Abstract
Background
Cardiac fibrosis is considered to be a crucial factor in the development of heart failure. Blockade of the mineralocorticoid receptor (MR) attenuated cardiac fibrosis and improved the prognosis of patients with chronic heart failure but the ligand for MR and the regulatory mechanism of MR pathway in the diseased heart are unclear. Here, we investigated whether glucocorticoids can promote cardiac fibrosis through MR in oxidative stress and the involvement of elongation factor eleven-nineteen lysine-rich leukemia (ELL), a co-activator of MR, in this pathway.
Methods and Results
The MR antagonist eplerenone attenuated corticosterone-induced collagen synthesis assessed by [3H]proline incorporation in rat neonatal cultured cardiac fibroblasts in the presence of H2O2, as an oxidative stress but not in the absence of H2O2. H2O2 increased the ELL expression levels and MR-bound ELL. ELL expression levels and MR-bound ELL were also increased in the left ventricle of heart failure model rats with significant fibrosis and enhanced oxidative stress. Eplerenone did not attenuate corticosterone-induced increase of [3H]proline incorporation in the presence of H2O2 after knockdown of ELL expression using small interfering RNA in cardiac fibroblasts.
Conclusion
Glucocorticoids can promote cardiac fibrosis via MR in oxidative stress, and oxidative stress modulates MR response to glucocorticoids through the interaction with ELL. Preventing cardiac fibrosis by modulating glucocorticoid-MR-ELL pathway may become a new therapeutic strategy for heart failure.
Keywords: co-activator, corticosterone, eplerenone, heart failure
Blockade of the mineralocorticoid receptor (MR) improved mortality of patients with chronic heart failure (HF)1, 2 and was effective in the reduction of myocardial damage even in HF patients with low to normal aldosterone levels.3, 4 However, the mechanism underlying the benefits of MR-blockade in those HF patients is still unclear.
In typical aldosterone targets such as renal, tubular or vascular smooth muscle cells, 11beta-hydroxysteroid dehydrogenase type II (11betaHSD2) converts glucocorticoid to inactive form, and thus aldosterone can exert specific actions on MR.5 In contrast, in several non-epithelial tissues such as heart, expression of 11betaHSD2 is negligibly low.5, 6 The physiological glucocorticoids are corisol in humans and corticosterone in rodents and they have similar affinity for MR as aldosterone.7 Cardiac MRs are overwhelmingly occupied by glucocorticoids rather than by aldosterone. 8 High serum levels of cortisol, the major glucocorticoid in humans are an independent predictor of increased mortality risk in patients with HF.9 We have shown that administration of eplerenone, an MR antagonist, attenuates cardiac fibrosis and prevents HF in hypertensive HF Dahl salt-sensitive rats, with corticosterone levels in the left ventricule (LV) approximately 800 times those of aldosterone.6 This suggests that corticosterone acts as an MR agonist and promotes cardiac fibrosis which plays an important role in the development of HF.10, 11 It has been postulated that if intracellular redox state changes with tissue damage and the generation of reactive oxygen species (ROS), the physiological glucocorticoids act as MR agonists.12– 14 Serum cortisol has also been reported as a useful predictor of cardiac events in patients with HF in the presence of oxidative stress.15 However, it is still unclear whether glucocorticoids promote cardiac fibrosis via MR in oxidative stress and the mechanisms.
Elongation factor eleven-nineteen lysine-rich leukemia (ELL) was reported to bind to MR and increase MR activity in response to glucocorticoids as well as to mineralocorticoids as a co-activator of MR.16 Therefore, ELL may be involved in glucocorticoid promotion of cardiac fibrosis via MR but the function in the diseased heart has not to be determined.
Here, we investigated whether glucocorticoid could promote cardiac fibrosis via MR in oxidative stress. In addition, the role of ELL in glucocorticoid-induced cardiac fibrosis via MR was investigated.
