Abstract
The current study examined potential mechanisms for altered circulating ghrelin levels observed in diet-induced obesity (DIO) and following weight loss resulting from Roux-en-Y gastric bypass (RYGB). We hypothesized that circulating ghrelin levels were altered in obesity and after weight loss through changes in ghrelin cell responsiveness to physiological cues. We confirmed lower ghrelin levels in DIO mice and demonstrated elevated ghrelin levels in mice 6 weeks post-RYGB. In both DIO and RYGB settings, these changes in ghrelin levels were associated with altered ghrelin cell responsiveness to two key physiological modulators of ghrelin secretion – glucose and norepinephrine. In DIO mice, increases in ghrelin cell density within both the stomach and duodenum and in somatostatin-immunoreactive D cell density in the duodenum were observed. Our findings provide new insights into the regulation of ghrelin secretion and its relation to circulating ghrelin within the contexts of obesity and weight loss.
Keywords: Ghrelin secretion, Obesity, Roux-en-Y gastric bypass, Norepinephrine, Sympathetic nervous system, Glucose
1. Introduction
Ghrelin is a 28-amino acid peptide hormone that was originally isolated from the stomach and found to potently stimulate growth hormone secretion [1]. Ghrelin cells within the oxyntic mucosa of the stomach have been confirmed as the predominant source of circulating ghrelin [2]. Ghrelin cells also exist in the gastrointestinal tract downstream of the stomach, including the duodenum, albeit in far fewer and gradually diminishing numbers, as well as in a few other sites such as pancreatic islets [3–6]. An acylated form of ghrelin, acyl-ghrelin is octonoylated at serine-3 by ghrelin O-acyltransferase (GOAT), which allows ghrelin to bind to its receptor, the growth hormone secretagogue receptor 1a (GHSR; ghrelin receptor), to exert its biologic actions [7,8]. A non-acylated form of ghrelin, desacyl-ghrelin, also has been shown to exert some biological activities, although these presumably occur via GHSR-independent mechanisms [9].
Ghrelin is best known for its orexigenic and glucoregulatory actions. Regarding its orexigenic activity, administered acyl-ghrelin potently increases the intake of freely-available food and also mediates several reward-based, hedonic eating behaviors [10–21]. While some studies have demonstrated little to no effect of genetic or pharmacologic interference with ghrelin signaling on body weight and food intake, other studies suggest that deficient or blocked ghrelin signaling results in a loss of the full complement of normal eating behaviors and body weight responses [20,22–36]. As just some examples, when placed on high fat diet (HFD) early in life, female GHSR-null mice accumulate 50% less fat mass than wild-types, in models of cancer cachexia, elevated ghrelin protects against worsened anorexia and accelerated death, and after an overnight fast, GHSR antagonist blocks rebound overeating [20,30,37]. In conditioned place preference studies, in which calorically-restricted wild-type mice prefer a chamber in which they had previously been conditioned to find a food reward, calorically-restricted GHSR-null mice show no such preference [17,20,38]. Regarding its glucoregulatory activity, acyl-ghrelin also independently increases blood glucose, lowers insulin levels, raises glucagon levels and attenuates insulin responses during glucose tolerance testing [39–44]. Although not corroborated by all trials [31,45], most studies have indicated that interference with the endogenous ghrelin system alters the normal glucose utilization phenotype. As examples, mice lacking ghrelin, GOAT, or GHSR, when individually-housed and exposed to a 7-day caloric restriction protocol consisting of daily access to only 40% of usual calories, demonstrate marked, life-threatening hypoglycemia compared to their control counterparts [27,36,46,47]. Taken together, these studies suggest that endogenous ghrelin signaling is important for several aspects of both feeding and blood glucose regulation.
Further suggestive of a role for ghrelin in eating and the management of energy stores is the observation that in healthy, lean individuals, plasma ghrelin levels fluctuate depending on energy intake: ghrelin levels show a preprandial rise and a postprandial fall [10,12,48–50]. Also, with few exceptions, ghrelin levels are inversely correlated with body weight, as shown in humans and rodents [48,51,52]. Although not observed in Prader–Willi Syndrome [53], obese individuals as a group exhibit lower circulating ghrelin levels and blunted meal-related fluctuations when compared to lean individuals [48,51,54,55]. This obesity-linked reduction in ghrelin levels can be reversed by weight loss achieved through caloric restriction [56]. Weight loss achieved by bariatric surgery – a far more effective method for weight loss than caloric restriction [57] – has been associated with varying plasma ghrelin results, depending on the procedure and the study. For instance, circulating ghrelin levels were initially demonstrated to drop dramatically following Roux-en-Y gastric bypass (RYGB) [58]. RYGB is a popular and efficacious form of bariatric surgery that, unlike conventional diet and behavioral therapies, results in sustained weight loss plus dramatic and expeditious metabolic improvements [59–61]. Although the exact mechanisms leading to the rapid, profound and long-lasting metabolic benefits observed post-RYGB are still an area of active investigation, changes in the levels and physiological responses to gastrointestinal hormones, such as glucagon-like peptide-1, peptide YY, cholecystokinin, and ghrelin, have been postulated to play central roles [62–66]. With respect to ghrelin, following the initial report of decreased levels, multiple subsequent reports of either increased, decreased or unchanged ghrelin levels after RYGB have left us without consensus regarding RYGB-associated changes to the ghrelin secretory machinery (as reviewed in Ref. [67]).
The molecular mechanisms regulating ghrelin secretion are another area of active investigation. Several models now indicate that adrenergic hormones, including norepinephrine, potently stimulate ghrelin release. For instance, direct delivery of norepinephrine and epinephrine to the oxyntic submucosa of awake rats via an implanted microdialysis probe stimulates ghrelin outflow, as does activation of gut sympathetic nerves by electrical sympathetic nerve stimulation and chemical sympathetic nerve activation using intravenous tyramine [68,69]. Exposure of immortalized ghrelinoma cell lines to norepinephrine, epinephrine or isoproterenol also stimulate both acyl-ghrelin and desacyl-ghrelin release, while pharmacologic adrenergic blockade with atenolol or depletion of sympathetic neuronal terminals of adrenergic neurotransmitters with reserpine prevent the usual fasting induced elevations in circulating ghrelin [27,70]. Furthermore, in primary cultures of dispersed gastric mucosal cells, of which 1–3% of the population is comprised of ghrelin cells, norepinephrine and epinephrine induce β1-adrenergic receptor-dependent ghrelin secretion [71–73]. On the other hand, glucose has been demonstrated to directly inhibit ghrelin secretion [71] and hyperglycemia is associated with lower ghrelin levels [74]. For example, in primary cultures of dispersed gastric mucosal cells, high glucose (10 mM) inhibits while low glucose (1 mM) stimulates ghrelin release [71]. In addition, somatostatin has been identified as a negative inhibitor of ghrelin secretion, likely occurring via direct engagement of one or more somatostatin receptors present on ghrelin cells [69,70,73,75–77].
Although we now know some of the physiologic states that control ghrelin levels and some of the molecules that mediate ghrelin release, links between the two remain mostly uncharacterized. For instance, possible altered responsiveness to modulators of ghrelin secretion during different pathophysiological conditions, such as in obesity and following RYGB, has not been explored. The goal of this study is to investigate mechanisms that contribute to altered circulating ghrelin levels observed during obesity and after weight loss either through calorie restriction or RYGB. We hypothesized that circulating ghrelin levels are altered in obesity and after weight loss through changes in ghrelin cell responsiveness to physiological cues. We tested this hypothesis in mice with diet-induced obesity (DIO) and after weight loss induced by RYGB. We also examined changes to ghrelin cell and somatostatin cell densities in the upper gastrointestinal tracts of these mice.
2. Material and methods
2.1. Animals
All procedures performed in the study were approved by the University of Texas Southwestern Institutional Animal Care and Use Committee. Mice were maintained in a conventional mouse holding facility with a 12-h light/dark cycle (6 AM/6 PM). Mice were fed either a standard rodent chow diet (12 kcal% fat, 2016, Teklad, Madison, WI), or a high-fat diet, (HFD; 60 kcal% fat, D12492, Research Diets, Inc., New Brunswick, NJ) for durations indicated below.
2.1.1. Lean and DIO mice
Male C57BL/6 and transgenic ghrelin reporter (ghrelin-hrGFP) mice on a pure C57BL/6 background [78] were used for this study. The ghrelin-hrGFP reporter expresses humanized Renilla reniformis green fluorescent protein (hrGFP) in nearly 100% of gastric ghrelin cells and approximately 12% of duodenal ghrelin cells [78]. Lean mice were fed standard rodent chow diet throughout the study period, while the DIO mice were switched to HFD between 4 and 5 weeks of age. Body weight was monitored weekly and food intake was monitored for 5 consecutive days between 12 and 14 weeks on the respective diets. At 17–18 weeks of age, mice were deeply anesthetized with intraperitoneal injection of chloral hydrate (500 mg/kg) and subsequently either perfused and fixed with formalin for immunohistochemistry or euthanized for isolation of gastric mucosa cells to establish primary cultures. All mice in these studies were sacrificed after a 4-h food deprivation starting at the beginning of the light cycle (6 AM–10 AM).
