimulation to the cortex at a younger age. Overall, the absence of the N1 component in the majority of (but not all) late implanted children is in marked contrast to the normal hearing, early implanted, and mid implanted children and indicates abnormal higher-level auditory cortical development (Gilley et al., 2005). Our findings are consistent with Eggermont and Ponton (2003) who reported that the N1 component in the CAEP was absent in cochlear implanted subjects who had been deaf for a period of at least 3 years.
3.3. Converging evidence for sensitive periods in the central auditory system
As described above, both P1 and N1 CAEP components point to similar characteristics of the sensitive period for central auditory development in deafness. Namely, implantation below the age of 3.5 allows for optimal use of the heightened neuroplasticity present in the early stages of the sensitive period, with an ending of the sensitive period occurring around7 years old. Since the current FDA approved age for pediatric cochlear implantation is 12 months, early implantation is highly possible in most cases. In all, implantation before age 3.5 years results in essentially normal development of the central auditory pathways. Furthermore, cochlear implantation at younger ages within this period takes maximal advantage of the high degree of developmental plasticity in the central auditory system. Of additional note are recent findings that early bilateral implantation within this timeframe with minimal age separation between the two implants is preferable (Gordon et al., 2011).
The results from children with cochlear implants described above are consistent with animal studies that also describe a sensitive period for cochlear implantation. Kral et al. (2002) showed that congenitally deaf cats that had received a cochlear implant by age ~3.5 months exhibited increased cortical activation and decreased cortical response latencies. On the other hand, cats that were implanted a short time later showed minimal cortical activation that was reminiscent of congenitally deaf cats that never received cochlear implants.
Further converging evidence for the sensitive period for central auditory maturation used positron emission tomography (PET) brain imaging to examine cortical development in deaf children who were cochlear implant candidates (Lee et al., 2001, 2005, 2007; Oh et al., 2003). In these studies, the investigators assessed resting cortical metabolic rate and regional density cerebral blood flow in children who had experienced different durations of deafness prior to implantation. Children who had undergone shorter durations of deafness (i.e., ~3–4 years) presented with decreased spontaneous glucose metabolism in the auditory cortices and good post-implantation behavioral outcomes. On the other hand, those who had experienced durations of deafness exceeding 6–7 years old demonstrated normal spontaneous metabolism in the auditory cortices and poor outcomes after implantation. Normal metabolic rates preceding implantation in the auditory cortices of children who had undergone long-term auditory deprivation suggested that the auditory cortices had been appropriated for processing by other sensory modalities. In contrast, decreased metabolic levels pointed to cortices that were available for the performance of auditory function.
A clear effect of age of implantation on functional outcome has also been borne out by behavioral assessment of children with cochlear implants. That is, numerous studies have established that children implanted before the age of 3–4 years tend to show superior speech and language outcomes in relation to children implanted after age 6–7 years (Dunn et al., 2014; Geers, 2006; Harrison et al., 2005; Holt and Svirsky, 2008; Niparko et al., 2010; Robbins et al., 2004; Svirsky et al., 2004; Wang et al., 2008). Though these data have proven highly informative, a more pertinent question may be whether children implanted before the FDA approved age of 12 months significantly outperform their peers who are implanted at or after age 1 year (but before age 3 years). Thus far, the results have been mixed, especially when duration of implant use is considered (e.g., Dettman et al., 2007; Havy et al., 2013; Miyamoto et al., 2008; Tajudeen et al., 2010). While age of implantation is an important factor, there are many variables which influence outcomes in spoken language for children with cochlear implants and these include socio-economic status, parent–child interaction, communication modality among others (Clark et al., 2012; Cruz et al., 2013; Niparko et al., 2010; Quittner et al., 2013).
3.4. Mechanisms that mediate sensitive periods
What is remarkable about ages 3.5 and 7 years during central auditory development in children? The 3.5 years age cut-off reflects, at least in part, the timeline for synaptogenesis in the temporal cortex. That is, synaptic density increases in infancy and childhood until it reaches its height in the auditory cortex around 3.5–4 years old. This intense proliferation of synapses is followed by a steep decline in number of synapses due to synaptic refinement (Huttenlocher and Dabholkar, 1997). Given that early synaptogenesis is, in large part, intrinsically driven, it appears to provide a great deal of flexibility concerning the introduction and effects of auditory stimulation (i.e., a kind of protective effect) for the first 3–4 years of life. In contrast, later synaptogenesis has been shown to be activity-dependent. In fact, congenitally deaf cats, which have not received cochlear implants within the sensitive period, exhibit significant delays in synaptogenesis, especially in infragranular cortical layers (Kral and Sharma, 2012; Kral et al., 2001, 2005). This is likely why, if implantation is carried out within the sensitive period, it provides the external stimulation necessary for synaptic refinement to occur via ‘pruning’ (Kral, 2007), and why late implantation leads to delays in maturation.