MATERIALS AND METHODS
This study was approved by the institutional ethics committee of Osaka University Graduate School of Medicine (Approval Number: 23-014-0, 23-030-1, 23-062-0), and conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
HF model rats
Male Dahl salt-sensitive rats (Japan SLC, Shizuoka, Japan) fed on 8% NaCl diet from 6 weeks old as HF group (n = 8) and male Dahl salt-sensitive rats fed on 0.3% NaCl diet throughout the study protocol as an age-matched control group (n = 8) were used for this study.17 This model presents HF around 19 to 20 weeks of age with significant cardiac fibrosis.18, 19 Rats were sacrificed in 22 weeks of age with established HF. Anesthesia included ketamine HCl (80 mg/kg, intraperitoneally) and xylazine HCl (10 mg/kg, intraperitoneally) and its adequacy monitored by the stability of blood pressure, heart rate, and lack of flexor responses to paw-pinch.20 The heart and lungs were rapidly harvested, and LV and lung weight corrected for tibial length. The LV was immediately placed in liquid nitrogen and stored at −80 ˚C for real-time quantitative reverse transcriptase-PCR (RT-qPCR) and Western blot analysis. LV samples were fixed with a phosphate-buffered 10% formalin solution for Azan-Mallory staining, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) on dry ice for immunohistochemistry.
Histological analysis
Azan Mallory staining was performed on LV transverse sections to evaluate LV fibrosis as previously described.18
To evaluate oxidative stress in the LV of the HF rats, tissue sections were incubated with mouse monoclonal anti-4-hydroxy-2-nonenal (HNE) antibody (1:50 dilution; NOF Medical Department, Tokyo) as previously described.18
Cultured cardiac fibroblasts
Neonatal rat cardiac fibroblasts were isolated from Wistar rats by standard techniques with some modification of the previously described method.21 Briefly, neonatal rats were deeply anesthetized by inhalation of 5% sevoflurane until loss of paw withdrawal reflex was obtained. After cervical dislocation was performed, they were opened by sternotomy and their hearts removed. LV samples from neonatal rats were cut into pieces of approximately 1 mm3 with scissors and subjected to collagenase (Wako Pure Chemical Industries, Osaka, Japan) digestion in phosphate-buffered saline. Dispersed cells were incubated on 100 mm culture dishes for 40 min in a 5% CO2 incubator. Non-myocytes attached to the bottom of the dishes were subsequently incubated with Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS) (26140, Invitrogen) for an additional 48 h. Confluent cardiac fibroblasts were treated with 0.05% trypsin-EDTA (Invitrogen) and subcultured. Virtually pure fibroblast cultures were confirmed by immunostaining with a monoclonal anti-vimentin (V9) antibody (1:250 dilution; sc-6260, Santa Cruz Biotechnology, Dallas, TX). 22 Cells were passaged twice and incubated in DMEM supplemented with 10% charcoal stripped FCS (12676, Invitrogen). The medium of subconfluent cells of the second passage was changed to DMEM without FCS 24 h before H2O2 administration (1 μmol/L; Wako Pure Chemical Industries), corticosterone (300 nmol/L; Sigma-Aldrich, St. Louis) or eplerenone (1 μmol/L; Tocris Bioscience, Ellisville, MO).
Western blot analysis
The protocol of Western blot analysis was the same as previously described.18, 19 LV tissue was homogenized in buffer (50 mM Tris-HCl, 0.1 mM sodium orthovanadate, 50 mM sodium fluoride, 150 mM sucrose, 1 mM benzamide, 5 mM EDTA and 2 mM EGTA) supplemented with protease inhibitor cocktail (Thermo Scientific, Waltham, MA). Cells were lysed with buffer (50 mM Tris-HCl, 0.1 mM sodium orthovanadate, 50 mM sodium fluoride, 150 mM sucrose, 1 mM benzamide, 5 mM EDTA, 2 mM EGTA and 1% Triton X-100) supplemented with the protease inhibitor cocktail. After centrifugation, the supernatant protein concentration was determined by the Lowry’s method. Aliquots were mixed with equal volumes of Laemmli buffer (250 mM Tris-HCl, 20% glycerol, 4% sodium dodecyl sulfate, 10% 2-mercaptoethanol and 0.01% bromophenol blue), heated for 5 min at 95 ˚C, subjected to electrophoresis on 7.5% SDS-PAGE and then transferred to Immobilon-P Transfer membranes (Millipore, Billerica, MA). The membrane was then incubated with rabbit polyclonal primary antibodies for ELL (1:200 dilution; sc-28702; Santa Cruz Biotechnology), MR (sc-11412; Santa Cruz Biotechnology) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000 dilution; sc-25778; Santa Cruz Biotechnology) at 4 ˚C overnight, followed by secondary antibody [111-035-144; peroxidase-conjugated AffiniPure goat anti-rabbit immunoglobulin G (H + L), Jackson ImmunoResearch Laboratories, West Grove, PA] at room temperature for 1 h. Blots were developed by enhanced chemiluminescence and expression levels quantified with LAS-4000 and MultiGauge software (Fujifilm, Tokyo). The band density of the protein of interest was normalized to GAPDH expression.20
RT-qPCR
Total RNA was extracted from the LV samples and cardiac fibroblasts stored at –80 ˚C and reverse- transcribed with oligo d(T)16 as a reverse primer as previously described. 23 RT-qPCR with the ABI PRISM 7900 HT Sequence Detection System and Software Version 2.3 (Applied Biosystems, Foster City, CA) was conducted to measure mRNA levels. The sequence of the primers and TaqMan probe for GAPDH were previously described.24 The primers and TaqMan probes for ELL, and MR were assays-on-demand gene expression products (assay ID Rn01420173_g1 and Rn00492539_m1, respectively; Applied Biosystems). The amount of each mRNA was divided by that of GAPDH mRNA to correct for the efficiency of cDNA synthesis and normalized to the mean of the control.