2.1.2. RYGB and sham operated mice
Male C57BL/6 mice were started on HFD between 4 and 5 weeks of age. After 12–15 weeks of HFD feeding, mice (approximately 45 g in body weight) were randomized to either RYGB- or sham-operated groups. Mice were allowed to recover under previously described post-operative care [79] during which a liquid diet (Vital HN; Abbott Laboratories, Abbott Park, IL) was provided on post-surgery days 2 through 7. On post-surgery days 6 and 7, 0.25 g HFD was reintroduced and on post-surgery day 8, HFD was provided ad libitum. Seven days post-surgery, a subset of sham-operated mice was calorically restricted and weight matched to RYGB counterparts (weight-matched sham; WMS). All RYGB-operated and the remaining sham-operated mice (ad libitum sham; ALS) were provided HFD ad libitum starting post-surgery day 8. All mice were weighed at 7, 11, 14, 21, 28 and 35 days post-surgery (Figure 3A). At 5 weeks post-surgery, food intake was measured on 5 consecutive days and body composition was evaluated using a Minispec mq10 nuclear magnetic resonance analyzer (NMR; Bruker Optics, Billerica, MA). At 6 weeks post-surgery, the mice were either perfused and formalin fixed for immunohistochemistry or euthanized for isolation of gastric mucosal cells to establish primary cultures. All mice in these studies were euthanized after an overnight fast (6 PM–10 AM).
Figure 3.
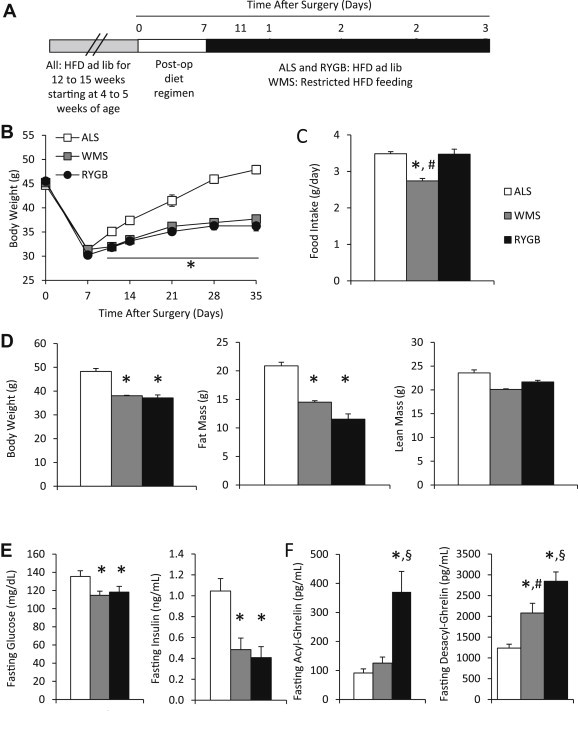
Elevated fasting plasma ghrelin levels in RYGB mice. (A) Study time line for ALS, WMS and RYGB mice. (B) Body weights were reduced post-operatively in RYGB compared to ALS mice. Body weights of WMS were maintained similar to RYGB mice through restricted caloric intake and are lower compared to ALS mice (n = 16–20 mice). Asterisk for this figure denotes difference compared to ALS mice for both RYGB and WMS mice at each time point. (C) Daily food intake measured over 5 consecutive days was similar in RYGB and ALS mice at 5 weeks post-surgery. Food measurements shown for the WMS mice are calorie requirements to match the body weight of RYGB mice (n = 8–15 mice). (D) Body composition measurements at 5 weeks post-surgery show reduction in fat mass in RYGB and WMS compared to ALS mice, but no change in lean mass (n = 16–17 mice). (E) Fasting blood glucose (left, n = 16–20 mice) and fasting insulin levels (right, n = 8 mice) were improved in RYGB and WMS mice compared to ALS mice. (F) Fasting acyl-ghrelin levels (right, n = 15–16 mice) were elevated in RYGB compared to WMS and ALS mice. Fasting desacyl-ghrelin levels (right, n = 11–15 mice) were different among RYGB, WMS and ALS mice. Data are represented as mean ± SEM. *P < 0.05 compared to ALS mice, #P < 0.05 compared to RYGB mice and §P < 0.05 compared to WMS mice.
2.2. Surgery
RYGB and sham surgeries were performed as previously described [79]. Isofluorane was used for induction and maintenance of general anesthesia, which was time-standardized between groups. Briefly, the RYGB procedure involved gastrointestinal reconstruction by attachment of the jejunal afferent limb to the restricted proximal gastric pouch. A hemostasis clip (Ethicon Endo-Surgery, Cincinnati, OH) was used to divide the stomach in two, restricting the gastric pouch and excluding the distal stomach and proximal intestine from alimentary flow. The sham procedure involved gastrotomy, enterotomy and surgical repair.
2.3. Immunohistochemistry
Tissues were prepared and immunohistochemistry was performed as previously published [78]. Briefly, mice were deeply anesthetized through intraperitoneal injection of chloral hydrate (500 mg/kg). Mice were perfused transcardially with DEPC-treated 0.9% PBS followed by 10% neutral buffered formalin. Stomach and duodenum were removed, and placed in the same fixative for 4–6 h at 4 °C, then immersed overnight in 20% sucrose (in DEPC-treated PBS) at 4 °C. Samples were then embedded in Tissue-Tek OCT compound (Sakura Finetechnical Co., Ltd., Tokyo) and sectioned at 12 μm thickness using a cryostat (Leica Biosystems, Buffalo Grove, IL). The sections were mounted onto SuperFrost slides (Fisher Scientific, Pittsburgh, PA), air-dried at room temperature and stored in desiccated boxes at −20 °C until use.
The sections were washed in PBS three times and incubated with a goat polyclonal anti-ghrelin antibody (1:1000; Lot# F0105, Santa Cruz Biotechnology, Dallas, TX) or rabbit polyclonal anti-somatostatin antibody (1:200; Lot # 216002, Immunostar, Hudson, WI) overnight at room temperature. After washes with PBS, the slides were incubated in Alexa Fluor 594® donkey anti-goat IgG or Alexa Fluor 350® goat anti-rabbit IgG (Molecular Probes, Carlsbad, CA) for 2 h at room temperature. Labeled slides were washed and coverslipped with Fluoromount G (Electron Microscopy Sciences, Hatfield, PA) and examined under a Zeiss fluorescence microscope with ApoTome attachment (Zeiss Axio Imager Z1; Thornwood, NY). Images taken using a camera (Zeiss) were processed with AxioVision software.
For analysis of the stomach, cell counts from the corpus and antrum region in each mouse were determined from 3 different sections separated by at least 300 μm, from 3 high power field (20×) images per each section. The imaged tissues used for counting were oriented such that the muscularis layer to the tip of the mucosa were visible in the frame. For analysis of the duodenum, cell counts in each mouse were performed from 3 different sections separated by at least 300 μm. Labeled duodenal cells were counted from entire cross sections. The mean number of cells per high power field or cross section was determined from 3 to 5 mice.
2.4. Tissue morphology
Stomach tissue morphology was analyzed via routine hematoxylin and eosin stain (H&E). H&E staining was performed on a Sakura DRS-601 x,y,z robot utilizing Leica-Surgipath Selectech reagents by the Molecular Pathology Core at UT Southwestern Medical Center.
2.5. Isolation of gastric mucosal cells and ghrelin secretion studies
Gastric mucosal cells were isolated by a combined enzymatic and mechanical dispersion method, as previously reported [78]. All primary gastric mucosal cell preparations were from inverted whole stomachs, except from mice that underwent RYGB, in which cells were isolated only from the inverted stomach proximal to the hemostasis clip. Each gastric mucosal cell preparation was derived from a single mouse (cells from multiple mice were not pooled). The primary cells were plated on to poly-d-lysine-coated 24-well plates (1 × 105 cells/1 ml medium/well) in DMEM/F12 medium (Meditech, Inc) containing 10% FBS (Atlanta Biologicals), supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin sulfate and 50 μM octanoate-BSA and incubated at 37 °C with 5% CO2. After overnight incubation, the serum-containing medium was aspirated and replaced with serum-free DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μM octanoate-BSA and either 1 mM glucose, 10 mM glucose, or 1 mM glucose with norepinephrine (10 μM; Sigma, St. Louis, MO). Each condition was tested in triplicate. The cells were exposed to test conditions for 6 h at 37 °C with 5% CO2 and then the medium was sampled and centrifuged immediately at 3000 rpm at 4 °C for 5 min. Acyl-ghrelin in the supernatants was stabilized by addition of HCl (to achieve a final concentration of 0.1 N) and the samples were stored at −80 °C, until further use. Ghrelin and desacyl-ghrelin concentrations were determined using rat acyl or desacyl-ghrelin ELISA kits (Cayman Chemical, Ann Arbor, MI) following manufacturer's instructions and the absorbance was read using a PowerWave XS spectrophotometer (BioTek Instruments, Inc., Winooski, VT).