Along with synaptogenesis, myelination of long fiber tracts is another developmental process which influences conduction times of cortical auditory evoked potentials in development. Myelination in the temporal cortex is adult-like by age 7–8 years (Eggermont and Moore, 2012; Su et al., 2008). Though speculative at this point, given the 7 year age cut-off, it is plausible that the dynamics of myelination may be related to the close of the sensitive period for central auditory development.
Structural measures reflecting auditory experience and development, such as the cortical thickness of Heschl’s Gyrus in auditory cortex, have also been correlated with the N1 response (Fu and Zuo, 2011; Liem et al., 2012). As children age through pre-adolescence, region-dependent decreases in gray matter volume occur, concurrent with white matter volume increases, possibly providing a structural basis for developmental changes reported for the N1 CAEP (Gogtay et al., 2004; Group B.D.C., 2012; Lenroot and Giedd, 2006; Muftuler et al., 2011). Age 7 years has been implicated as an age at which major structural changes are taking place, allowing cortical networks to form and function appropriately (Nie et al., 2013). It is interesting to note that it is around this age (7 years) that the N1 CAEP becomes clearly apparent in typical stimulation paradigms in normal hearing children.
Thus, it appears that by age 7 years, a turning point is reached in the development of cortical synaptic formation and myelination when gray matter begins to decrease, cortical folding is stable, and global cortical connections become more efficient (Nie et al., 2013). These massive developmental cortical changes coincide with, and are likely related to, an age after which auditory developmental plasticity, while present, is likely reduced.
4. Cross-modal re-organization in the auditory cortex
4.1. De-coupling in auditory cortex
Kral et al. (2005) have described a sensitive period in congenitally deaf cats of approximately 3.5 months. When electrical stimulation is introduced via a cochlear implant following four months of deafness, delays are seen in the activation of supragranular layers of the cortex, and activity at longer latencies and in infragranular layers (layers V and VI) is virtually absent (Kral et al., 2005). In these subjects, the near-absence of afferent currents in layers IV and III suggests immaturity of inhibitory synapses and an alteration of information flow from layer IV to supragranular layers. In typically developing cats, the higher-order auditory cortex projects back to A1 (primary auditory cortex), mainly to the infragranular layers (V and VI), and then sends long-range feedback projections to the subcortical auditory areas. Thus, the absence of activity in infragranular layers in congenitally deaf cats seems to suggest a functional decoupling of primary cortex from higher-order auditory cortex, which, in turn, affects the aforementioned feedback projections to the subcortical auditory system (Kral et al., 2000, 2002, 2005). Kral has speculated that a similar partial or complete decoupling between A1 and higher-order cortex may occur in congenitally deaf children at the close of the sensitive period (Kral, 2007; Kral and Sharma, 2012) due to the lack of extrinsic input spurring development.
The N1 CAEP provides a biomarker of higher order auditory cortical development as it is predominantly generated in higher-order auditory cortex, with input from cortico-cortical reciprocal loops between primary and secondary auditory cortices (Godey et al., 2001; Kral and Eggermont, 2007; Liegeois-Chauvel et al., 1994; Wunderlich and Cone-Wesson, 2006). As reported above, the N1 CAEP data from cochlear implanted children show that most children who are implanted after age 7 years never develop an N1 response (consistent with Eggermont and Ponton, 2003). This finding is in stark contrast to children implanted before a sensitive period of 3.5 years who develop an N1 component that is similar in morphology and latency to that found in normal hearing children (Sharma and Dorman, 2006). Given the higher order origins of the N1, it is likely that the missing N1 wave in late implanted children is indicative of improper activation of higher-order areas, which, taken together with the above data from congenitally deaf cats, is likely due to partial or total decoupling of higher-order areas from the primary auditory cortex. That three children in the late-implanted group showed development of an N1 response (consistent with Gordon et al., 2008) suggests that at least some late implanted children can exhibit partially developed cortico-cortical connections, despite less than optimal timing of implant fitting. It is likely that factors such as an early period of normal hearing, good aided hearing, and intensive auditory rehabilitation may mitigate complete cortico-cortical and intra-hemispheric decoupling in long-term deafness (Dinces et al., 2009; Gordon et al., 2008; Waltzman et al., 2002).
The functional consequences of de-coupling of auditory cortices may be quite serious for learning. That is, with longer-term deprivation of auditory input, not only does the bottom-up capacity for information processing decrease, but there is also an inability to integrate the afferent information with cognitive top-down modulatory influences from higher-order cortices (Kral, 2007). Late implanted children, therefore, may have difficulty attaching meaning to the auditory stimuli encoded by their cochlear implants, resulting in deficient oral language learning.
4.2. Cortical re-organization
When higher-order auditory cortex, which is inherently multimodal, lacks normal auditory input through typical neural connectivity to primary auditory cortex, it may become dominated by other sensory input in long-term deafened children. Using current density reconstructions, Gilley et al. (2008) reported that early implanted children showed temporal activation that was similar to normal hearing children. On the other hand, late implanted children (i.e. implanted after age 7 years) primarily showed activation of parietotemporal cortex (in response to auditory stimulation). This finding points to an atypically distributed network of brain areas that is associated with the poor auditory processing and deficient oral language acquisition typically seen in late-implanted children.
