Abstract
Metabolic flexibility allows rapid adaptation to dietary change, however, little is known about the CNS mechanisms regulating this process. Neurons in the hypothalamic ventromedial nucleus (VMN) participate in energy balance and are the target of the metabolically relevant hormone leptin. Cannabinoid type-1 (CB1) receptors are expressed in VMN neurons, but the specific contribution of endocannabinoid signaling in this neuronal population to energy balance regulation is unknown. Here we demonstrate that VMN CB1 receptors regulate metabolic flexibility and actions of leptin. In chow-fed mice, conditional deletion of CB1 in VMN neurons (expressing the steroidogenic factor 1, SF1) decreases adiposity by increasing sympathetic activity and lipolysis, and facilitates metabolic effects of leptin. Conversely, under high-fat diet, lack of CB1 in VMN neurons produces leptin resistance, blunts peripheral use of lipid substrates and increases adiposity. Thus, CB1 receptors in VMN neurons provide a molecular switch adapting the organism to dietary change.
Keywords: CB1 receptor, Endocannabinoid, Ventromedial nucleus, Hypothalamus, Obesity, Leptin
Abbreviations: CB1, cannabinoid type-1; FDG, fluorodeoxyglucose; FISH, fluorescent in situ hybridization; PET, positron emission tomography; SF1, steroidogenic factor 1; vGlut2, vesicular transporter of glutamate 2 receptor; VMN, ventromedial nucleus
Graphical abstract
1. Introduction
Diet composition importantly influences metabolic responses whose dysregulation leads to obesity, diabetes and cancer [1]. But how exactly different diets modulate underlying biological functions remains unclear. In order to appropriately face the dietary change, the organism needs to regulate metabolic flexibility, which is the capacity to continuously adjust use of energy substrates (primarily glucose and fatty acids) based on energy supply and demand, and to achieve stability through physiological and behavioral adaptation to the new diet. However, these adaptive responses can lead to pathology if not adequately regulated. Consequently, a high-fat diet (HFD) will not only provide high levels of energy, but will also cause a set of allostatic modifications influencing both energy intake and storage so as to ultimately maintain the newly acquired weight. This is likely one of the reasons why fighting against obesity is a very difficult and challenging task. Thus, understanding the biological mechanisms regulating metabolic flexibility might lead to better therapeutic strategies tackling metabolic disorders.
HFD consumption plays a significant role in the development of insulin and leptin resistance in the central nervous system (CNS) [2]. In addition, recent studies have demonstrated that nutrients are not only a source of calories, but also work as intracellular signals capable of modifying the activity of specific molecular cascades [3,4]. However, little is known about the underlying CNS mechanisms that regulate metabolic flexibility in response to dietary changes.
Endocannabinoid signaling through the cannabinoid type-1 (CB1) receptor plays a key role in energy balance regulation [5,6] and, importantly, its physiological functions are influenced by the diet and especially by the consumption of fat [7,8]. Within the rodent hypothalamus, CB1 receptor mRNA is highly expressed in the ventromedial nucleus (VMN) [9,10]. This structure has been long associated with the regulation of food intake and body weight [11].
VMN neurons, particularly those expressing the steroidogenic factor 1 (SF1, also named NR5A1; [12,13]) are critically involved in the regulation of energy balance [14] and exert this function by projecting both locally, within the VMN, as well as to other hypothalamic and extra-hypothalamic sites. In particular, recent studies have shown that SF1-positive neurons project to proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (ARC) and to a number of autonomic centers, thus regulating sympathetic nervous system (SNS) outflow [15]. The adipocyte-secreted hormone leptin directly activates SF1-positive neurons in the VMN and this action is required for normal body weight regulation, since deletion of the leptin receptor in SF1 neurons favors diet-induced obesity (DIO) [16]. Additionally, the expression of the phosphatidyl inositol 3-kinase (PI3K, p110alpha), a downstream molecular target of leptin, in SF1-positive neurons is required for the anorectic action of leptin and the appropriate regulation of energy expenditure in response to an HFD [17]. Finally, selective inactivation of the suppressor of cytokine signaling 3 (SOCS3), a negative regulator of leptin signaling, in SF1 neurons facilitates leptin-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3) in this neuronal population, leading to enhanced anorectic and weight-reducing effects of exogenous leptin [18].
Intra-VMN administration of the endocannabinoid anandamide stimulates food intake in rats [19] and treatment of hypothalamic slice preparations with a specific CB1 receptor agonist inhibits the electrical activity of SF1-positive neurons [20]. However, the physiological relevance of CB1 receptors in the VMN in the regulation of metabolic homeostasis remains to be established. Here we used mice carrying a deletion of the CB1 receptor gene in SF1-positive neurons (SF1-CB1-KO) [21] and studied their behavioral and metabolic responses to a normocaloric standard chow and to a 40% HFD, respectively. These studies reveal that CB1 receptors in VMN neurons exert a diet-dependent bidirectional role in regulating energy balance and metabolic responses to leptin, thereby representing a molecular switch for correct metabolic flexibility.