MR IP
Samples were homogenized with immunoprecipitation (IP) buffer (50 mM Tris-HCl, 0.1 mM sodium orthovanadate, 50 mM sodium fluoride, 150 mM sucrose, 1 mM benzamide, 5 mM EDTA, 2mM EGTA and 1% Triton X100) supplemented with the protease inhibitor cocktail. The same amount of samples was pre-cleared with protein A-agarose IP reagent (sc-2001; Santa Cruz Biotechnology), and incubated with 2 µg of rabbit polyclonal primary antibodies for MR (sc-11412; Santa Cruz Biotechnology) for 1 h at 4 ˚C. MR-associated proteins were pulled-down with protein A-agarose IP reagent overnight at 4 ˚C, and washed 4 times in IP buffer. Immunoprecipitated proteins were eluted in Laemmli buffer and Western blot analysis performed to detect ELL bound to MR by using the antibody for ELL.
[3H]proline incorporation into cardiac fibroblasts
To evaluate collagen synthesis, cardiac fibroblasts were incubated in medium supplemented with [3H]proline (1 μCi/mL; Perkin Elmer, Waltham, MA) in the absence or presence of corticosterone (300 nmol/L).20 The effects of MR antagonist (eplerenone, 1 μmol/L) on corticosterone-stimulated [3H]proline incorporation were assessed in the absence or the presence of H2O2 (1 μmol/L). After 48 h treatment, extracellular proline was removed by aspiration of media, plates were washed twice with phosphate-buffered saline, and 5% cold trichloroacetic acid was added to solubilize intracellular free [3H]proline and precipitate [3H]proline incorporated into collagen. After 30 min, trichloroacetic acid was aspirated, plates were washed twice with 95% ethanol, and 0.5 N NaOH was added to solubilize the precipitated proteins. After an additional 30 min, scintillation cocktail (Insta-Gel Plus; Perkin Elmer) was added, and the radioactivity was measured in a liquid scintillation counter (LS 6500; Beckman Coulter, Brea, CA). The amount of [3H]proline incorporation was normalized to the mean of the control.
Small interfering RNA-mediated knockdown of ELL
Cardiac fibroblasts from the second passage were transfected with ELL small interfering RNA (siRNA) or with non-targeting control siRNA (B-Bridge International, Cupertino, CA) at a final concentration of 50 nmol/L with Lipofectamine RNAiMAX (Invitrogen) in DMEM supplemented with 10% charcoal stripped FCS without antibiotics according to the manufacturer’s instructions. The media were changed to DMEM supplemented with 10 % charcoal stripped FCS and antibiotics 24 h after of siRNA transfection. The inhibitory efficiency of each siRNA was examined by measuring ELL mRNA levels by RT-qPCR 108 h after of siRNA transfection. Measurement of [3H]proline incorporation into siRNA transfected cardiac fibroblasts was performed.
Statistics
Results are expressed as mean ± SD. Differences among groups were analyzed by one-way analysis of variance, followed by the Fisher protected least significant difference post hoc test. A P value < 0.05 was regarded as statistically significant.
RESULTS
Corticosterone promotes collagen production via MR in oxidative stress in cardiac fibroblasts
To investigate whether glucocorticoid promotes collagen production through MR, [3H]proline incorporation into cardiac fibroblasts was measured.