Cross-modal plasticity is another form of cortical re-organization associated with deafness. This form of plasticity occurs when an intact sensory modality recruits cortical resources from a deprived sensory modality to increase the processing capabilities of the intact modality as compensation for the effects of sensory deprivation. Converging evidence suggests that the visual and somatosensory systems are both involved in the cross-modal recruitment of higher-order auditory cortex in deafness. For instance, some studies have shown activation of the higher-order auditory cortex by visual motion stimuli in deaf adults (Buckley and Tobey, 2011; Doucet et al., 2006; Fine et al., 2005; Finney et al., 2001, 2003; Neville et al., 1983; Rebillard et al., 1977; Sadato et al., 2005) and adults with mild-moderate hearing loss (Campbell and Sharma, 2014). Other investigators have presented evidence of activation of higher-order auditory and association cortices in response to somatosensory (i.e., vibrotactile) stimulation in similar participants (Baldwin, 2001; Levanen, 1998; Sharma et al., 2007). Furthermore, both auditory and somatosensory stimuli (e.g., Braille reading) activate visual cortex in blind adults (Cohen et al., 1997; Hamilton and Pascual-Leone, 1998; Hyvärinen et al., 1981a,b; Kujala et al., 1995; Sadato et al., 1996; Uhl et al., 1991).
Despite routine early implantation in current clinical practice, there exists tremendous variability in behavioral outcomes for cochlear implanted children. Geers et al. (2009) reported that less than 50% of the variability in speech and language performance of implanted children could be accounted for, using demographic factors, such as age of implantation. Henkin et al. (2008) described poor speech perception scores for many children implanted within 3.5 years, which provides further evidence of the variability in behavioral outcome in even early implanted children. Compensatory cross-modal re-organization, which results in areas of auditory cortex being re-purposed by vision or somatosensation, has been implicated as a factor that may further explain some of the variable outcomes for auditory processing in children with cochlear implants.
Recent studies have described a strong correlation between cross-modal re-organization and poor outcomes with cochlear implants in deaf adults (Buckley and Tobey, 2011; Doucet et al., 2006; Sandmann et al., 2012). Here we present new preliminary evidence that cross-modal plasticity may influence speech perception performance in pediatric cochlear implant recipients. In ongoing investigations in our laboratory, we are conducting large-scale studies of visual and somatosensory cross-modal re-organization and whether these phenomena predict behavioral outcomes in cochlear implanted children. We use high-density 128-channel EEG, with methods identical to Campbell and Sharma (2013, 2014).
In Fig. 3, current density reconstructions (CDRs) computed via sLORETA show activated regions as illustrated on sagittal and coronal MRI slices. Yellow regions reflect maximal cortical activation, while brown/black regions reflect the areas of least activation. In Fig. 3A we show cortical activation elicited by visual stimuli in a normal hearing (NH) child (left panel), a high-performing cochlear implanted child (middle panel), and an average-performing cochlear implanted child (right panel). The visual stimuli used have been shown to activate higher-order visual cortices, including both dorsal and ventral streams due to the percept of apparent motion and shape change (Bertrand et al., 2012; Campbell & Sharma, 2014; Doucet et al., 2006; Wilkinson et al., 2000). As can be seen for the P2 cortical visual evoked potential (VEP) component, the NH child and the high-performing cochlear implanted child (96% speech perception score) show expected occipital activation in response to visual stimulation, including higher-order visual cortex (middle occipital gyrus, fusiform gyrus, and lingual gyrus). On the other hand, the average-performing cochlear implanted child (67% speech perception score) exhibits occipital activation and activity in superior temporal gyrus and medial temporal gyrus.
Fig. 3.