2. Material and methods
2.1. Study approval
All experiments were conducted in strict compliance with the European Union recommendations (2010/63/EU) and were approved by the French Ministry of Agriculture and Fisheries (animal experimentation authorization n° 3309004) and the local ethical committee of the University of Bordeaux (project authorization n° 5012062A). Maximal efforts were made to reduce the suffering and the number of animals used.
2.2. Animals
Conditional mutant mice lacking CB1 receptor gene in SF1-positive cells (CB1SF1Cre;f/f) hereafter called SF1-CB1-KO and their SF1-Cre negative, wild-type littermates (CB1f/f), hereafter called SF1-CB1-WT, were generated, maintained and genotyped as previously described in Ref. [21]. All mice used in the study were male littermates and they were on a C57BL/6N genetic background (at least seven backcrossings). Effective Cre-mediated deletion of CB1 in the VMN was assessed by in situ hybridization (see below). 7-week-old male mice were individually housed in a thermo-regulated animal facility (22 C ± 2 °C), with a 12 h/12 h light/dark cycle (light on at 1 a.m., light off at 1 p.m.). Animals had ad libitum access to water and a standard rodent diet (Standard Rodent Diet A03, 3.2 kcal/g, SAFE, France) unless otherwise specified. For high-fat diet (HFD) studies, 7-weeks old male mice were placed on an HFD with 40% of calories from fat (40% HFD, Research Diets, 4.54 kcal/g, New Brunswick, NJ). Food intake and body weight were recorded daily. Feed efficiency was calculated as body weight gained over caloric intake × 100. At the end of the study, mice were sacrificed by cervical dislocation or anesthetized to undergo perfusion, and tissues collected for further molecular and biochemical analysis. Number of animals used for each experiment is detailed in the figures legends. Experimenters were always blind to genotypes and, when possible, to the treatments.
2.3. Body composition analysis
Assessment of lean and fat mass in 15-weeks old conscious male mice was performed using a nuclear echo magnetic resonance imaging whole-body composition analyzer (Echo MRI 900; EchoMedical Systems, Houston, TX, USA).
2.4. Food intake response to leptin
Hoppers containing chow were removed from the cages 1 h before the administration of leptin [depending on studies, 5 mg/kg or 2.5 mg/kg, ip; mouse recombinant leptin obtained from Dr. A.F. Parlow, National Hormone and Pituitary Program (Torrance, CA)] or its vehicle (phosphate buffer saline, PBS), which occurred 4 h before the onset of the dark phase, when food hoppers were returned to the cages, as in Ref. [22,23]. Food intake was recorded 1, 2 and 24 h afterwards. Body weight was measured immediately before and 24 h after the treatment. The leptin food intake studies were performed using a within subjects design in which mice received both vehicle and leptin in counterbalanced order.
2.5. Glucose tolerance test (GTT) and insulin tolerance test (ITT)
For the GTT, 16–17 weeks old male mice underwent an overnight fast and were injected ip with 2 g/kg of d-Glucose (Sigma Aldrich, St Louis, MO). For the ITT, animals underwent a 6 h fast and were injected ip with 0.5 U/kg of insulin (Humulin, Lilly, France). Blood samples were collected from the tail vein and glucose was measured using glucose sticks (OneTouch, Vita, France).
2.6. Indirect calorimetry
Indirect calorimetry, in-cage locomotor activity and gas exchange analysis were carried out in calorimetric chambers (TSE systems, Bad Homburg, Germany) after 72 h of acclimatization, as previously described in Ref. [22]. VO2 values reported in figures were expressed per animal. Unless specified, studies were carried out at 22 °C. To evaluate metabolic changes in response to cold (15 °C) or to an acute injection of leptin, recordings were respectively carried out in chow-fed male mice exposed for 24 h to 15 °C starting at the onset of the dark or in male mice maintained at 22 °C and injected with vehicle or leptin (2.5 mg/kg) in the light phase, 4 h before the onset of the dark.
2.7. Positron emission tomography (PET)
For the studies using the [18F]- fluorodeoxyglucose (FDG), chow-fed 16-week-old male SF1-CB1-KO and their WT littermates were analyzed in three different sessions. In the first session, mice were placed at 24 °C and then treated ip with a vehicle solution and a reference CT scan with PET images was recorded as in Ref. [24] to properly evaluate subsequent tracer absorption in the BAT. Scans were performed with a PET system (Explore Vista, GE). The second session begun 1 h later. Mice were placed in a cold room (6 °C) for 3 h, then slightly anesthetized with sevoflurane, injected with [18F]-FDG (15 MBq) and placed back in the cold room for 1 h to evaluate the tracer's absorption. Five days later, the experiment was repeated in the same animals treated with the β3-adrenergic receptor agonist CL 316,243 and maintained at 24 °C. [18F]-FDG accumulation data on PET images are expressed as standard absorption values (SUV), representing the radioactivity per gram of tissue, divided by the radioactivity dose injected per gram of animal.