2.6. Blood parameters
Blood samples for glucose measurement were obtained via the tail vein. Blood glucose levels were measured using a hand-held glucometer (OneTouch, LifeScan Inc., Milipitas, CA). Blood samples for all other parameters were obtained either from the submandibular vein in live mice or via cardiac puncture under chloral hydrate anesthesia, prior to euthanasia. Blood samples were placed on ice and treated with the cysteine protease inhibitor PHMB (p-hydroxymercuribenzoate) and dipeptidyl peptidase IV inhibitor. Plasma was separated by centrifugation at 1500 × g and stored at −80 °C. Plasma samples aliquoted for ghrelin measurements were acid stabilized, as described above for cell culture supernatants, and stored at −80 °C. Plasma insulin levels were measured using a high sensitivity mouse enzyme-linked immunosorbent assay (Crystal Chem, Downers Grove, IL). Plasma acyl-ghrelin and desacyl-ghrelin levels were determined as described above.
2.7. Data and statistics
All data are expressed as mean ± SEM. Statistical differences were tested using Prism 6 (GraphPad Software, Inc., San Diego, CA). If unequal variance among groups was detected by Bartlett's test, data were log transformed before analysis, as required. Student's “t”-test was used to compare differences between two sets of means. One-way ANOVA followed by Tukey's post-hoc multiple comparisons test was used to compare multiple groups. Two-way ANOVA followed by Bonferroni post-hoc multiple comparison was used to analyze body weight curves. P < 0.05 was considered statistically significant.
3. Results
3.1. Altered metabolic characteristics and reduced plasma ghrelin levels in DIO mice
To study the mechanisms underlying obesity-associated reductions in plasma ghrelin levels, we used a DIO mouse model. C57BL/6 mice fed HFD for 7 weeks (DIO mice) had higher body weights compared to those fed standard rodent chow (lean mice) (Figure 1A). DIO mice consumed more calories per day compared to lean mice (Figure 1B). DIO mice had higher fat mass but similar lean mass compared to lean mice (Figure 1C). There was no difference in stomach weight between DIO and lean mice (0.142 ± 0.007 g vs. 0.145 ± 0.007 g, P = 0.77, n = 8–9 mice). Fasting blood glucose was significantly higher in DIO mice compared to lean mice (Figure 1D). Fasting plasma acyl-ghrelin and fasting plasma desacyl-ghrelin were both significantly lower in DIO mice compared to lean mice (Figure 1D).
Figure 1.
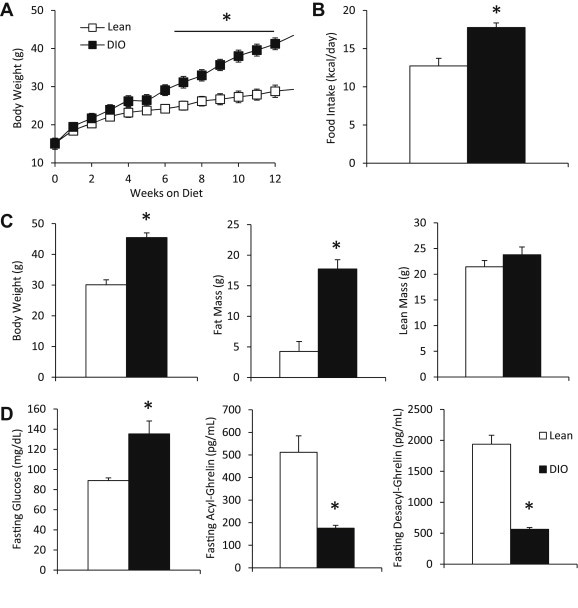
Reduced fasting ghrelin levels in DIO mice. (A) Body weight was increased in DIO (HFD-fed) compared to lean (standard chow-fed) mice starting at 7 weeks on respective diets. Asterisk for this figure denotes difference compared to lean mice at each time point (n = 6–7 mice). (B) Daily food intake was increased in DIO compared to lean mice. (n = 6–7 mice). (C) Body composition was changed in DIO compared to lean mice. DIO mice had increased body weight and fat mass, but similar lean mass.(n = 6–7 mice). (D) Fasting blood glucose levels (left, n = 5–6 mice) were elevated, fasting acyl-ghrelin levels (middle, n = 6–7 mice) were reduced, and fasting desacyl-ghrelin levels (right, n = 5–6 mice) were reduced in DIO compared to lean mice. Data are represented as mean ± SEM. *P < 0.05 compared to lean mice.
3.2. Altered responsiveness of ghrelin cells to mediators of ghrelin secretion in DIO mice
Ghrelin secretion in response to two established direct mediators of ghrelin secretion was tested in primary cultures of dispersed gastric mucosal cells generated from either lean or DIO mice. In preparations from lean mice, 10 mM glucose reduced acyl-ghrelin secretion while norepinephrine (10 μM) stimulated acyl-ghrelin secretion, as compared to that observed in the setting of 1 mM glucose in the culture medium (Figure 2A, left). In contrast, the effects of these mediators on acyl-ghrelin release were lost in gastric mucosal cells derived from DIO mice (Figure 2A, right).
Figure 2.
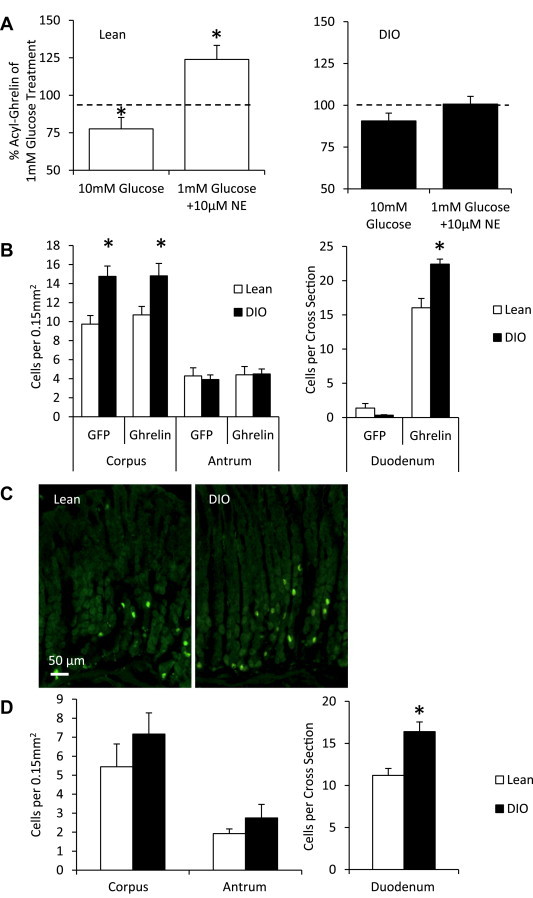
Altered acyl-ghrelin secretion response and ghrelin cell density in DIO mice. (A) Acyl-ghrelin secretion from primary gastric mucosal cell cultures in response to 1 mM glucose, 10 mM glucose and 1 mM glucose with 10 μM norepinephrine were altered in DIO compared to lean mice. Results are shown relative to 1 mM glucose treatment within respective weight groups (n = 6–7 mice). Data are represented as mean ± SEM. *P < 0.05 compared to ghrelin levels from 1 mM glucose treatment in its respective groups. (B) Average ghrelin cell numbers as determined by endogenous GFP signal and ghrelin-immunoreactivity in the stomach (left, n = 3–5 mice) per high power field and in the duodenum (right, n = 4 mice) per cross section were higher in DIO compared to lean mice. Data are represented as mean ± SEM. *P < 0.05 compared to lean mice. (C) Representative images of the endogenous GFP signal in the corpus region of the stomach in DIO and lean mice (n = 3–5 mice). (D) Average somatostatin-immunoreactive cell numbers in the stomach (left, n = 3–5 mice) per high power field were similar and in the duodenum (right, n = 4 mice) per cross section were increased in DIO compared to lean mice. Data are represented as mean ± SEM. *P < 0.05 compared to lean mice.
Desacyl-ghrelin levels were also measured (Supplementary Figure 1A). Similar to their effects on acyl-ghrelin release from gastric mucosal cells derived from lean mice, 10 mM glucose and norepinephrine resulted in a pattern of reduced desacyl-ghrelin secretion and of increased desacyl-ghrelin secretion, respectively. However, while this pattern was similar, and unlike our previous report using younger mice [71], these effects did not meet statistical significance in the present study. Furthermore, while a loss of acyl-ghrelin secretion in response to norepinephrine in gastric mucosal cells derived from DIO mice was observed, desacyl-ghrelin secretion persisted.