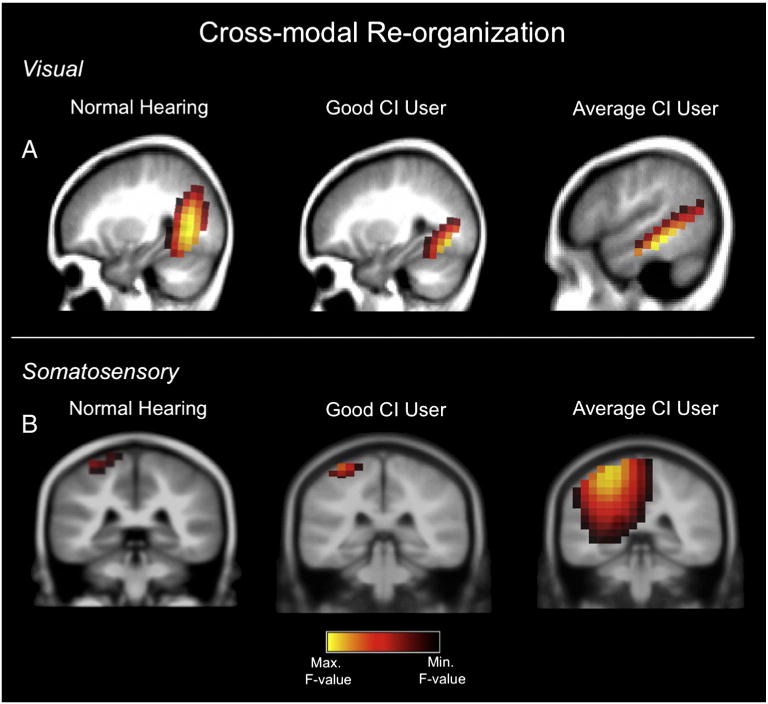
A. Visual cross-modal re-organization in children with cochlear implants. Visual gradient stimulation was presented to a child with normal hearing and two children with cochlear implants. Current density reconstructions (CDRs) of the cortical visual P2 component computed via sLORETA show activated regions as illustrated on sagittal MRI slices. Yellow regions reflect maximal cortical activation, while brown/black regions reflect the areas of least activation. Left panel: a 10 year-old child with normal hearing shows activation of higher-order occipital cortices in response to visual stimuli. Middle panel: an 8 year-old cochlear implanted child with a speech perception score of 96% on the Lexical Neighborhood Test, shows similar activation of higher-order visual areas, such as middle occipital gyrus, fusiform gyrus, and lingual gyrus. Right panel: in contrast, a 7 year-old cochlear implanted child with a speech perception score of 67% on the Multisyllabic Lexical Neighborhood Test shows activation of occipital areas and superior temporal gyrus and medial temporal gyrus. B. Somatosensory cross-modal re-organization in children with cochlear implants. Vibrotactile stimulation of the right index finger was presented to a child with normal hearing and two children with cochlear implants. Current density reconstructions (CDRs) of the cortical somatosensory N70 component computed via sLORETA show activated regions as illustrated in coronal MRI slices. Left panel: a normal hearing 7 year-old child shows activation of somatosensory cortex in the post-central gyrus. Middle panel: a 13 year-old cochlear implanted child with a speech perception score of 94% on the Consonant Nucleus Consonant (CNC) test shows similar activation of somatosensory cortex in post-central gyrus. Right panel: in contrast, a 15 year-old cochlear implanted child who showed average performance on the CNC speech perception test (76%) exhibited activation of the somatosensory cortex, superior and transverse temporal gyri, and parietal cortex.
Fig. 3B describes case results evaluating somatosensory activation in cochlear implanted children. In response to vibrotactile stimulation of the right index finger, a NH child (left panel) and a high-performing cochlear implanted child (94% speech perception score; middle panel) show activation of somatosensory cortex in the post-central gyrus at 76 ms post-stimulation (i.e., the N70 cortical somatosensory evoked potential component; Hämäläinen et al., 1990). On the other hand, an average-performing cochlear implanted child (72% speech perception score; right panel) presented with activity in somatosensory cortex, superior and transverse temporal gyri, and parietal association cortical areas at a similar post-stimulus latency. Given that superior temporal gyrus is associated with auditory processing, results from Figure 3A and B may be suggestive of cross-modal re-organization in the average-performing cochlear implanted children.
While the data from Fig. 3 should be interpreted cautiously, since they reflect individual cases, they show that both visual and somatosensory stimulation can activate auditory cortical areas in cochlear implanted children. Furthermore, it appears from these data that such cross-modal re-organization may be related to less than optimal outcomes with the cochlear implant. Although preliminary, these results are consistent with a recent study showing a negative correlation between visual cross-modal re-organization and speech-in-noise perception in adult-onset, mild-to-moderate hearing loss (Campbell and Sharma, 2014). Large-scale studies are needed to determine the extent to which cross-modal re-organization may be a predictive factor in pediatric cochlear implant success. Future research should also determine whether cross-modal plasticity might be utilized in a beneficial manner, for example in a multimodal auditory training paradigm, to customize rehabilitation for individual deaf children.
5. Clinical assessment of developmental plasticity for improved clinical outcomes
From determination of candidacy for cochlear implantation, to verification of the efficacy of the implant in promoting cortical maturation, the developmental status of the auditory cortex can be monitored and used in clinical decision-making (Carter et al., 2010; He et al., 2012; Hossain et al., 2013; Thabet and Said, 2012). For instance, the P1 CAEP has been employed as a tool in determining whether a patient is a candidate for a cochlear implant. That is, if a deaf child who has been using hearing aids does not show evidence of developmental progress (i.e., delayed or absent P1 CAEP latency) with amplification, then cochlear implantation may be a viable option. This technique is especially useful in infants and young children in whom the P1 is expected to be the most robust CAEP component and in whom behavioral data, such as measures of speech and language development, are limited (Campbell et al., 2011; Cardon et al., 2012; Cardon and Sharma, 2013; Golding et al., 2007; Pearce et al., 2007; Purdy and Gardner-Berry, 2009; Sharma et al., 2005a,b, 2011a,b, 2013). CAEPs can also be used following implantation to evaluate the effectiveness of this treatment. Based on the N1 CAEP data from cochlear implanted children that we report in this paper, it would be useful to measure the N1 in school-age implanted children to monitor long-term maturational status of their higher-order auditory cortex and cortico-cortical coupling, as a function of their speech, language, academic outcomes and auditory training. Thus, the P1 and N1 CAEPs can be used as biomarkers at different stages in childhood to examine developmental plasticity of the central auditory system in individual patients and guide clinical decision-making regarding treatments and rehabilitation.