2.8. Quantitative real time PCR (qPCR)
qPCR assays on epididymal white adipose tissue (WAT), adrenals, testis, pituitary and hypothalamus (defined caudally by the mammillary bodies, rostrally by the optic chiasm, laterally by the optic tract, and superiorly by the apex of the hypothalamic third ventricle) were performed with a LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Meylan, France). qPCR reactions and analysis were carried out as in Ref. [25]. For qPCR studies done after leptin treatment, chow-fed male mice were sacrificed 30 min after the administration of the hormone (2.5 mg/kg, ip). Primers sequences are reported in Supplementary Table 1 in the Supplementary Information. For determination of the reference genes, the GeNorm method was used. Relative expression analysis was corrected for PCR efficiency and normalized against two reference genes. The relative level of expression was calculated using the comparative (2−ΔΔCT) method.
2.9. Double fluorescent in situ hybridization (FISH)
FISH was performed as previously described in Ref. [21]. Slides were analyzed by epifluorescence microscopy (Leica, Nanterre, France).
2.10. Immunohistochemistry (IHC)
Chow-fed male SF1-CB1-KO and their WT littermates received either leptin (2.5 mg/kg, ip) or its vehicle 4 h before the onset of the dark and 1 h later were anesthetized with 0.1 ml pentobarbital and perfused transcardially with ice-cold PBS and 4% paraformaldehyde (PFA). In a different series of experiments, male SF1-CB1-KO and their WT littermates maintained on 40% HFD for 2 weeks received leptin (5 mg/kg, ip) or its vehicle as described above and 1 h later were anesthetized and perfused transcardially. Brains were collected and maintained in a 4% PFA solution for 24 h at 4 °C and subsequently transferred to a 30% sucrose solution for one week at 4 °C. Brains were then cut with a cryostat (30 μm, CM 1950, Leica) and sections stored at −20 °C in antifreeze solution until further histological processing. Sections were washed in PBS (pH = 7.4) for 1 h before being treated with a solution of 1% NaOH (1 M), 1% H2O2 in PBS for 20 min, washed in PBS, and then incubated for 10 min in a 0.3% glycine solution. Sections were then placed in a 20% SDS solution for 10 min, washed in PBS, and then incubated in 8% goat serum (Dako, Trappes, France), 0.4% Triton X 100 (Sigma Aldrich, St Louis, MO) solution for 2 h. Sections were incubated overnight at 4 °C in a solution containing rabbit serum phospho-STAT3 tyr 705 (1:500, catalog # 9131, Cell Signaling technologies, Beverly, MA), 4% goat serum and 0.4% Triton X 100. The following day, sections were washed in a 1% goat serum, 0.02% Triton X 100 solution, then incubated in a 4% goat serum solution containing 1:250 biotinylated goat anti-rabbit secondary antibody for 1 h, then washed in a 1% goat serum solution, followed by 1 h incubation in an avidin-biotin complex solution (Vectastain Elite ABC Kit, Vector laboratories, Burlingame, CA). Sections were incubated in a diaminobenzidine tetrahydrochloride (DAB; Vectastain Peroxidase Substrate Kit, Vector Laboratories) solution. Sections were finally washed, dehydrated and mounted on SuperFrost slides, then visualized at the microscope (DM5000, Leica). All sections containing the VMN were rostro-caudally (from Bregma −1.22 mm to Bregma −1.82 mm) analyzed to examine distribution of phospho-STAT3 staining. The number of phospho-STAT3 positive cells was counted by an observer blind to the genotype or treatment using a grid reticule (520 × 520 μm) under a 20× microscope objective (Leitz Aristoplan, Leitz Wetzlar, Germany).
2.11. Western blot analysis
Proteins from epididymal WAT were extracted and quantified and western blots carried out as in Ref. [26]. Membranes were incubated with the following primary antibodies: β3-adrenergic receptor (1:2000, catalog # 15688, Millipore, Molsheim, France), adipose triglyceride lipase (ATGL, 1:1000, catalog # 2439, Cell Signaling), hormone-sensitive lipase (HSL, 1:1000, catalog # 4107, Cell Signaling), phospho-HSL ser 563 (1:1000, catalog # 4139, Cell Signaling), phospho-HSL ser 660 (1:1000, catalog # 4126, Cell Signaling) and β-actin (1:4000, catalog # 4967, Cell Signaling). β-actin was used as loading control. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL plus, PerkinElmer) then exposed on radiographic films. Bands quantification was performed using ImageJ software (NIH, Bethesda, MA).
2.12. Data collection
No statistical methods were used to predetermine sample sizes, but they are similar to those reported previously [21]. In some experiments (PET analysis, leptin food intake studies and leptin effect on RQ), the same animals were also their own controls. In experiments with different treatment conditions (IHC following leptin or vehicle administration) mice were randomly assigned to the treatment.
2.13. Statistical analysis
All results are expressed as mean ± SEM. Data were analyzed using Statistica 9 software (StatSoft, USA). For multiple group designs, data were analyzed by two-way or repeated measure ANOVA followed by Fisher LSD post-hoc test. For designs with only two groups, statistical validity was assessed with unpaired t test or Mann–Whitney U test. P values less than 0.05 denote statistical significance.