3.3. Ghrelin cell density is increased in the corpus of DIO mice
We next compared ghrelin cell density in the stomachs and duodena of DIO and lean ghrelin hrGFP reporter mice. The corpus (body; central region of the stomach) and the antrum (distal region of the stomach) were analyzed separately since ghrelin cell densities differ by stomach region [80]. The forestomach (non-glandular region) was not analyzed since glandular mucosa and ghrelin cells are absent from this region. Ghrelin cell density, as demonstrated by either endogenous hrGFP-fluorescence or ghrelin-immunoreactivity, was significantly increased in the corpus, but not in the antrum of DIO mice, when compared to lean mice (Figure 2B, left and C). In the duodenum, very few hrGFP-fluorescent cells were observed per cross section, as had been described previously for the ghrelin-hrGFP line [78]. However, similar to the corpus, ghrelin cell density in the duodenum, as determined by ghrelin-immunoreactivity, was higher in DIO mice than in lean mice (Figure 2B, right). We also analyzed the density of somatostatin-producing cells in the corpus and antral regions of the stomach via somatostatin-immunoreactivity, finding no differences between DIO and lean mice (Figure 2D, left). The density of somatostatin-producing cells in the duodenum was greater in DIO mice than in lean mice (Figure 2D, right).
3.4. Metabolic characteristics and ghrelin levels of RYGB, WMS and ALS mice
To study the mechanisms underlying RYGB-induced changes in plasma ghrelin levels, DIO mice were subjected to either RYGB or sham surgery. Following standard post-operative recovery, mice that underwent sham surgery were subsequently allowed to eat HFD ad libitum (ad lib-fed sham; ALS) or were fed a restricted amount of HFD to achieve body weights matching those of RYGB-operated mice (weight-matched sham; WMS) (Figure 3A). RYGB and WMS mice weighed less when compared to ALS mice starting day 11 post-surgery, and the weight loss was sustained for the remainder of the monitored duration of 35 days post-surgery (Figure 3B). As previously reported, in order to match the body weights of the post-RYGB mice, WMS were fed significantly less HFD (Figure 3C) [79]. Similar to our previous report, ALS mice ate similar amounts of HFD as the RYGB-treated mice (Figure 3C) [79]. Also as previously reported, fat mass was reduced but lean mass was similar in RYGB and WMS mice, as compared to ALS mice, at 5 weeks post-surgery (Figure 3D) [79]. Fasting blood glucose and plasma insulin levels decreased significantly in both WMS and RYGB mice, as compared to ALS mice (Figure 3E) [79]. Furthermore, at 6 weeks post-surgery, fasting plasma acyl-ghrelin levels measured were significantly increased in post-RYGB mice, as compared to both ALS and WMS mice. Lastly, fasting plasma desacyl-ghrelin levels were significantly different among all groups, with RYGB mice having the highest and the ALS mice having the lowest desacyl-ghrelin levels (Figure 3F).
3.5. Altered responsiveness of ghrelin cells to mediators of ghrelin secretion in post-RYGB mice compared to WMS and ALS mice
Acyl-ghrelin secretion in primary cultures of dispersed gastric mucosal cells from ALS mice decreased in the presence of 10 mM glucose, when compared to secretion in the presence of 1 mM glucose; the cells did not respond to addition of 10 μM norepinephrine (Figure 4A, left). Similar to the responses observed in primary cultures of dispersed gastric mucosal cells from lean mice, cells from WMS mice decreased acyl-ghrelin secretion upon exposure to 10 mM glucose and increased acyl-ghrelin secretion in response to 10 μM norepinephrine (Figure 4A, middle). However, acyl-ghrelin secretion by cells derived from the proximal stomach of RYGB mice was unchanged by 10 mM glucose but was stimulated by addition of 10 μM norepinephrine (Figure 4A, right).
Figure 4.
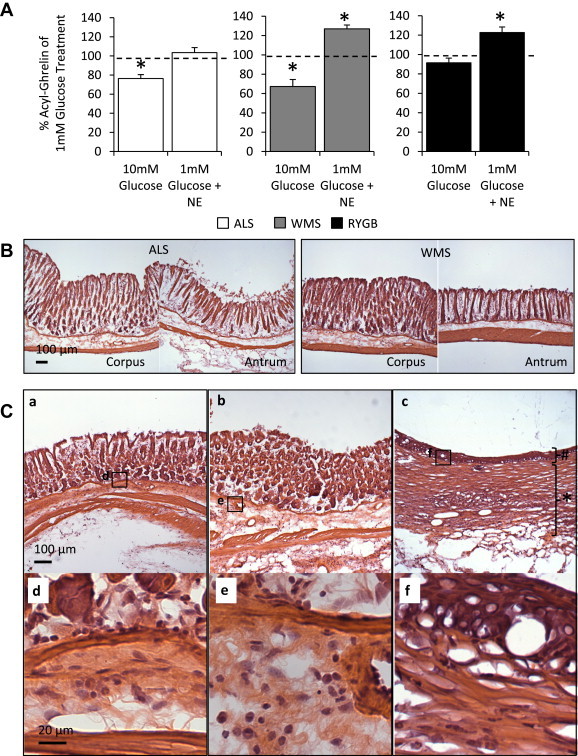
Altered acyl-ghrelin secretion response and distal stomach morphology in RYGB mice. (A) Primary gastric mucosal cell cultures from ALS, WMS and RYGB mice treated with 1 mM glucose, 10 mM glucose and 1 mM glucose with 10 μM norepinephrine responded differentially. Results are shown relative to 1 mM glucose treatment within it respective weight groups (n = 6–11 mice). Data are represented as mean ± SEM. *P < 0.05 compared to ghrelin levels in the media of 1 mM glucose treatment in its respective groups. (B) Representative H&E stained sections of the corpus and antrum structures in distal stomachs in ALS (left) and WMS (right) mice (n = 3 mice). (C) Representative H&E stained sections of the stomachs distal to the hemostasis clip in three different RYGB-operated mice. Gastric glands were effaced in the distal aspect of the stomachs post-RYGB (Panel a and c), and squamous metaplasia was prominent in the distal stomach of one of these mice (Panel c). There was evidence of inflammation in the submucosa and mucosa layers of the RYGB stomachs. The inflammatory infiltrate consisted of neutrophils (Panels d–f). In addition, fibrosis was observed in the muscle layers and extended to the serosal surface (Panel a–c). Hash tag symbol highlights the squamous cell metaplasia. Asterisk denotes extensive and thickened fibrosis. (n = 3 mice).
Desacyl-ghrelin levels were also measured (Supplementary Figure 1B). The patterns of desacyl-ghrelin secretion from mucosal cells derived from the three surgery groups, in response to glucose and norepinephrine, were fairly similar to those patterns described above for acyl-ghrelin. Exceptions included stimulation of desacyl-ghrelin but not of acyl-ghrelin release by norepinephrine in ALS mice. Also, the pattern of reduced desacyl-ghrelin secretion and of increased desacyl-ghrelin secretion from WMS mucosal cells, in response to glucose and norepinephrine, respectively, matched that of acyl-ghrelin, although did not meet statistical significance. Desacyl-ghrelin responses in RYGB mucosal cells were identical to those of acyl-ghrelin. Also of note, in none of the in vivo or in vitro experiments in this report was there a treatment effect on acyl-ghrelin:desacyl-ghrelin ratio (data not shown).
3.6. Post-surgical pathologic findings in RYGB, WMS, and ALS mice
Upon isolation of the stomach, there were no gross pathological changes observed in the ALS and WMS mice. However, the stomachs of the RYGB mice were found to be thickened and fixed to the surrounding viscera by fibrous adhesions. Furthermore, while microscopic analysis of H&E stained sections showed no consistent histological alterations in ALS and WMS stomachs, the sections from RYGB stomachs revealed several changes. The gastric glands were effaced in the distal aspect of the stomachs post-RYGB and squamous metaplasia was prominent in the distal stomach of one of these mice (Figure 4C, Panel c). Also, there was evidence of inflammation in the submucosa and mucosa layers of the RYGB stomachs. The inflammatory infiltrate consisted of neutrophils (Figure 4C, Panels d–f). In addition, fibrosis was observed in the muscle layers, extending to the serosal surface (Figure 4C, Panels a–c).