6. Summary and conclusion
Optimal development of oral speech and language skills is dependent upon auditory cortical development, which is in turn dependent on sufficient auditory experience. In children with congenital and early-onset deafness, higher-order auditory cortices do not have the opportunity to develop normally unless adequate auditory stimulation is received, usually via cochlear implantation. If auditory stimulation is initiated within a brief sensitive period early in childhood, maturation of the primary auditory cortex progresses, providing input for higher-order auditory cortices and allowing feedback circuitry to develop. This maturation, in turn, typically leads to improved acquisition of speech and oral language. Long-term deafness extending beyond the early school-age years may result in significant cortical re-organization, including a lack of connectivity between primary and higher order cortices. Cross-modal repurposing of areas of auditory cortex by visual and somatosensory modalities is another form of re-organization that may influence behavioral outcomes for deaf children. Future research is needed to determine the extent to which cross-modal plasticity can be harnessed to develop individually customized rehabilitation programs targeted at improving clinical outcomes for cochlear implanted children.
Acknowledgments
This research was supported by NIH R01 DC06257 to A.S., NIH F31 DC011970 to J.C., and NIH F31 DC013218 to G.C. We would like to acknowledge Jehan Alsalmi, who worked on the N1 data.
References
- Baldwin RL. Doctoral dissertation. Gallaudet University; 2001. Functional Reallocation of the Auditory Cortex in Individuals who are Deaf. [Google Scholar]
- Bertrand JA, Lassonde M, Robert M, Nguyen DK, Bertone A, Doucet ME, et al. An intracranial event-related potential study on transformational apparent motion. Does its neural processing differ from real motion? Exp Brain Res. 2012;216(1):145–153. doi: 10.1007/s00221-011-2920-8. [DOI] [PubMed] [Google Scholar]
- Buckley KA, Tobey EA. Cross-modal plasticity and speech perception in pre- and postlingually deaf cochlear implant users. Ear Hear. 2011;32(1):2–15. doi: 10.1097/AUD.0b013e3181e8534c. [DOI] [PubMed] [Google Scholar]
- Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci. 2013;7(71) doi: 10.3389/fnsys.2013.00071. (np) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Sharma A. Cross-modal re-organization in adults with early stage hearing loss. PLoS ONE. 2014;9(2):2014. doi: 10.1371/journal.pone.0090594. (np) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Cardon G, Sharma A. Clinical application of the P1 cortical auditory evoked potential biomarker in children with sensorineural hearing loss and Auditory Neuropathy Spectrum Disorder. Semin Hear. 2011;32(2):147–155. doi: 10.1055/s-0031-1277236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon G, Sharma A. Central auditory maturation and behavioral outcome in children with Auditory Neuropathy Spectrum Disorder who use cochlear implants. Int J Audiol. 2013;52(9):577–586. doi: 10.3109/14992027.2013.799786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon G, Campbell J, Sharma A. Plasticity in the developing auditory cortex: evidence from children with sensorineural hearing loss and Auditory Neuropathy Spectrum Disorder. J Am Acad Audiol. 2012;23(6):396–411. doi: 10.3766/jaaa.23.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L, Golding M, Dillon H, Seymour J. The detection of infant cortical auditory evoked potentials (CAEPs) using statistical and visual detection techniques. J Am Acad Audiol. 2010;21(5):347–356. doi: 10.3766/jaaa.21.5.6. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Cheour M, Naatanen R. Interstimulus interval and auditory event-related potentials in children: evidence for multiple generators. Electroencephalogr Clin Neurophysiol. 1998;108(4):345–354. doi: 10.1016/s0168-5597(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113(6):870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Clark JH, Wang NY, Riley AW, Carson CM, Meserole RL, Lin FR, et al. Timing of cochlear implantation and parents’ global ratings of children’s health and development. Otol Neurotol. 2012;33(4):545–552. doi: 10.1097/MAO.0b013e3182522906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Cruz I, Quittner AL, Marker C, DesJardin JL, CDaCI Investigative Team Identification of effective strategies to promote language in deaf children with cochlear implants. Child Dev. 2013;84(2):543–559. doi: 10.1111/j.1467-8624.2012.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman SJ, Pinder D, Briggs RJS, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28(2 Suppl):11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- Dinces E, Chobot-Rhodd J, Sussman E. Behavioral and electrophysiological measures of auditory change detection in children following late cochlear implantation: a preliminary study. Int J Pediatr Otorhinolaryngol. 2009;73(6):843–851. doi: 10.1016/j.ijporl.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet ME, Bergeron F, Lassonde M, Ferron P, Lepore F. Cross-modal reorganization and speech perception in cochlear implant users. Brain. 2006;129(Pt 12):3376–3383. doi: 10.1093/brain/awl264. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Walker EA, Oleson J, Kenworthy M, Van Voorst T, Tomblin JB, et al. Longitudinal speech perception and language performance in pediatric cochlear implant users: the effect of age at implantation. Ear Hear. 2014;35(2):148–160. doi: 10.1097/AUD.0b013e3182a4a8f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. On the rate of maturation of sensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1988;70(4):293–305. doi: 10.1016/0013-4694(88)90048-x. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Moore JK. Morphological and functional development of the auditory nervous system. In: Werner L, Fay RR, Popper AN, editors. Human Auditory Development. Springer; US: 2012. pp. 535–559. [Google Scholar]