3. Results
3.1. Characterization of CB1 receptor expression in SF1-CB1-KO mice
SF1-CB1-WT and SF1-CB1-KO littermates were obtained as previously described in Ref. [21]. FISH analysis revealed a specific strong decrease of CB1 receptor mRNA expression in the VMN of SF1-CB1-KO mice as compared to SF1-CB1-WT littermates (Figure 1A). Reduction in CB1 mRNA expression was present throughout the VMN, and particularly in the dorsomedial part of the nucleus, where leptin receptors are localized [27]. Importantly, mRNA expression of the vesicular transporter of glutamate 2 receptor (vGlut2) did not differ between genotypes, excluding the possibility of a defective cytoarchitecture of the VMN in SF1-CB1-KO mice (Figure 1A). As expected [16], no appreciable reduction of CB1 receptor mRNA was observed in other brain regions (data not shown).
Figure 1.
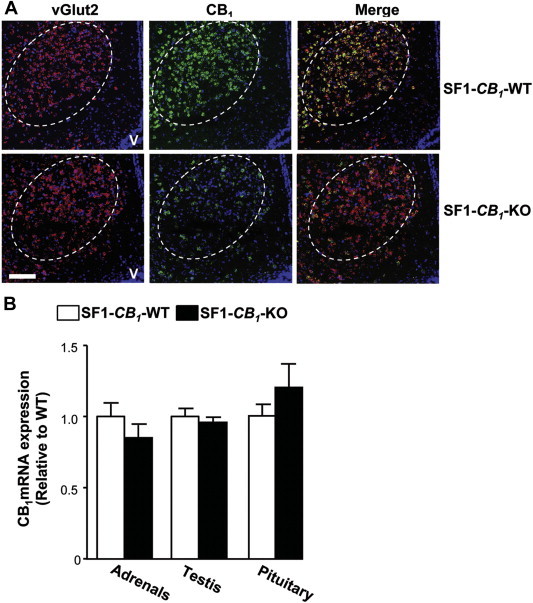
Characterization of CB1 receptor mRNA expression in SF1-CB1-KO mice. (A) Representative images taken at the level of the ventromedial nucleus of the hypothalamus from fluorescent in situ hybridization studies showing the marker of glutamatergic neurons vesicular glutamate transporter 2 (vGlut2, red) and CB1 receptor (green) mRNA expression in SF1-CB1-WT and SF1-CB1-KO mice (n = 3–4 per genotype). Nuclear counter stain carried out with DAPI. V: third ventricle. Scale bar: 400 μm. (B) CB1 receptor mRNA expression evaluated by qPCR in the adrenals, testis and pituitary of chow-fed male SF1-CB1-WT and SF1-CB1-KO littermates (n = 5). Data are represented as mean ± SEM.
SF1 is also present in the gonads, the adrenals and the pituitary [12,13], leaving open the possibility that Cre-mediated recombination of CB1 might have occurred in these organs. However, CB1 receptor mRNA expression was not altered in the testis, the adrenals or the pituitary of SF1-CB1-KO mice when compared with SF1-CB1-WT littermates (Figure 1B), suggesting that the expression of the CB1 receptor was preserved in these organs. Thus, SF1-CB1-KO mice carry a specific deletion of CB1 receptor in the VMN, thereby representing an ideal tool to study the roles of the endocannabinoid signaling in neurons located in this hypothalamic nucleus.
3.2. SF1-CB1-KO mice on standard chow are lean and have increased insulin sensitivity
As measured from 7 until 15 weeks of age, CB1 deletion in SF1-positive neurons did not affect body weight in male mice maintained on a normocaloric, standard chow (Figure 2A). Even at younger age (4 weeks old), body weight was comparable between genotypes [SF1-CB1-WT (n = 8): 11.6 ± 0.7 g vs. SF1-CB1-KO: 11.2 ± 0.7 g (n = 14), P = 0.68; t(20) = 0.4093]. However, body composition analysis carried out at 15 weeks of age revealed a significant decrease in fat mass in SF1-CB1-KO mice as compared with their WT littermates (Figure 2B), associated with a slight, non-significant increase in lean mass (Figure 2C). No differences were found in either cumulative food intake (Figure 2D) or weekly food intake during weeks 7–15 of age (SF1-CB1-WT: 5.2 ± 0.1 g vs. SF1-CB1-KO: 5.4 ± 0.1 g, n = 7, repeated measures ANOVA, genotype effect: P = 0.65; F(1) = 0.214).
Figure 2.