4. Discussion
The current study used mouse models to examine potential mechanisms for altered circulating ghrelin levels observed in obesity and following weight loss resulting from RYGB. We confirmed lower acyl-ghrelin and desacyl-ghrelin levels in DIO mice and demonstrated, using a recently described mouse model of RYGB [79], elevated acyl-ghrelin and desacyl-ghrelin levels in mice 6 weeks post-RYGB, by which time they had attained weight stability. Interestingly, although WMS control mice, whose body weights were matched to the RYGB mice by restricted HFD feeding, demonstrated a rise in desacyl-ghrelin levels, as would be expected upon caloric restriction, they did not demonstrate a rise in acyl-ghrelin. Altered ghrelin cell responsiveness to two key physiological modulators of ghrelin secretion, glucose and norepinephrine, correlated with some of the observed changes in ghrelin levels. Increases in ghrelin cell density and/or somatostatin-producing D cells within the stomach and duodenum were observed in DIO mice. These findings provide new insight for the regulation of ghrelin secretion and its relation to circulating ghrelin, in the contexts of obesity and weight loss.
4.1. Plasma ghrelin levels observed in DIO, post-RYGB and in WMS mice
The finding of lowered circulating acyl-ghrelin and desacyl-ghrelin in DIO was as expected, as this has been reported fairly consistently in multiple previous rodent and human studies [48,51,52,81–83]. In the setting of obesity, the associated reduction of acyl-ghrelin, together with observed ghrelin resistance, is presumed to serve as a counter-regulatory mechanism to minimize pro-orexigenic and pro-adipogenic acyl-ghrelin actions [84,85]. Exceptions to the finding of lowered ghrelin with DIO are rare in the literature [86].
Our observation of raised plasma acyl-ghrelin and desacyl-ghrelin in RYGB compared to sham mice contradicts the first report of total ghrelin levels after RYGB by Cummings et al., which showed a marked decrease [58]. However, subsequent human data have been mixed, with reported ghrelin levels post-weight loss surgery being either lower, unchanged or higher [67]. Similar to human studies, rat RYGB models also have shown variability, with one study demonstrating unchanged acyl-ghrelin and desacyl-ghrelin levels 11 months post-surgery [87], a second study demonstrating decreased total ghrelin levels in weight-stable rats 3 months post-surgery [88], and another two studies showing an increase in total ghrelin levels after RYGB [89,90]. The exact reasons for the differences observed in post-RYGB ghrelin levels among studies are not clear, although this variability may represent differences in operative technique, types of ghrelin assayed, the use of measures to stabilize acyl-ghrelin in plasma, and timing of assays post-surgery. Regarding the latter possibility, one study demonstrated that fasting total ghrelin levels in human subjects who underwent RYGB decreased immediately post-operatively, followed by successive increases to presurgical levels after 1 month and further increases over the next 11 months [91]. A second human trial demonstrated a fall in random total ghrelin 1 day post-RYGB, with a return to presurgical levels at 7 days and one month, and then an increase at 3 months [83]. Obese Long-Evans rats displayed increased fasting acyl-ghrelin 30 days following RYGB but equivalent fasting ghrelin 110 days following RYGB, relative to sham-operated animals [92]. Furthermore, although RYGB did not change overnight-fasted plasma acyl-ghrelin in comparison to sham rats, postprandial suppression of ghrelin was significantly greater after RYGB [93].
As circulating ghrelin levels following RYGB have been variable, it is more difficult to assign a role for the post-RYGB ghrelin response. That said, in the Stylopoulos et al. study, weight loss in rats at 3 months following RYGB was noted to correlate with the magnitude of the decrease in circulating total ghrelin, suggesting that altered ghrelin signaling contributes to post-RYGB weight loss, just as had been suggested in the earlier Cummings et al. study [58,88]. Of interest, human subjects homozygous for a particular single nucleotide polymorphism in the gene encoding GHSR, which decreases promoter activity when tested in vitro, have been shown to lose the most weight after RYGB [94]. Another bariatric surgical model, vertical sleeve gastrectomy (VSG), results in significantly decreased ghrelin levels in rodents [87], consistent with the excision of the majority of the corpus and part of the antrum during this surgical procedure. Nonetheless, as ghrelin-deficient mice had similar metabolic outcomes post-VSG compared to wild-type mice, it has been proposed that changes in ghrelin are not required for the metabolic effects of VSG [87].
The increased acyl-ghrelin that we observed post-RYGB, which attained levels similar to those present in lean mice, concurs with many other forms of weight loss, including that induced by caloric restriction [56]. Just as the decrease in acyl-ghrelin levels in DIO may be a counter-regulatory mechanism to limit further ghrelin-engaged processes that could exaggerate DIO, the increase in acyl-ghrelin levels induced by weight loss may be a means to defend body weight. As an extreme example, in cancer cachexia, which is also associated with elevated plasma ghrelin, pharmacologic antagonism of GHSR results in several negative outcomes, including worsened anorexia and accelerated death, suggesting a key protective role of raised ghrelin [37]. Alternatively, the raised ghrelin associated with extreme forms of weight loss could serve other important functions, such as those related to preservation of sustainable blood glucose levels and defense against depressed mood [27,95]. That said, despite the use of caloric restriction to match the weight loss induced by RYGB, WMS mice demonstrated only higher desacyl-ghrelin but not higher acyl-ghrelin levels than those observed in the heavier, ad lib-fed ALS mice. We hypothesize that the absence of calorie restriction- and weight loss-induced acyl-ghrelin elevation in the WMS animals relates to the sole availability of HFD as food. Suggesting a direct inhibitory effect of HFD on the usual caloric restriction-induced ghrelin secretion, a previous human trial using healthy lean subjects demonstrated that three weeks of overfeeding with high fat dietary supplements resulted in an 18% decrease in ghrelin, despite only a subtle increase in body weight [96]. Furthermore, receptors for fatty acids are highly enriched in ghrelin cells and in vitro studies suggest the ability of both short-chain and long-chain fatty acids to directly inhibit ghrelin secretion [73,97–99].
4.2. Altered ghrelin cell responsiveness in DIO and post-RYGB
Our observation of altered cellular responsiveness to norepinephrine and/or glucose in ghrelin cells from physiologically manipulated mice has not previously been reported. Regarding norepinephrine, several previous in vitro and in vivo studies using lean, healthy animals, cells and tissues derived from those animals, and immortalized ghrelin cell lines have identified adrenergic hormones released from sympathetic nerves as important regulators of ghrelin secretion [68,70,71,73,100–102]. For example, purified populations of gastric ghrelin cells and ghrelinoma cell lines express high levels of mRNA encoding the β1-adrenergic receptor, which is the only adrenergic receptor enriched within ghrelin cells, as compared to non-ghrelin gastric mucosal cells [100]. Furthermore, the β1-adrenergic receptor is the most highly expressed of all the non-odorant, seven transmembrane G protein-coupled receptors within ghrelin cells [73]. As mentioned above, when mice are treated with reserpine to deplete adrenergic neurotransmitters from sympathetic neurons, the overnight fast-induced increase in plasma ghrelin is blocked [100]. Atenolol, a β1-adrenergic receptor selective blocker has the same effect and also decreases baseline plasma ghrelin levels [100].
Regarding glucose, several rodent and human studies have demonstrated its importance in regulating ghrelin secretion. The regulation of ghrelin secretion by glucose synchronizes with a fundamental feature of enteroendocrine cells – their ability to sense nutrients and dietary metabolites. Food intake, intralipid infusion and experimental induction of hyperglycemia all reduce ghrelin levels [48,58,74]. As mentioned above, ghrelin release in primary cultures of dispersed gastric mucosal cells is negatively correlated with glucose concentration in the culture medium [71]. As compared to 5 mM glucose exposure, ghrelin release from gastric mucosal cells taken from lean mice is reduced by 10 mM glucose (mimicking a hyperglycemic state) and is stimulated by 1 mM glucose (mimicking a hypoglycemic state), but is unaffected by the non-metabolizable glucose enantiomer l-glucose [71]. Furthermore, the glucoprivic agent 2-deoxy-d-glucose prevents the inhibitory effect of high glucose exposure on ghrelin release, suggesting a requirement for glucose entry into the ghrelin cell and subsequent metabolism for its inhibitory effects on ghrelin secretion [71]. This is further supported by the expression by ghrelin cells of mRNAs encoding several channels and enzymes responsible for mediating the effects glucose responsiveness and metabolism in other cell types, including several facilitative glucose transporters (GLUT1, GLUT4, and GLUT5), hexokinases (including glucokinase), and both components of the pancreatic β-cell KATP channel (the ATP-sensitive potassium channel subunit Kir6.2 and the sulfonylurea type 1 receptor SUR1) [71].
In evaluating the observed lower ghrelin levels in DIO, one might expect known stimulators of ghrelin secretion – such as norepinephrine – to no longer be potent and/or inhibitors of ghrelin secretion – such as glucose – to be more potent. The current study indeed demonstrated DIO-associated changes in the usual ghrelin secretion responses to norepinephrine and glucose: no longer did acyl-ghrelin or desacyl-ghrelin secretion change with glucose and no longer did acyl-ghrelin secretion change with norepinephrine. In regard to acyl-ghrelin secretion, this suggests that ghrelin cells in DIO mice are desensitized to these physiological signals of caloric restriction (norepinephrine) and food intake or dysregulated glucose handling (glucose). This desensitization to norepinephrine might parallel presumed defects in sympathetic nervous system-driven energy expenditure in obesity [103]. Similarly, in evaluating the observed higher ghrelin levels following RYGB, one might expect ghrelin secretagogues to be more potent and/or negative modulators of ghrelin secretion to be less potent following RYGB. As predicted, ghrelin cells from post-RYGB mice regained their sensitivity to norepinephrine – secreting more acyl-ghrelin in response to norepinephrine – but not to changes in glucose concentrations. The recovered sensitivity to norepinephrine might parallel the improved sympathetic outflow that has been demonstrated via indirect measures after different forms of surgical weight loss [104–106].