- Eggermont JJ, Ponton CW. The neurophysiology of auditory perception: from single units to evoked potentials. Audiol Neurootol. 2002;7(2):71–99. doi: 10.1159/000057656. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123(2):249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW, Don M, Waring MD, Kwong B. Maturational delays in cortical evoked potentials in cochlear implant users. Acta Otolaryngol. 1997;117(2):161–163. doi: 10.3109/00016489709117760. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11(6):528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine I, Finney EM, Boynton GM, Dobkins KR. Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. J Cogn Neurosci. 2005;17(10):1621–1637. doi: 10.1162/089892905774597173. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4(12):1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Finney EM, Clementz BA, Hickok G, Dobkins KR. Visual stimuli activate auditory cortex in deaf subjects: evidence from MEG. Neuroreport. 2003;14(11):1425–1427. doi: 10.1097/00001756-200308060-00004. [DOI] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34(4):177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. J Deaf Stud Deaf Educ. 2009;14(3):371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2005;116(3):648–657. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Finley CC, Panch AS, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006;117(8):1772–1882. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112(10):1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding M, Pearce W, Seymour J, Cooper A, Ching T, Dillon H. The relationship between obligatory cortical auditory evoked potentials (CAEPs) and functional measures in young infants. J Am Acad Audiol. 2007;18(2):117–125. doi: 10.3766/jaaa.18.2.4. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Tanaka S, Wong DD, Papsin BC. Characterizing responses from auditory cortex in young people with several years of cochlear implant experience. Clin Neurophysiol. 2008;119(10):2347–2362. doi: 10.1016/j.clinph.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Jiwani S, Papsin BC. What is the optimal timing for bilateral cochlear implantation in children? Cochlear Implants Int. 2011;12(Suppl. 2):S8–S14. doi: 10.1179/146701011X13074645127199. [DOI] [PubMed] [Google Scholar]
- BDC Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development Cereb. Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen H, Kekoni J, Sams M, Reinikainen K, Näätänen R. Human somatosensory evoked potentials to mechanical pulses and vibration: contributions of SI and SII somatosensory cortices to P50 and P100 components. Electroencephalogr Clin Neurophysiol. 1990;75(2):13–21. doi: 10.1016/0013-4694(90)90148-d. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends Cogn Sci. 1998;2(5):168–174. doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Shepherd RK, Heid S, Klinke R. Response of the primary auditory cortex to electrical stimulation of the auditory nerve in the congenitally deaf white cat. Hear Res. 1997;112:1–2. 115–133. doi: 10.1016/s0378-5955(97)00114-7. [DOI] [PubMed] [Google Scholar]
- Havy M, Nazzi T, Bertoncini J. Phonetic processing during the acquisition of new words in 3-to-6-year-old French-speaking deaf children with cochlear implants. J Commun Disord. 2013;46(2):181–192. doi: 10.1016/j.jcomdis.2012.12.002. [DOI] [PubMed] [Google Scholar]
- He S, Grose J, Hang AX, Buchman CA. Cochlear implant-evoked cortical activation in children with cochlear nerve deficiency. Otol Neurotol. 2012;33(7):1188–1196. doi: 10.1097/MAO.0b013e31826426d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin Y, Kileny PR, Hildesheimer M, Kishon-Rabin L. Phonetic processing in children with cochlear implants: an auditory event-related potentials study. Ear Hear. 2008;29(2):239–249. doi: 10.1097/aud.0b013e3181645304. [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MD, Raghunandhan S, Kameswaran M, Ranjith R. A clinical study of cortical auditory evoked potentials in cochlear implantees. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl. 3):587–593. doi: 10.1007/s12070-012-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Carlson S, Hyvärinen L. Early visual deprivation alters modality of neuronal responses in area 19 of monkey cortex. Neurosci Lett. 1981a;26(3):239–243. doi: 10.1016/0304-3940(81)90139-7. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Hyvärinen L, Linnankoski I. Modification of parietal association cortex and functional blindness after binocular deprivation in young monkeys. Exp Brain Res. 1981b;42(1):1–8. doi: 10.1007/BF00235723. [DOI] [PubMed] [Google Scholar]
- King KA, Campbell J, Sharma A, Martin K, Dorman M, Langran J. The representation of voice onset time in the cortical auditory evoked potentials of young children. Clin Neurophysiol. 2008;119(12):2855–2861. doi: 10.1016/j.clinph.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kral A. Unimodal and cross-modal plasticity in the “deaf auditory cortex. Int J Audiol. 2007;46(9):479–493. doi: 10.1080/14992020701383027. [DOI] [PubMed] [Google Scholar]