Corticosterone increased [3H]proline incorporation into cardiac fibroblasts (Figs. 1A and B). The corticosterone-induced increase of [3H]proline incorporation was not changed by eplerenone in the absence of H2O2 (Fig. 1A), but attenuated by eplerenone in the presence of H2O2 (Fig. 1B). Eplerenone alone did not affect the [3H]proline incorporation in either the absence or the presence of H2O2 (Figs. 1A and B). H2O2 alone did not affect the [3H]proline incorporation in the cardiac fibroblasts (data not shown). These results indicate that corticosterone increases collagen production through MR in cardiac fibroblasts under oxidative stress.
Fig. 1.
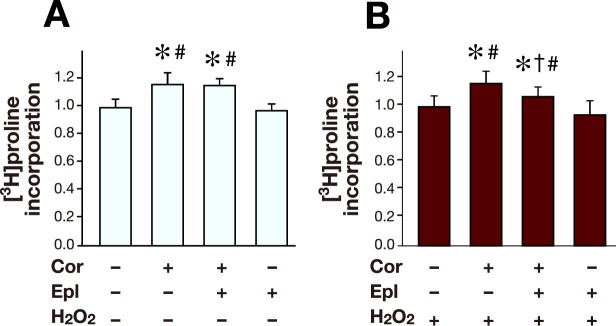
[3H]proline incorporation into cardiac fibroblasts under oxidative stress.
A: Cardiac fibroblasts were treated with Cor (300 nmol/L) and/or Epl (1 μmol/L) in the absence of H2O2. Untreated cardiac fibroblasts were served as control. n = 8 for each group.
B: Cardiac fibroblasts were treated with Cor (300 nmol/L) and/or Epl (1 μmol/L) in the presence of H2O2 (1 μmol/L). Cardiac fibroblasts treated with H2O2 alone were served as control. n = 8 for each group.
Control was arbitrarily set to 1. Data are expressed as mean values ± SD.
*P < 0.05 versus control.
†P < 0.05 versus Cor (+) Epl (–).
#P < 0.05 versus Cor (–) Epl (+).
Cor, corticosterone; Epl, eplerenone.
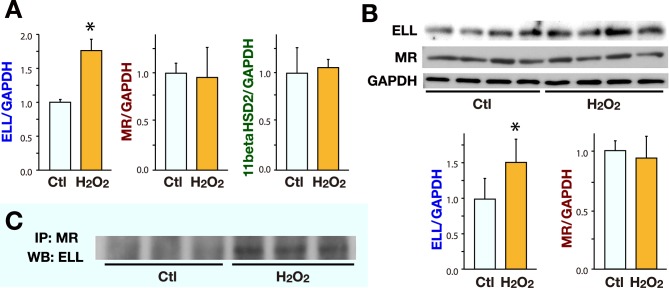
ELL expression and binding to MR are increased by oxidative stress in cardiac fibroblasts
To investigate the effect of oxidative stress on the ELL expression levels and the ELL-MR interaction, cardiac fibroblasts were incubated in the presence or absence of H2O2 (1 μmol/L) for 48 h.
The mRNA and the protein levels of ELL were increased in the cultured cardiac fibroblasts under oxidative stress with H2O2 (Figs. 2A and B). IP assay revealed the presence of ELL-MR binding and that the amount of ELL bound to MR was increased in the cardiac fibroblasts incubated in the presence of H2O2 (Fig. 2C).
Fig. 2.
The effect of H2O2 on the expression of ELL, MR and 11betaHSD2, and ELL-MR interaction in cultured cardiac fibroblasts. Cardiac fibroblasts were treated with H2O2 (1 μmol/L) for 48 h. Cardiac fibroblasts without H2O2 were served as Ctl.
A: RT-qPCR was performed to evaluate the mRNA levels of ELL, MR and 11betaHSD2 in the cardiac fibroblasts (n = 5 for each group).
B: WB analysis was performed to evaluate the protein levels of ELL and MR in the cardiac fibroblasts. The images (upper) and the results of densitometry (lower) of WB analysis (n = 4 for each group).
Ctl was arbitrarily set to 1. Data are expressed as mean values ± SD.
*P < 0.05 versus Ctl.
C: Cell lysate of cardiac fibroblasts was subjected to IP with anti-MR antibody. Immunoprecipitates were subsequently analyzed by Western blotting with anti-ELL antibody.
Ctl, control; ELL, elongation factor eleven-nineteen lysine-rich leukemia; IP, immunoprecipitation; RT-qPCR, real-time quantitative reverse transcriptase-PCR; MR, mineralocorticoid receptor; WB, Western blot.