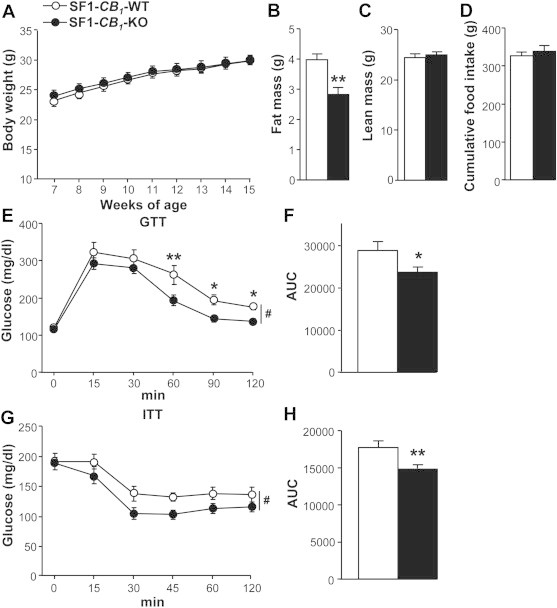
Lack of CB1 receptors in SF1-positive neurons leads to leanness and improved glucose metabolism under standard chow. (A) Weekly body weight gain, (B) fat mass, (C) lean mass and (D) cumulative food intake in chow-fed male SF1-CB1-WT and SF1-CB1-KO littermates (n = 7). (E) Glucose tolerance test, (G) insulin tolerance test and (F, H) related area under the curve (AUC) carried out in chow-fed male SF1-CB1-WT and SF1-CB1-KO mice (n = 7–9). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; #P < 0.05 genotype effect.
The amount of adiposity plays a role in glucose homeostasis and insulin sensitivity [28]. Having found a decrease in fat mass in chow-fed SF1-CB1-KO mice, we studied glucose responses to glucose- or insulin-tolerance test (GTT or ITT, respectively). While fasting glucose levels were not different between genotypes (Figure 2E, values at time 0), SF1-CB1-KO mice had better glucose tolerance (Figure 2E,F) and increased insulin sensitivity (Figure 2G,H) as compared with their WT littermates.
Thus, in chow, deletion of CB1 receptors in VMN neurons decreases adiposity and improves glucose metabolism.
3.3. CB1 receptors in the VMN regulate SNS activity, lipid oxidation and WAT lipolysis under standard chow
Since there were no obvious alterations in food intake that could explain the decreased adiposity of SF1-CB1-KO mice, we assessed whether this phenomenon could be due to changes in locomotor activity or in the use of energy substrates. Chow-fed SF1-CB1-KO and WT littermates had similar activity (Figure 3A) and VO2 consumption (Figure 3B) at 22 °C ambient temperature, but SF1-CB1-KO mice showed decreased respiratory quotient (RQ, Figure 3C), suggesting a higher consumption rate of lipids as energy source.
Figure 3.
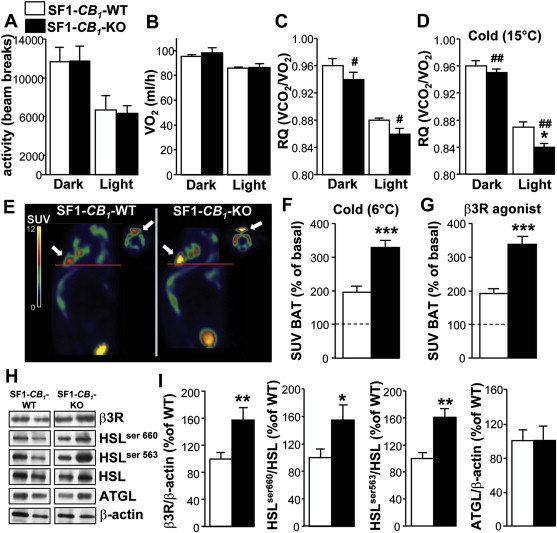
CB1 receptors in SF1-positive neurons regulate SNS activity, lipid oxidation and WAT lipolysis. (A) In-cage locomotor activity, (B) VO2 consumption and (C) respiratory quotient (RQ) during the dark and light phases determined in 15-weeks old chow-fed male SF1-CB1-WT and SF1-CB1-KO mice at 22 °C ambient temperature (n = 5–6). (d) RQ during the dark and light phases assessed in 15-weeks old chow-fed male SF1-CB1-WT and SF1-CB1-KO mice at 15 °C ambient temperature (n = 6). (E) Representative sagittal (main figure) and transverse (smaller insets) PET images showing 18F-FDG accumulation expressed as standard absorption values (SUV) in the BAT of chow-fed male SF1-CB1-WT and SF1-CB1-KO mice. Red lines indicate the image sections reported in the transverse views; images were from the study with the β3R agonist CL 316,243. SUV quantification after (F) 4 h exposure to 6 °C or (G) treatment with β3R agonist of chow-fed male SF1-CB1-WT and SF1-CB1-KO mice expressed as % of basal non-stimulated condition in the same animals (n = 6). (H) Representative western blot scans and (I) quantification of β3R, phospho-HSL ser 660, phospho-HSL ser 563, HSL and ATGL protein expression in the WAT of chow-fed male SF1-CB1-WT and SF1-CB1-KO mice maintained at 22 °C (n = 6–11; β-actin: loading control). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.05 and ##P < 0.01 genotype effect.