Complicating the interpretation of the results, not all of the in vitro desacyl-ghrelin secretion data matched that of the in vitro acyl-ghrelin secretion data. Most notable were differential acyl-ghrelin vs. desacyl-ghrelin responses to norepinephrine in gastric mucosal cells from DIO mice and in gastric mucosal cells from ALS mice. As mentioned in the Results, in cells from WMS mice, the patterns of the acyl-ghrelin and desacyl-ghrelin responses to glucose and norepinephrine matched but did not meet statistical significance for desacyl-ghrelin. The discrepancy between acyl-ghrelin and desacyl-ghrelin in those few instances is unclear at this time. Presumably, some situations might exist in which in addition to differential regulation of secretion, there might also or instead be differential regulation of GOAT expression or activity, which in turn could influence acyl-ghrelin but not desacyl-ghrelin. Evidence for fasting-dependent, separable regulation of ghrelin secretion and acylation has been provided in the form of a trial in lean young men in whom acyl-ghrelin and desacyl-ghrelin displayed similar dynamics when not calorically restricted but displayed marked differences following a prolonged fast, despite unchanged total ghrelin levels [107]. That said, gastric GOAT mRNA levels had been shown by others to remain unchanged in response to DIO in mice, although that was one of the few studies in which neither plasma acyl-ghrelin nor total ghrelin levels differed between lean and DIO subjects [86]. Another study in rats, though, demonstrated increased gastric GOAT mRNA levels in response to leptin administration and in response to chronic malnutrition although unchanged levels in response to a 2-day fast [108]. Also worth considering is the potential influence of desacyl-ghrelin as a functional acyl-ghrelin antagonist, which has been suggested by some studies, as well as a role for changes in acyl-ghrelin:desacyl-ghrelin ratio, although such changes were not observed here [9].
Two other observations that are not easy to explain include the regained sensitivity to both norepinephrine and glucose by gastric mucosal cells derived from WMS mice and the regained sensitivity to glucose by cells from ALS mice. As discussed above, WMS mice do not show elevated acyl-ghrelin and yet have similar patterns of norepinephrine and glucose sensitivity as lean mice. We speculate that weight loss might be a main driver of the recovered sensitivity to norepinephrine, in both post-RYGB and WMS mice, although as-of-yet unknown processes might intervene, resulting in the observed differential fasting ghrelin levels. Also, mice from both WMS and ALS groups lost substantial weight during the first week of the post-operative period, however, unlike WMS mice, ALS mice were provided free access to HFD following surgeries and rapidly reached pre-operative weights. We speculate that the rapid weight loss during the first week after operation may be responsible for the regained response to glucose that is otherwise missing in DIO mice. With time, this improved response to glucose in the ALS mice is predicted to wane, although future studies are needed to test this hypothesis.
A caveat of the presented ghrelin secretion data from gastric mucosal cells derived from RYGB mice is that only the gastric mucosal cells proximal to the surgical clip transecting the stomach were used, whereas the gastric mucosal cells from the other groups were derived from the entire stomachs. In non-surgery-exposed and sham groups, cells were isolated from the whole stomach via inversion and inflation of the entire stomach, followed by gentle enzymatic digestion. This was not technically possible with the stomachs of RYGB mice, due to scarring that occurred where the hemostasis clip was placed, permanently dividing the stomach in two. Gross and microscopic examination of the tissue demonstrated varying degrees of fibrosis as well as metaplasia in the distal stomach. Therefore, we inverted, inflated and enzymatically digested the proximal stomach, hence only isolating cells from the proximal stomach. Although not determined, this fibrosis potentially could have altered ghrelin secretion by the ghrelin cells in the distal stomach, influencing the overall net circulating ghrelin levels.
4.3. Enteroendocrine cells in DIO and post-RYGB ghrelin levels
Another potential mechanism contributing to the observed ghrelin levels in DIO and following RYGB could involve changes in ghrelin cell numbers and distribution relative to other enteroendocrine cells. We found an increase in ghrelin cell density in the corpus of the DIO mice, which may be a compensatory reaction to the cells' altered sensitivities to norepinephrine and glucose. This observation was similar to what has been previously shown in two human studies looking at ghrelin levels in severely obese individuals [54,80]. The finding in a separate study of lower plasma ghrelin yet increased intensity of ghrelin-immunoreactivity in the gastric mucosa from obese humans as compared to that from control subjects was interpreted as being suggestive of “hypoactive” ghrelin cells, although no comment was made regarding ghrelin cell density [83]. This contrasts with a study in mice in which DIO was associated with slightly less numerous and less intensely immunoreactive (for ghrelin) gastric ghrelin cells [82].
We further investigated changes to somatostatin-producing cells, which normally reside in proximity to a subset of ghrelin cells [75] and which inhibit ghrelin secretion in a paracrine/endocrine fashion via interaction of somatostatin with somatostatin receptors on the ghrelin cells [73]. Although no change was observed in the density of somatostatin producing cells in the stomach, an increase was observed in duodenal somatostatin cell density in DIO mice. This could potentially provide an increased inhibitory tone to ghrelin cells in the DIO setting.
In addition to ghrelin, RYGB has been associated with changes in levels of several other gastrointestinal hormones including glucagon-like peptide-1, peptide YY, and cholecystokinin [62–65]. A recent study in rats demonstrated increased numbers of CCK-, GLP-1-, serotonin- and neurotensin-immunoreactive cells in the Roux-limb, but not in the biliopancreatic limb of RYGB-treated mice; furthermore, ghrelin-immunoreactive cell numbers were not altered in distal stomach, nor were there any obvious differences in the small intestine [109]. Unfortunately we were not able to confirm these findings regarding ghrelin cell number in the distal stomach due to the above-mentioned altered, post-RYGB morphology of the distal stomach (from inflammation, metaplasia and fibrosis), which prevented accurate comparisons in cell numbers with the sham stomachs.
4.4. Conclusions
Overall, our findings provide new insight into the regulation of ghrelin secretion and its relation to circulating ghrelin, in the context of obesity and weight loss. We now know that obesity and different methods of weight loss alter sensitivities to physiological cues, such as glucose and norepinephrine, and that changes to overall ghrelin cell density and the density of somatostatin-producing enteroendocrine cells, which provide inhibitory input onto ghrelin cells, likely work together to result in the net change in ghrelin levels. Future studies will be needed to determine the exact molecular mechanisms resulting in the observed obesity-, RYGB-, and caloric restriction-induced changes to norepinephrine and glucose sensitivity in ghrelin cells, including the possible roles of changed GOAT expression and/or activity, glucose entry and/or metabolism, and signaling by targets downstream of the β1-adrenergic receptors within ghrelin cells.
Acknowledgments
We would like to thank Dr. James Richardson at the UT Southwestern Medical Center for assistance in analyzing the histology of the post-surgical stomachs, the Molecular Pathology Core for assistance in preparing the H&E stains of those sections, and Dr. Joyce Repa for helpful discussions regarding the statistical analyses. This work was supported by the National Institutes of Health (T32DK007307-33 and F32DK100085-01 to A.U., K08DK091511 to V.A.), the Hilda and Preston Davis Foundation Postdoctoral Fellowship in Eating Disorders Research (to B.K.M.), the UT Southwestern Department of Internal Medicine (to V.A.), and an International Research Alliance Grant with the Novo Nordisk Foundation Center for Basic Metabolic Research (to J.M.Z.).