The mRNA and protein levels of MR were unchanged by H2O2 (Figs. 2A and B).
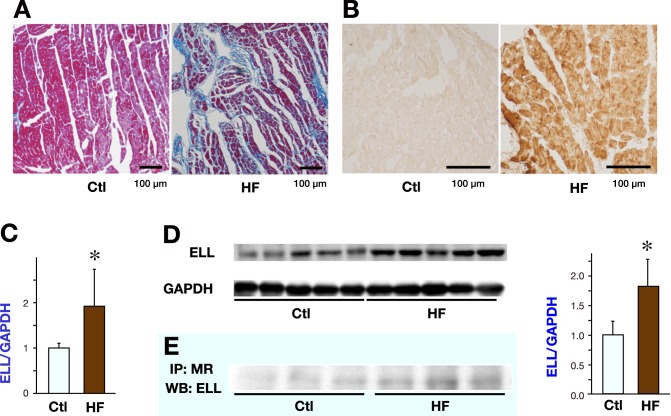
ELL expression and binding to MR are increased in LV of HF rats with enhanced oxidative stress
To evaluate the ELL expression levels and the interaction between ELL and MR in in vivo heart, the LV of the HF rats were compared with those of control rats.
Systolic blood pressure was elevated in the HF group than in the control group (224 ± 15 mmHg in the HF group versus 128 ± 7 mmHg in the control group, P < 0.05). The increased ratio of lung weight to tibial length indicated that the HF group developed overt congestive HF (83.3 ± 15.3 mg/mm in the HF group versus 36.8 ± 1.5 mg/mm in the control group, P < 0.05). Significant interstitial fibrosis was observed in the HF group (Fig. 3A) and the percent area of LV fibrosis was increased in the HF group compared with the control group (4.9 ± 0.4% in the HF group versus 2.2 ± 0.4% in the control group, P < 0.05). HNE staining revealed the increased ROS generation in the LV of the HF group (Fig. 3B).
Fig. 3.
LV fibrosis, ROS generation, ELL expression and ELL-MR interaction in the LVs of 22 weeks old Dahl salt-sensitive rats.
Representative photomicrographs of Azan Mallory staining to evaluate LV fibrosis (A) and representative immunohistochemical staining for 4-hydroxy-2-nonenal to evaluate ROS generation (B) in the LV of a Dahl-salt sensitive rat in the Ctl group and the HF group.
C: RT-qPCR analysis of the mRNA levels of ELL in the LVs of Dahl salt-sensitive rats (n = 8 for each group).
D: WB analysis of the protein levels of ELL in the LVs of Dahl salt-sensitive rats. The images (left) and the results of densitometry (right) of WB analysis (n = 5 for each group).
Data are expressed as mean values ± SD. The Ctl group was arbitrarily set to 1.
*P < 0.05 versus the Ctl group.
E: LV lysate of Dahl salt-sensitive rats was subjected to IP with anti-MR antibody. Immunoprecipitates were subsequently analyzed by Western blotting with anti-ELL antibody.
Ctl, control; ELL, elongation factor eleven-nineteen lysine-rich leukemia; HF, heart failure; IP, immunoprecipitation; LV, left ventricle; MR, mineralocorticoid receptor; ROS, reactive oxygen species; WB, Western blot.
Both mRNA and the protein levels of ELL were increased in the LV of the HF group compared with control (Figs. 3C and D ). IP assays revealed that the amount of ELL bound to MR was increased in the LVs of the HF group (Fig. 3E ).
MR blockade did not inhibit corticosterone-induced collagen production after knockdown of ELL in cardiac fibroblasts
[3H]proline incorporation into cardiac fibroblasts induced by corticosterone was evaluated after knockdown of ELL expression using siRNA for ELL, to investigate the role of ELL in glucocorticoid-induced collagen production.
The ELL mRNA levels in cardiac fibroblasts were significantly decreased after the transfection of ELL siRNA compared with non-targeting control siRNA (Fig. 4A). Corticosterone increased [3H]proline incorporation, and this increase was attenuated by eplerenone in the presence of H2O2 with non-targeting control siRNA (Fig. 4B). However, the corticosterone-induced increase of [3H]proline incorporation was not attenuated by eplerenone in the presence of H2O2 after knockdown of ELL by siRNA (Fig. 4C). These results suggest that ELL enables corticosterone to promote collagen production through MR under oxidative stress.