Activation of the SNS favors the oxidation of lipids rather than carbohydrates and induces lipolysis in the white adipose tissue (WAT) [29,30]. Several SNS functions are negatively affected by CB1 receptor signaling [21,31], whilst excitation of neurons of the VMN, particularly those located in the dorsomedial part of the nucleus, induces SNS activity [15,32]. To assess the impact of VMN CB1 receptors on SNS activity under chow, we physiologically and pharmacologically induced SNS activity, by exposing SF1-CB1-KO and their SF1-CB1-WT littermates to cold or by administering a β3-adrenergic receptor agonist, respectively. Changes in in vivo lipid oxidation and in the metabolic activity of the brown adipose tissue (BAT) were used as an indirect measure of SNS activity.
RQ differences between chow-fed SF1-CB1-KO and SF1-CB1-WT littermates were maintained when mice were exposed to 15 °C ambient temperature for 24 h and became particularly evident during the light phase of the diurnal cycle (Figure 3D). Additionally, positron emission tomography (PET) analysis showed that BAT 18F-FDG accumulation was greater in SF1-CB1-KO than in their SF1-CB1-WT littermates following short-term (4 h) exposure to 6 °C or the acute administration of the β3-adrenergic receptor agonist CL 316,223 (Figure 3E–G). These data thus strongly suggest that VMN CB1 receptors control SNS activity.
To then determine whether the enhanced SNS activity found in SF1-CB1-KO mice was associated with increased lipolysis, we studied the expression of the β3-adrenergic receptor and the activity of lipolytic markers in the epididymal WAT. As compared with SF1-CB1-WT littermates, SF1-CB1-KO mice had increased β3-adrenergic receptor protein expression in the WAT (Figure 3H,I). This was associated with increased phosphorylation of the lipolytic enzyme hormone-sensitive lipase (HSL) on ser 660 and 563 (Figure 3H,I), two phosphorylation sites induced by the activation of β3-adrenergic receptor in the WAT [33]. Conversely, protein expression of the adipose triglyceride lipase (ATGL), another enzyme participating in lipolysis [34], was similar between genotypes (Figure 3H,I).
3.4. CB1 receptors in the VMN determine the ability of leptin to decrease food intake and induce lipolysis in the WAT under standard chow
A close relationship exists between leptin and CB1 receptor signaling in the regulation of energy balance [5]. Knowing that SF1-positive neurons critically mediate the actions of leptin on metabolism [16], we investigated the effects of the hormone in chow-fed SF1-CB1-KO and SF1-CB1-WT littermates.
Hypothalamic mRNA expression of the leptin receptor and of its downstream targets STAT3 and SOCS3 [35] was similar between chow-fed SF1-CB1-WT and SF1-CB1-KO mice (Supplementary Figure 1), and both genotypes responded to the dose of 5 mg/kg of leptin with a comparable 24 h decrease in food intake [SF1-CB1-WT (n = 9): 25.2 ± 5.9% FI decrease vs. SF1-CB1-KO (n = 8): 25.5 ± 2.6% FI decrease, P = 0.96; t(15) = 0.0474]. However, the administration of a lower dose of the hormone (2.5 mg/kg) decreased 24 h food intake in SF1-CB1-KO mice only (Figure 4A) and led to a strong increase in STAT3 phosphorylation in the dorsomedial part of the VMN of SF1-CB1-KO but not SF1-CB1-WT mice (Figure 4B,C). Whilst no phospho-STAT3 labeling was found in the VMN of vehicle-treated mice (Figure 4B,C). Moreover, at metabolic level, leptin at 2.5 mg/kg rapidly increased in vivo lipid oxidation in SF1-CB1-KO but not in SF1-CB1-WT littermates (Figure 4D).
Figure 4.
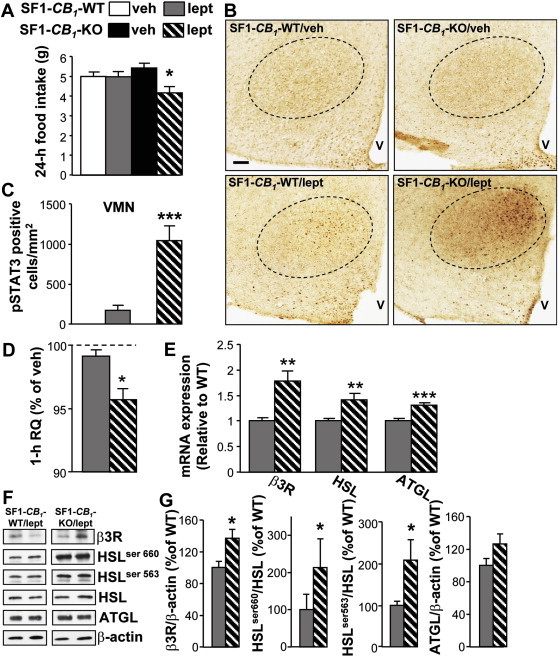
Leptin actions on food intake and WAT lipolysis depend upon CB1 receptors in SF1-positive neurons. (A) 24 h food intake of chow-fed male SF1-CB1-WT and SF1-CB1-KO mice treated with leptin (2.5 mg/kg) or vehicle (n = 5–7). (B) Representative staining for phospho-STAT3 (dark brown nuclear dots) in the VMN (dotted line) of chow-fed male SF1-CB1-WT and SF1-CB1-KO mice treated with leptin (2.5 mg/kg) or vehicle and (C) related quantification in the VMN (n = 4). Scale bar in (A): 50 μm. (D) 1 h RQ changes recorded during the light phase in chow-fed male SF1-CB1-WT and SF1-CB1-KO mice in response to leptin (2.5 mg/kg) (n = 5). Data are expressed as % of change vs. vehicle administration carried out in the same animals the day before. (E) mRNA expression levels of β3R, HSL and ATGL assessed 30 min after leptin administration (2.5 mg/kg) in chow-fed male SF1-CB1-WT and SF1-CB1-KO mice (n = 5–6). (F) Representative western blot scans and (G) quantification of β3R, phospho-HSL ser 660, phospho-HSL ser 563, HSL and ATGL protein expression in the WAT of leptin-treated (2.5 mg/kg) chow-fed male SF1-CB1-WT and SF1-CB1-KO mice (n = 6–7; β-actin: loading control). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001.