Conflict of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
References
- 1.Kojima M., Kangawa K. Ghrelin: from gene to physiological function. Results and Problems in Cell Differentiation. 2010;50:185–205. doi: 10.1007/400_2009_28. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. Journal of Clinical Endocrinology & Metabolism. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 3.Date Y., Kojima M., Hosoda H., Sawaguchi A., Mondal M.S., Suganuma T. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 4.Sakata I., Nakamura K., Yamazaki M., Matsubara M., Hayashi Y., Kangawa K. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–536. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- 5.Wierup N., Svensson H., Mulder H., Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regulatory Peptides. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.M., Wang G., Englander E.W., Kojima M., Greeley G.H., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez J.A., Solenberg P.J., Perkins D.R., Willency J.A., Knierman M.D., Jin Z. Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Brown M.S., Liang G., Grishin N.V., Goldstein J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Delhanty P.J., Neggers S.J., van der Lely A.J. Mechanisms in endocrinology: ghrelin: the differences between acyl- and des-acyl ghrelin. European Journal of Endocrinology. 2012;167:601–608. doi: 10.1530/EJE-12-0456. [DOI] [PubMed] [Google Scholar]
- 10.Cummings D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology & Behavior. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Strassburg S., Anker S.D., Castaneda T.R., Burget L., Perez-Tilve D., Pfluger P.T. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. American Journal of Physiology. Endocrinology and Metabolism. 2008;295:E78–E84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 13.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 14.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 15.Wren A.M., Small C.J., Abbott C.R., Dhillo W.S., Seal L.J., Cohen M.A. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 16.Uchida A., Zigman J.M., Perello M. Ghrelin and eating behavior: evidence and insights from genetically-modified mouse models. Frontiers in Neuroscience. 2013;7:121. doi: 10.3389/fnins.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egecioglu E., Jerlhag E., Salome N., Skibicka K.P., Haage D., Bohlooly Y.M. Ghrelin increases intake of rewarding food in rodents. Addiction Biology. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker A.K., Ibia I.E., Zigman J.M. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiology & Behavior. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanoski S.E., Fortin S.M., Ricks K.M., Grill H.J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biological Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perello M., Sakata I., Birnbaum S., Chuang J.C., Osborne-Lawrence S., Rovinsky S.A. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biological Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disse E., Bussier A.L., Deblon N., Pfluger P.T., Tschop M.H., Laville M. Systemic ghrelin and reward: effect of cholinergic blockade. Physiology & Behavior. 2011;102:481–484. doi: 10.1016/j.physbeh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Butte N.F., Garcia J.M., Smith R.G. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albarran-Zeckler R.G., Sun Y., Smith R.G. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides. 2011;32:2229–2235. doi: 10.1016/j.peptides.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y., Ahmed S., Smith R.G. Deletion of ghrelin impairs neither growth nor appetite. Molecular and Cellular Biology. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Wang P., Zheng H., Smith R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wortley K.E., Anderson K.D., Garcia K., Murray J.D., Malinova L., Liu R. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao T.J., Liang G., Li R.L., Xie X., Sleeman M.W., Murphy A.J. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirchner H., Gutierrez J.A., Solenberg P.J., Pfluger P.T., Czyzyk T.A., Willency J.A. GOAT links dietary lipids with the endocrine control of energy balance. Nature Medicine. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wortley K.E., del Rincon J.P., Murray J.D., Garcia K., Iida K., Thorner M.O. Absence of ghrelin protects against early-onset obesity. Journal of Clinical Investigation. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigman J.M., Nakano Y., Coppari R., Balthasar N., Marcus J.N., Lee C.E. Mice lacking ghrelin receptors resist the development of diet-induced obesity. Journal of Clinical Investigation. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfluger P.T., Kirchner H., Gunnel S., Schrott B., Perez-Tilve D., Fu S. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 32.Shearman L.P., Wang S.P., Helmling S., Stribling D.S., Mazur P., Ge L. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology. 2006;147:1517–1526. doi: 10.1210/en.2005-0993. [DOI] [PubMed] [Google Scholar]
- 33.Zorrilla E.P., Iwasaki S., Moss J.A., Chang J., Otsuji J., Inoue K. Vaccination against weight gain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolph J., Esler W.P., O'Connor S., Coish P.D., Wickens P.L., Brands M. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. Journal of Medicinal Chemistry. 2007;50:5202–5216. doi: 10.1021/jm070071+. [DOI] [PubMed] [Google Scholar]
- 35.Esler W.P., Rudolph J., Claus T.H., Tang W., Barucci N., Brown S.E. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148:5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 36.McFarlane M.R., Brown M.S., Goldstein J.L., Zhao T.J. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metabolism. 2014;20:54–60. doi: 10.1016/j.cmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujitsuka N., Asakawa A., Uezono Y., Minami K., Yamaguchi T., Niijima A. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Translational Psychiatry. 2011;1:e23. doi: 10.1038/tp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang J.C., Perello M., Sakata I., Osborne-Lawrence S., Savitt J.M., Lutter M. Ghrelin mediates stress-induced food-reward behavior in mice. Journal of Clinical Investigation. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 40.Dezaki K., Kakei M., Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007;56:2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 41.Broglio F., Arvat E., Benso A., Gottero C., Muccioli G., Papotti M. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. Journal of Clinical Endocrinology & Metabolism. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 42.Reed J.A., Benoit S.C., Pfluger P.T., Tschop M.H., D'Alessio D.A., Seeley R.J. Mice with chronically increased circulating ghrelin develop age-related glucose intolerance. American Journal of Physiology. Endocrinology and Metabolism. 2008;294:E752–E760. doi: 10.1152/ajpendo.00463.2007. [DOI] [PubMed] [Google Scholar]
- 43.Colombo M., Gregersen S., Xiao J., Hermansen K. Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas. 2003;27:161–166. doi: 10.1097/00006676-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Chuang J.C., Sakata I., Kohno D., Perello M., Osborne-Lawrence S., Repa J.J. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Molecular Endocrinology. 2011;25:1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi C.X., Heppner K.M., Kirchner H., Tong J., Bielohuby M., Gaylinn B.D. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS ONE. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R.L., Sherbet D.P., Elsbernd B.L., Goldstein J.L., Brown M.S., Zhao T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. Journal of Biological Chemistry. 2012;287:17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Liu C., Uchida A., Chuang J.C., Walker A., Liu T. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Molecular Metabolism. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 49.Tschop M., Wawarta R., Riepl R.L., Friedrich S., Bidlingmaier M., Landgraf R. Post-prandial decrease of circulating human ghrelin levels. Journal of Endocrinological Investigation. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 50.Wren A.M., Seal L.J., Cohen M.A., Brynes A.E., Frost G.S., Murphy K.G. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology & Metabolism. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 51.Tschop M., Weyer C., Tataranni P.A., Devanarayan V., Ravussin E., Heiman M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 52.Williams D.L., Grill H.J., Cummings D.E., Kaplan J.M. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. Journal of Endocrinological Investigation. 2006;29:863–868. doi: 10.1007/BF03349188. [DOI] [PubMed] [Google Scholar]
- 53.DelParigi A., Tschop M., Heiman M.L., Salbe A.D., Vozarova B., Sell S.M. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. Journal of Clinical Endocrinology & Metabolism. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 54.Maksud F.A., Alves J.S., Diniz M.T., Barbosa A.J. Density of ghrelin-producing cells is higher in the gastric mucosa of morbidly obese patients. European Journal of Endocrinology. 2011;165:57–62. doi: 10.1530/EJE-11-0201. [DOI] [PubMed] [Google Scholar]
- 55.le Roux C.W., Patterson M., Vincent R.P., Hunt C., Ghatei M.A., Bloom S.R. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. Journal of Clinical Endocrinology & Metabolism. 2005;90:1068–1071. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- 56.Hansen T.K., Dall R., Hosoda H., Kojima M., Kangawa K., Christiansen J.S. Weight loss increases circulating levels of ghrelin in human obesity. Clinical Endocrinology. 2002;56:203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 57.Schauer P.R., Kashyap S.R., Wolski K., Brethauer S.A., Kirwan J.P., Pothier C.E. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New England Journal of Medicine. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings D.E., Weigle D.S., Frayo R.S., Breen P.A., Ma M.K., Dellinger E.P. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New England Journal of Medicine. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen N.T., Masoomi H., Magno C.P., Nguyen X.M., Laugenour K., Lane J. Trends in use of bariatric surgery, 2003–2008. Journal of the American College of Surgeons. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 60.Kushner R.F. Weight loss strategies for treatment of obesity. Progress in Cardiovascular Diseases. 2014;56:465–472. doi: 10.1016/j.pcad.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Schauer P.R., Burguera B., Ikramuddin S., Cottam D., Gourash W., Hamad G. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Annals of Surgery. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cummings D.E., Overduin J., Shannon M.H., Foster-Schubert K.E. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surgery for Obesity and Related Diseases. 2005;1:358–368. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 63.Jacobsen S.H., Olesen S.C., Dirksen C., Jorgensen N.B., Bojsen-Moller K.N., Kielgast U. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obesity Surgery. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 64.Dirksen C., Jorgensen N.B., Bojsen-Moller K.N., Kielgast U., Jacobsen S.H., Clausen T.R. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. International Journal of Obesity (London) 2013;37:1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 65.Korner J., Bessler M., Inabnet W., Taveras C., Holst J.J. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surgery for Obesity and Related Diseases. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubino F., Gagner M., Gentileschi P., Kini S., Fukuyama S., Feng J. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of Surgery. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tymitz K., Engel A., McDonough S., Hendy M.P., Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obesity Surgery. 2011;21:125–130. doi: 10.1007/s11695-010-0311-z. [DOI] [PubMed] [Google Scholar]