Fig. 4.
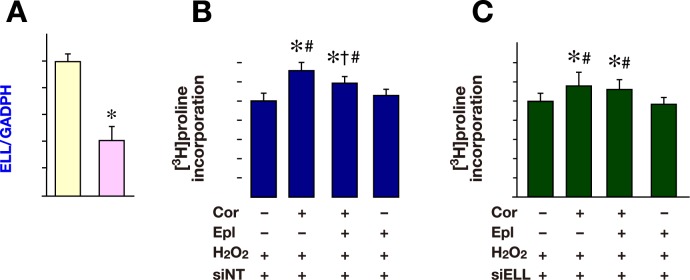
[3H]proline incorporation into cardiac fibroblasts after knockdown of ELL under oxidative stress.
A: Cardiac fibroblasts were transfected with siELL (50 nmol/L, n = 3). Cardiac fibroblasts transfected with siNT (50 nmol/L, n = 3) were served as control.
B: Cardiac fibroblasts were transfected with siNT (50 nmol/L) with Cor (300 nmol/L) and/or Epl (1 μmol/L) in the presence of H2O2 (1 μmol/L). Cardiac fibroblasts transfected with siNT in the presence of H2O2 were served as control. n = 8 for each group.
C: siELL transfected cardiac fibroblasts were treated with Cor (300 nmol/L) and/or Epl (1 μmol/L) in the presence of H2O2 (1 μmol/L). siELL transfected cardiac fibroblasts with H2O2 alone were served as control. n = 8 for each group.
Control was arbitrarily set to 1. Data are expressed as mean values ± SD.
*P < 0.05 versus control.
†P < 0.05 versus Cor (+) Epl (–) H2O2 (+).
#P < 0.05 versus Cor (–) Epl (+) H2O2 (+).
Cor, corticosterone; ELL, elongation factor eleven-nineteen lysine-rich leukemia; Epl, eplerenone; siELL, ELL siRNA; siNT, non-targeting control siRNA.
DISCUSSION
In this study, we showed that i) corticosterone promoted collagen production via MR in the presence of oxidative stress in cardiac fibroblasts, ii) ELL expression levels and MR-bound ELL were increased in cardiac fibroblasts in oxidative stress, iii) ELL expression levels and MR-bound ELL were increased in LV of HF rats by oxidative stress and iv) knockdown of ELL expression blunted the corticosterone-induced collagen production via MR in oxidative stress in cardiac fibroblasts.
Although it has been suggested that glucocorticoids can function to work as an MR ligand in the heart, it is still uncertain how glucocorticoids act via MR in the diseased heart and contribute to the development of HF. Glucocorticoids occupy cardiac MR without obvious mineralocorticoid-like effects in normal conditions,8 but may activate MR under particular condition such as oxidative stress.5, 12– 14 Our previous study demonstrated that blockade of MR attenuated cardiac fibrosis and prevented HF in the HF model rats with low cardiac aldosterone levels. 6 In the present study, we expanded the previous studies by demonstrating that corticosterone can increase collagen production via MR in oxidative stress. Therefore, blockade of MR may protect cardiac MR from corticosterone, reduce cardiac fibrosis, and prevent the development of HF with enhanced oxidative stress. In the absence of H2O2, corticosterone induced collagen production without the inhibition by eplerenon in this in vitro study. Corticosterone can also bind to glucocorticoid receptor. Our previous study showed that the expression of glucocorticoid receptor was upregulated in the hypertrophied heart but the role of the glucocorticoid receptor on cardiac fibrosis was not clear.19 Further studies are required to clarify the role of glucocorticoid receptor and MR on in vivo cardiac fibrosis.
In non-epithelial tissues such as heart, the expression of 11betaHSD2 which inactivates glucocorticoid to inactive form is negligibly low.5, 6 In the present study, 11betaHSD2 protein levels were undetermined because of its very low expression level in cultured cardiac fibroblasts (data not shown). The MR expression levels were also unchanged by oxidative stress in cultured cardiac fibroblasts. Therefore, it suggests that corticosterone can promote collagen synthesis via MR under oxidative stress independently of 11betaHSD2 or MR expression levels. At the post-receptor level several co-activators of MR other than ELL have been reported;25– 29 we have not investigated the involvement of these factors and their interaction with ELL in the present study.