Leptin is known to activate neuronal circuits in the medio-basal hypothalamus leading to increased SNS outflow to the WAT [36,37] and to stimulate HSL in this tissue [38]. Analysis of the expression of key lipolytic genes in the WAT of chow-fed SF1-CB1-KO and SF1-CB1-WT littermates after the administration of leptin at 2.5 mg/kg showed increased mRNA expression of the β3-adrenergic receptor, HSL, and ATGL in SF1-CB1-KO only (Figure 4E). Western blot studies confirmed that the acute administration of the hormone significantly increased protein expression of the β3-adrenergic receptor and induced HSL phosphorylation on ser 660 and 563 in the WAT of SF1-CB1-KO mice as compared with SF1-CB1-WT littermates (Figure 4F,G).
Overall, these findings imply that CB1 receptors in VMN neurons specifically modulate the sensitivity of the VMN to the action of leptin and determine leptin's ability to decrease food intake and to stimulate SNS activity and lipolysis in the WAT.
3.5. SF1-CB1-KO mice are obesity-prone when chronically exposed to an HFD
Deletion of the CB1 gene, either complete or limited to the principal forebrain neurons and SNS, protects from DIO [31,39]. SF1-positive neurons in the VMN play a role in the response to DIO [16,18,40]. In particular, deletion of the leptin receptor in these neurons favors body weight gain during exposure to an HFD [16]. To evaluate the specific role of CB1 receptors in the VMN in DIO, male SF1-CB1-KO and SF1-CB1-WT littermates were maintained for 8 weeks on a mild HFD (40% of calories from fat). Surprisingly, SF1-CB1-KO mice gained significantly more weight than their SF1-CB1-WT controls (Figure 5A), resulting in a significant increase in fat mass (Figure 5B), while lean mass was similar between genotypes (Figure 5C). To determine whether the increased adiposity had a detrimental effect on glucose metabolism, GTT and ITT were carried out. HFD-fed SF1-CB1-KO mice showed an overall worsened glucose tolerance in response to the acute administration of glucose during the GTT (Figure 5D), whilst no differences were observed between genotypes in the response to the ITT (Figure 5E). Thus, despite the mild lean phenotype of mutant mice under chow, SF1-CB1-KO mice are paradoxically more prone to the deleterious effects of an HFD.
Figure 5.
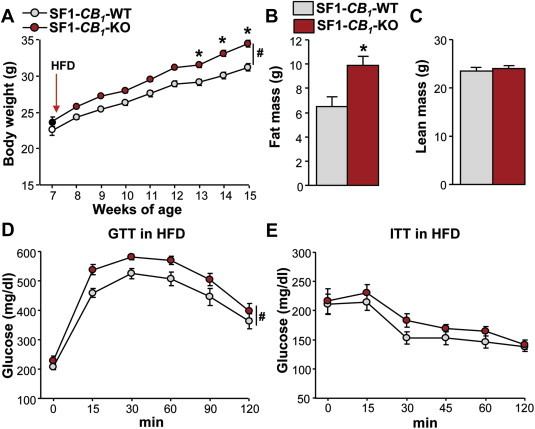
SF1-CB1-KO mice are obesity-prone when chronically exposed to a mild HFD. (A) Weekly body weight gain, (B) fat mass and (C) lean mass in male SF1-CB1-WT and SF1-CB1-KO littermates maintained on HFD for 8 weeks (n = 6–10). (D) Glucose tolerance test and (E) insulin tolerance test carried out in male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 10–14). Data are represented as mean ± SEM. *P < 0.05; #P < 0.05 genotype effect.