- Kral A. Auditory critical periods: a review from system’s perspective. Neuroscience. 2013;247:117–133. doi: 10.1016/j.neuroscience.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Eggermont JJ. What’s to lose and what’s to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007;56(1):259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35(2):111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000;10(7):714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurootol. 2001;6(6):346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12(8):797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15(5):552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Kekoni J, Hamalainen H, Reinikainen K, Salonen O, Standertskjold-Nordenstam CG, Naatanen R. Auditory and somatosensory event-related brain potentials in early blind humans. Exp Brain Res. 1995;104(3):519–526. doi: 10.1007/BF00231986. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kang E, Oh SH, Kang H, Lee DS, Lee MC, Kim CS. Preoperative differences of cerebral metabolism relate to the outcome of cochlear implants in congenitally deaf children. Hear Res. 2005;203:1–2. 2–9. doi: 10.1016/j.heares.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, Lee DS. Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex. 2007;17(4):909–917. doi: 10.1093/cercor/bhl001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levanen S. Neuromagnetic studies of human auditory cortex function and reorganization. Scand Audiol. 1998;27(4):1–6. doi: 10.1080/010503998420595. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92(3):204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Liem F, Zaehle T, Burkhard A, Jäncke L, Meyer M. Cortical thickness of supratemporal plane predicts auditory N1 amplitude. Neuroreport. 2012;23(17):1026–1030. doi: 10.1097/WNR.0b013e32835abc5c. [DOI] [PubMed] [Google Scholar]
- Makela JP, Hari R. Neuromagnetic auditory evoked responses after a stroke in the right temporal lobe. Neuroreport. 1992;3(1):94–96. doi: 10.1097/00001756-199201000-00025. [DOI] [PubMed] [Google Scholar]
- Makela JP, McEvoy L. Auditory evoked fields to illusory sound source movements. Exp Brain Res. 1996;110(3):446–454. doi: 10.1007/BF00229144. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol. 2008;128(4):373–377. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Guan YL. Cytoarchitectural and axonal maturation in human auditory cortex. J Assoc Res Otolaryngol. 2001;2(4):297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuler LT, Davis EP, Buss C, Head K, Hasso AN, Sandman CA. Cortical and subcortical changes in typically developing preadolescent children. Brain Res. 2011;1399:15–24. doi: 10.1016/j.brainres.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Schmidt A, Kutas M. Altered visual-evoked potentials in congenitally deaf adults. Brain Res. 1983;266(1):127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- Nie J, Li G, Shen D. Development of cortical anatomical properties from early childhood to early adulthood. Neuroimage. 2013;76:216–224. doi: 10.1016/j.neuroimage.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, Fink NE. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Kim CS, Kang EJ, Lee DS, Lee HJ, Chang SO, et al. Speech perception after cochlear implantation over a 4-year time period. Acta Otolaryngol. 2003;123(2):148–153. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- Pallas SL. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001;24(7):417–423. doi: 10.1016/s0166-2236(00)01853-1. [DOI] [PubMed] [Google Scholar]
- Pearce W, Golding M, Dillon H. Cortical auditory evoked potentials in the assessment of auditory neuropathy: two case studies. J Am Acad Audiol. 2007;18:380–390. doi: 10.3766/jaaa.18.5.3. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6(6):363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Don M, Waring MD, Eggermont JJ, Masuda A. Spatio-temporal source modeling of evoked potentials to acoustic and cochlear implant stimulation. Electroencephalogr Clin Neurophysiol. 1993;88(6):478–493. doi: 10.1016/0168-5597(93)90037-p. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Masuda A. Maturation of human cortical auditory function: differences between normal-hearing children and children with cochlear implants. Ear Hear. 1996a;17(5):430–437. doi: 10.1097/00003446-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Kwong B, Masuda A. Auditory system plasticity in children after long periods of complete deafness. Neuroreport. 1996b;8(1):61–65. doi: 10.1097/00001756-199612200-00013. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Moore JK, Eggermont JJ. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scand Audiol Suppl. 1999;51:13–22. [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Don M, Waring MD, Kwong B, Cunningham J, Trautwein P. Maturation of the mismatch negativity: effects of profound deafness and cochlear implant use. Audiol Neurootol. 2000a;5:3–4. 167–185. doi: 10.1159/000013878. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000b;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol. 2002;113(3):407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Purdy SC, Gardner-Berry K. Auditory evoked potentials and cochlear implants: research findings and clinical applications in children. Perspect Hear Disord Child. 2009;19(1):14–21. [Google Scholar]