In cardiac myocytes, glucocorticoids have been reported to increase beating frequency via MR in oxidative stress,30, 31 but has not been elucidated how oxidative stress modulates MR response to glucocorticoid. In the present study, knockdown of ELL can at least partially decrease the MR response to corticosterone under oxidative stress. ELL, a co-activator of MR, enhances MR transcriptional activity by both glucocorticoids and mineralocorticoids.16 In the present study, ELL expression levels and the amount of ELL bound to MR were increased in cultured cardiac fibroblasts in oxidative stress; corticosterone did not promote collagen synthesis through MR after knockdown of ELL expression. These results indicate that ELL may contribute to the development of cardiac fibrosis by co-activating MR with corticosterone under oxidative stress. In this study, we did not show how ELL expression levels and ELL-MR binding were changed under oxidative stress and further investigation should clarify those mechanisms. The ELL expression and the amount of ELL bound to MR were increased in the LV of the HF model rats, and associated with increased cardiac fibrosis and enhanced oxidative stress. Given that glucocorticoid activity via MR and ELL expression were increased together in oxidative stress, it suggests that ELL may contribute to the MR activation in the development of cardiac fibrosis and HF in vivo. Although the mechanism of the regulation of ELL expression was unknown, it is possible that some therapeutic approaches reduce ELL expression and affect the MR dependent cardiac fibrosis. Cardiac conditional KO of ELL gene might also elucidate the function of ELL and coticosterone in cardiac fibrosis of in vivo heart.
Serum cortisol has also been reported as a useful predictor of cardiac events in patients with HF, but there is not enough evidence showing cardiac fibrosis in patients with high serum glucocorticoid such as Cushing syndrome or connective tissue diseases with steroid therapy. It is possible that high serum concentration of glucocorticoid has a different effect on the heart from a local increase of corticosterone in cardiac tissue while a local increase of corticosterone in cardiac tissue has a role in cardiac fibrosis in the presence of oxidative stress in the in vivo heart.
In summary, our present study indicates that corticosterone promotes cardiac fibrosis via MR in oxidative stress, and this corticosterone-induced cardiac fibrosis is at least partially modulated by ELL. Preventing cardiac fibrosis by modulating glucocorticoid-MR-ELL pathways may thus become a new therapeutic strategy for HF.
Acknowledgments
Acknowledgments:The authors are grateful to Ms. Saori Nanbu for the excellent technical assistance of the experiments.
This study was supported in part by grants from the Japanese Society for the Promotion of Science (No. 23591042 and No. 21590893).
The authors declare no conflict of interest.
REFERENCES
- 1.Pitt B , Zannad F , Remme WJ , Cody R , Castaigne A , Perez A .The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341: 709-17. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B , Remme W , Zannad F , Neaton J , Martinez F , Roniker B .Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348: 1309-21. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B , Reichek N , Willenbrock R , Zannad F , Phillips RA , Roniker B .Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003; 108: 1831-8. [DOI] [PubMed] [Google Scholar]
- 4.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996; 78: 902-7. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW . Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009; 53: 286-90. [DOI] [PubMed] [Google Scholar]
- 6.Ohtani T , Ohta M , Yamamoto K , Mano T , Sakata Y , Nishio M .Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol. 2007; 292: R946-54. [DOI] [PubMed] [Google Scholar]
- 7.Arriza JL , Weinberger C , Cerelli G , Glaser TM , Handelin BL , Housman DE .Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987; 237: 268-75. [DOI] [PubMed] [Google Scholar]
- 8.Funder J , Myles K . Exclusion of corticosterone from epithelial mineralocorticoid receptors is insufficient for selectivity of aldosterone action: in vivo binding studies. Endocrinology. 1996; 137: 5264-8. [DOI] [PubMed] [Google Scholar]
- 9.Guder G , Bauersachs J , Frantz S , Weismann D , Allolio B , Ertl G .Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007; 115: 1754-61. [DOI] [PubMed] [Google Scholar]