- 68.Mundinger T.O., Cummings D.E., Taborsky G.J., Jr. Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- 69.de la Cour C.D., Norlen P., Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regulatory Peptides. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Iwakura H., Ariyasu H., Hosoda H., Yamada G., Hosoda K., Nakao K. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology. 2011;152:2619–2625. doi: 10.1210/en.2010-1455. [DOI] [PubMed] [Google Scholar]
- 71.Sakata I., Park W.M., Walker A.K., Piper P.K., Chuang J.C., Osborne-Lawrence S. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. American Journal of Physiology. Endocrinology and Metabolism. 2012;302:E1300–E1310. doi: 10.1152/ajpendo.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gagnon J., Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154:666–674. doi: 10.1210/en.2012-1994. [DOI] [PubMed] [Google Scholar]
- 73.Engelstoft M.S., Park W.M., Sakata I., Kristensen L.V., Husted A.S., Osborne-Lawrence S. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Molecular Metabolism. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flanagan D.E., Evans M.L., Monsod T.P., Rife F., Heptulla R.A., Tamborlane W.V. The influence of insulin on circulating ghrelin. American Journal of Physiology. Endocrinology and Metabolism. 2003;284:E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 75.Shimada M., Date Y., Mondal M.S., Toshinai K., Shimbara T., Fukunaga K. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochemical and Biophysical Research Communications. 2003;302:520–525. doi: 10.1016/s0006-291x(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 76.Schaller G., Schmidt A., Pleiner J., Woloszczuk W., Wolzt M., Luger A. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes. 2003;52:16–20. doi: 10.2337/diabetes.52.1.16. [DOI] [PubMed] [Google Scholar]
- 77.Seoane L.M., Al-Massadi O., Barreiro F., Dieguez C., Casanueva F.F. Growth hormone and somatostatin directly inhibit gastric ghrelin secretion. An in vitro organ culture system. Journal of Endocrinological Investigation. 2007;30:RC22–25. doi: 10.1007/BF03350806. [DOI] [PubMed] [Google Scholar]
- 78.Sakata I., Nakano Y., Osborne-Lawrence S., Rovinsky S.A., Lee C.E., Perello M. Characterization of a novel ghrelin cell reporter mouse. Regulatory Peptides. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zechner J.F., Mirshahi U.L., Satapati S., Berglund E.D., Rossi J., Scott M.M. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144:580–590. doi: 10.1053/j.gastro.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdemur A., Slone J., Berho M., Gianos M., Szomstein S., Rosenthal R.J. Morphology, localization, and patterns of ghrelin-producing cells in stomachs of a morbidly obese population. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2014;24:122–126. doi: 10.1097/SLE.0b013e318290167a. [DOI] [PubMed] [Google Scholar]
- 81.Chandarana K., Gelegen C., Karra E., Choudhury A.I., Drew M.E., Fauveau V. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60:810–818. doi: 10.2337/db10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moesgaard S.G., Ahren B., Carr R.D., Gram D.X., Brand C.L., Sundler F. Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regulatory Peptides. 2004;120:261–267. doi: 10.1016/j.regpep.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Dadan J., Hady H.R., Zbucki R.L., Iwacewicz P., Bossowski A., Kasacka I. The activity of gastric ghrelin positive cells in obese patients treated surgically. Folia Histochemica et Cytobiologica. 2009;47:307–313. doi: 10.2478/v10042-009-0033-z. [DOI] [PubMed] [Google Scholar]
- 84.Andrews Z.B., Liu Z.W., Walllingford N., Erion D.M., Borok E., Friedman J.M. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez M., Lage R., Saha A.K., Perez-Tilve D., Vazquez M.J., Varela L. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metabolism. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Gahete M.D., Cordoba-Chacon J., Salvatori R., Castano J.P., Kineman R.D., Luque R.M. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Molecular and Cellular Endocrinology. 2010;317:154–160. doi: 10.1016/j.mce.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chambers A.P., Kirchner H., Wilson-Perez H.E., Willency J.A., Hale J.E., Gaylinn B.D. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50–52. doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stylopoulos N., Davis P., Pettit J.D., Rattner D.W., Kaplan L.M. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surgical Endoscopy. 2005;19:942–946. doi: 10.1007/s00464-004-8825-x. [DOI] [PubMed] [Google Scholar]
- 89.Zhou D., Jiang X., Ding W., Zhang D., Yang L., Zhen C. Impact of bariatric surgery on ghrelin and obestatin levels in obesity or type 2 diabetes mellitus rat model. Journal of Diabetes Research. 2014;2014:569435. doi: 10.1155/2014/569435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aprahamian C.J., Tekant G., Chen M., Yagmurlu A., Yang Y.K., Loux T. A rat model of childhood diet-induced obesity: Roux-en-Y gastric bypass induced changes in metabolic parameters and gastric peptide ghrelin. Pediatric Surgery International. 2007;23:653–657. doi: 10.1007/s00383-007-1944-4. [DOI] [PubMed] [Google Scholar]
- 91.Sundbom M., Holdstock C., Engstrom B.E., Karlsson F.A. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obesity Surgery. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 92.Davis J.F., Tracy A.L., Schurdak J.D., Magrisso I.J., Grayson B.E., Seeley R.J. Roux en Y gastric bypass increases ethanol intake in the rat. Obesity Surgery. 2013;23:920–930. doi: 10.1007/s11695-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin A.C., Zheng H., Townsend R.L., Sigalet D.L., Berthoud H.R. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matzko M.E., Argyropoulos G., Wood G.C., Chu X., McCarter R.J., Still C.D. Association of ghrelin receptor promoter polymorphisms with weight loss following Roux-en-Y gastric bypass surgery. Obesity Surgery. 2012;22:783–790. doi: 10.1007/s11695-012-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature Neuroscience. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robertson M.D., Henderson R.A., Vist G.E., Rumsey R.D. Plasma ghrelin response following a period of acute overfeeding in normal weight men. International Journal of Obesity and Related Metabolic Disorders. 2004;28:727–733. doi: 10.1038/sj.ijo.0802637. [DOI] [PubMed] [Google Scholar]
- 97.Gong Z., Yoshimura M., Aizawa S., Kurotani R., Zigman J.M., Sakai T. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. American Journal of Physiology. Endocrinology and Metabolism. 2014;306:E28–E35. doi: 10.1152/ajpendo.00306.2013. [DOI] [PubMed] [Google Scholar]
- 98.Lu X., Zhao X., Feng J., Liou A.P., Anthony S., Pechhold S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2012;303:G367–G376. doi: 10.1152/ajpgi.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al Massadi O., Pardo M., Roca-Rivada A., Castelao C., Casanueva F.F., Seoane L.M. Macronutrients act directly on the stomach to regulate gastric ghrelin release. Journal of Endocrinological Investigation. 2010;33:599–602. doi: 10.1007/BF03346655. [DOI] [PubMed] [Google Scholar]
- 100.Zhao T.J., Sakata I., Li R.L., Liang G., Richardson J.A., Brown M.S. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gagnon J., Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology. 2012;153:3646–3656. doi: 10.1210/en.2012-1040. [DOI] [PubMed] [Google Scholar]
- 102.Mani B.K., Chuang J.C., Kjalarsdottir L., Sakata I., Walker A.K., Kuperman A. Role of calcium and EPAC in norepinephrine-induced ghrelin secretion. Endocrinology. 2014;155:98–107. doi: 10.1210/en.2013-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lowell B.B., Bachman E.S. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. Journal of Biological Chemistry. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- 104.Pontiroli A.E., Pizzocri P., Paroni R., Folli F. Sympathetic overactivity, endothelial dysfunction, inflammation, and metabolic abnormalities cluster in grade III (World Health Organization) obesity: reversal through sustained weight loss obtained with laparoscopic adjustable gastric banding. Diabetes Care. 2006;29:2735–2738. doi: 10.2337/dc06-1417. [DOI] [PubMed] [Google Scholar]
- 105.Karason K., Molgaard H., Wikstrand J., Sjostrom L. Heart rate variability in obesity and the effect of weight loss. American Journal of Cardiology. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 106.Machado M.B., Velasco I.T., Scalabrini-Neto A. Gastric bypass and cardiac autonomic activity: influence of gender and age. Obesity Surgery. 2009;19:332–338. doi: 10.1007/s11695-008-9665-x. [DOI] [PubMed] [Google Scholar]
- 107.Liu J., Prudom C.E., Nass R., Pezzoli S.S., Oliveri M.C., Johnson M.L. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. Journal of Clinical Endocrinology & Metabolism. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gonzalez C.R., Vazquez M.J., Lopez M., Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. Journal of Molecular Endocrinology. 2008;41:415–421. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- 109.Mumphrey M.B., Patterson L.M., Zheng H., Berthoud H.R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterology and Motility. 2013;25:E70–E79. doi: 10.1111/nmo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