- Quittner AL, Cruz I, Barker DH, Tobey E, Eisenberg LS, Niparko JK, et al. Effects of maternal sensitivity and cognitive and linguistic stimulation on cochlear implant users’ language development over four years. J Pediatr. 2013;162(2):343–348. doi: 10.1016/j.jpeds.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebillard G, Carlier E, Rebillard M, Pujol R. Enhancement of visual responses on the primary auditory cortex of the cat after an early destruction of cochlear receptors. Brain Res. 1977;129(1):162–164. doi: 10.1016/0006-8993(77)90980-5. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Green JE, Waltzman SB. Bilingual oral language proficiency in children with cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130(5):644–647. doi: 10.1001/archotol.130.5.644. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380(6574):526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Honda M, Matsuki K, Yoshida M, Kashikura K, Takei W, Sato T, Kochiyama T, Yonekura Y. Cross-modal integration and plastic changes revealed by lip movement, random-dot motion and sign languages in the hearing and deaf. Cereb Cortex. 2005;15(8):1113–1122. doi: 10.1093/cercor/bhh210. [DOI] [PubMed] [Google Scholar]
- Sandmann P, Dillier N, Eichele T, Meyer M, Kegel A, Pascual-Marqui RD, Marcar VL, Jancke L, Debener S. Visual activation of auditory cortex reflects maladaptive plasticity in cochlear implant users. Brain. 2012;135(2):555–568. doi: 10.1093/brain/awr329. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Central auditory development in children with cochlear implants: clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant–vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002a;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002b;13(10):1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman M, Spahr A, Todd NW. Early cochlear implantation in children allows normal development of central auditory pathways. Ann Otol Rhinol Laryngol. 2002c;(Suppl. 189):38–41. doi: 10.1177/00034894021110s508. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005a;203:1–2. 134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Sharma A, Martin K, Roland P, Bauer M, Sweeney H, Gilley P, Dorman M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad Audiol. 2005b;16(8):564–573. doi: 10.3766/jaaa.16.8.5. [DOI] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46(9):494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and reorganization in children with cochlear implants. J Commun Disord. 2009;42(4):272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Cardon G, Henion K, Roland P. Cortical maturation and behavioral outcomes in children with Auditory Neuropathy Spectrum Disorder. Int J Audiol. 2011;50(2):98–106. doi: 10.3109/14992027.2010.542492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Glick H, Campbell J. Central auditory development in children with hearing impairment: clinical relevance of the P1 CAEP biomarker in children with multiple disabilities. Hear Balance Commun. 2013;11(3):110–120. doi: 10.3109/21695717.2013.812378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Liegeois-Chauvel C, Brugge JF. Auditory evoked potentials and their utility in the assessment of complex sound processing. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; US: 2011. pp. 535–559. [Google Scholar]
- Su P, Kuan CC, Kaga K, Sano M, Mima K. Myelination progression in language-correlated regions in brain of normal children determined by quantitative MRI assessment. Int J Pediatr Otorhinolaryngol. 2008;72(12):1751–1763. doi: 10.1016/j.ijporl.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M, Gumenyuk V, Grushko J, Lawson K. The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear Res. 2008;236:1–2. 61–79. doi: 10.1016/j.heares.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9(4):224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Tajudeen BA, Waltzman SB, Jethanamest D, Svirsky MA. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otol Neurotol. 2010;31(8):1254–1260. doi: 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet MT, Said NM. Cortical auditory evoked potential (P1): a potential objective indicator for auditory rehabilitation outcome. Int J Pediatr Otorhinolaryngol. 2012;76(12):1712–1718. doi: 10.1016/j.ijporl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Uhl F, Franzen P, Lindinger G, Lang W, Deecke L. On the functionality of the visually deprived occipital cortex in early blind persons. Neurosci Lett. 1991;124(2):256–259. doi: 10.1016/0304-3940(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Roland JT, Jr, Cohen NL. Delayed implantation in congenitally deaf children and adults. Otol Neurotol. 2002;23(3):333–340. doi: 10.1097/00129492-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Wang NY, Eisenberg LS, Johnson KC, Fink NE, Tobey EA, Quittner AL, Niparko JK. Tracking development of speech recognition: longitudinal data from hierarchical assessments in the childhood development after Cochlear Implantation Study. Otol Neurotol. 2008;29(2):240–245. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson F, James TW, Wilson HR, Gati JS, Menon RS, Goodale MA. An fMRI study of the selective activation of human extrastriate form vision areas by radial and concentric gratings. Curr Biol. 2000;10(22):1455–1458. doi: 10.1016/s0960-9822(00)00800-9. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: a review. Hear Res. 2006;212:1–2. 212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas from intracerebral auditory evoked potentials using distributed source models. Neuroimage. 2005;28(1):140–153. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]