- 10.Masuyama T , Yamamoto K , Sakata Y , Doi R , Nishikawa N , Kondo H .Evolving changes in doppler mitral flow velocity pattern in rats with hypertensive hypertrophy. J Am Coll Cardiol. 2000; 36: 2333-8. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K , Masuyama T , Sakata Y , Nishikawa N , Mano T , Yoshida J .Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res. 2002; 55: 76-82. [DOI] [PubMed] [Google Scholar]
- 12.Funder JW . Rales, ephesus and redox. J Steroid Biochem Mol Biol. 2005; 93: 121-5. [DOI] [PubMed] [Google Scholar]
- 13.Funder JW . Mineralocorticoid receptor activation and oxidative stress. Hypertension. 2007; 50: 840-1. [DOI] [PubMed] [Google Scholar]
- 14.Mihailidou AS , Loan Le TY , Mardini M , Funder JW . Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009; 54: 1306-12. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji M , Tsutamoto T , Kawahara C , Nishiyama K , Yamamoto T , Fujii M .Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009; 2: 608-15. [DOI] [PubMed] [Google Scholar]
- 16.Pascual-Le Tallec L , Simone F , Viengchareun S , Meduri G , Thirman MJ , Lombes M . The elongation factor ell (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions.. Mol Endocrinol. 2005; 19: 1158-69. [DOI] [PubMed] [Google Scholar]
- 17.Doi R , Masuyama T , Yamamoto K , Doi Y , Mano T , Sakata Y .Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000; 18: 111-20. [DOI] [PubMed] [Google Scholar]
- 18.Nishio M , Sakata Y , Mano T , Ohtani T , Takeda Y , Miwa T .Beneficial effects of bisoprolol on the survival of hypertensive diastolic heart failure model rats. Eur J Heart Fail. 2008; 10: 446-53. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani T , Mano T , Hikoso S , Sakata Y , Nishio M , Takeda Y .Cardiac steroidogenesis and glucocorticoid in the development of cardiac hypertrophy during the progression to heart failure. J Hypertens. 2009; 27: 1074-83. [DOI] [PubMed] [Google Scholar]
- 20.Omori Y , Ohtani T , Sakata Y , Mano T , Takeda Y , Tamaki S .L-carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J Hypertens. 2012; 30: 1834-44. [DOI] [PubMed] [Google Scholar]
- 21.Toyofuku T , Hong Z , Kuzuya T , Tada M , Hori M . Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000; 150: 225-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J , Chen H , Seth A , McCulloch CA . Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003; 285: H1871-81. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K , Masuyama T , Sakata Y , Doi R , Ono K , Mano T .Local neurohumoral regulation in the transition to isolated diastolic heart failure in hypertensive heart disease: absence of AT1 receptor downregulation and ‘overdrive’ of the endothelin system. Cardiovasc Res. 2000; 46: 421-32. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa N , Yamamoto K , Sakata Y , Mano T , Yoshida J , Miwa T .Differential activation of matrix metalloproteinases in heart failure with and without ventricular dilatation. Cardiovasc Res. 2003; 57: 766-74. [DOI] [PubMed] [Google Scholar]
- 25.Tallec LP , Kirsh O , Lecomte MC , Viengchareun S , Zennaro MC , Dejean A .Protein inhibitor of activated signal transducer and activator of transcription 1 interacts with the N-terminal domain of mineralocorticoid receptor and represses its transcriptional activity: implication of small ubiquitin-related modifier 1 modification. Mol Endocrinol. 2003; 17: 2529-42. [DOI] [PubMed] [Google Scholar]
- 26.Meijer OC , Kalkhoven E , van der Laan S , Steenbergen PJ , Houtman SH , Dijkmans TF .Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005; 146: 1438-48. [DOI] [PubMed] [Google Scholar]
- 27.Pascual-Le Tallec L , Lombès M . The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol. 2005; 19: 2211-21. [DOI] [PubMed] [Google Scholar]
- 28.Tirard M , Almeida OF , Hutzler P , Melchior F , Michaelidis TM . Sumoylation and proteasomal activity determine the transactivation properties of the mineralocorticoid receptor. Mol Cell Endocrinol. 2007; 268: 20-9. [DOI] [PubMed] [Google Scholar]
- 29.Yang J , Young MJ . The mineralocorticoid receptor and its coregulators. J Mol Endocrinol. 2009; 43: 53-64. [DOI] [PubMed] [Google Scholar]
- 30.Rossier MF , Lenglet S , Vetterli L , Python M , Maturana A . Corticosteroids and redox potential modulate spontaneous contractions in isolated rat ventricular cardiomyocytes. Hypertension. 2008; 52: 721-8. [DOI] [PubMed] [Google Scholar]
- 31.Rossier MF , Python M , Maturana AD . Contribution of mineralocorticoid and glucocorticoid receptors to the chronotropic and hypertrophic actions of aldosterone in neonatal rat ventricular myocytes. Endocrinology. 2010; 151: 2777-87. [DOI] [PubMed] [Google Scholar]