3.6. SF1-CB1-KO mice are hyperphagic and have reduced lipid oxidation and lipolysis when chronically exposed to an HFD diet
We then investigated the possible causes of the SF1-CB1-KO obese phenotype. HFD-fed SF1-CB1-KO mice had a significant increase in cumulative food intake as compared with HFD-fed SF1-CB1-WT littermates (Figure 6A). This increase was the result of a slight increase in daily food intake, present during both the dark and the light phase of the diurnal cycle (data not shown). We therefore went on to assess the mRNA expression levels of several hypothalamic neuropeptides involved in the regulation of food intake and found that only POMC mRNA expression was significantly decreased in the hypothalamus of obese SF1-CB1-KO mice (Figure 6B).
Figure 6.
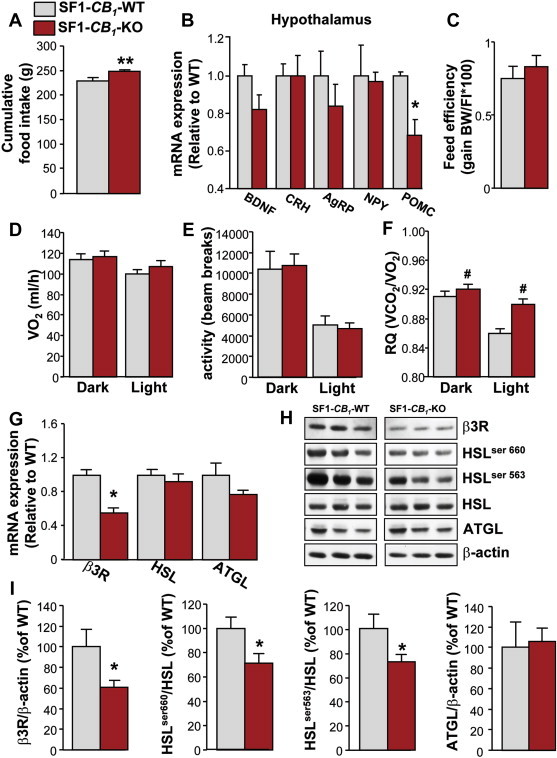
SF1-CB1-KO mice are hyperphagic and have reduced lipid oxidation and lipolysis when chronically exposed to HFD. (A) Cumulative food intake of male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 6–10). (B) mRNA expression levels of BDNF, CRH, AgRP, NPY and POMC in the hypothalamus of male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 5–9). (C) Feed efficiency expressed as body weight gain over caloric intake × 100 in male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 6–10). (D) VO2 consumption, (E) in-cage locomotor activity and (F) RQ during the dark and light phases determined at 22 °C ambient temperature in 15-weeks old male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 7–12). (G) mRNA expression levels of β3R, HSL and ATGL in the WAT of male SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 6). (H) Representative western blot scans and (I) quantification of β3R, phospho-HSL ser 660, phospho-HSL ser 563, HSL and ATGL protein expression in the WAT of male WT and SF1-CB1-KO mice maintained on HFD for 8 weeks (n = 7–10; β-actin: loading control). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; #P < 0.05 genotype effect.
To further determine whether mechanisms other than the observed hyperphagia might have participated to the SF1-CB1-KO obese-prone phenotype, we studied changes in energy expenditure, locomotor activity and use of fuel substrates. No significant differences in feed efficiency or VO2 consumption were observed between obese SF1-CB1-KO mice and SF1-CB1-WT littermates (Figure 6C,D). In-cage activity was also similar between genotypes (Figure 6E). However, use of substrates was different, with HFD-fed SF1-CB1-KO mice having a significant decrease in lipid oxidation, as revealed by the increased RQ (Figure 6F), possibly favoring fat accumulation. Accordingly, SF1-CB1-KO mice had an almost 50% decrease in the mRNA expression of the β3-adrenergic receptor in the WAT as compared with control littermates (Figure 6G). Subsequent protein analysis confirmed the significant reduction in β3-adrenergic receptor in the WAT of HFD-fed SF1-CB1-KO mice only (Figure 6H,I), together with a decreased HSL phosphorylation at ser 660 and ser 563 (Figure 6H,I).
Thus, during chronic HFD exposure, lack of CB1 receptors in SF1-positive neurons leads to body weight gain and obesity by inducing hyperphagia and decreased use of lipid substrates, likely through decreased SNS outflow to the WAT.
3.7. SF1-CB1-KO mice develop impaired VMN leptin signaling when consuming an HFD
Resistance to the appetite-suppressant action of leptin and decreased hypothalamic leptin signaling are features commonly associated with DIO. However, once obesity is established it is difficult to determine whether hypothalamic alterations in leptin signaling are the cause or the consequence of the increased adiposity [41]. To determine whether decreased leptin signaling might have a causal role in the SF1-CB1-KO obese-prone phenotype described during chronic HFD exposure, we investigated leptin-induced STAT3 phosphorylation in the VMN of SF1-CB1-WT and SF1-CB1-KO mice maintained on HFD for only 2 weeks before the acute administration of leptin (5 mg/kg). Animals were matched by body weight (SF1-CB1-WT, 29.1 ± 1 g vs. SF1-CB1-KO, 28.8 ± 2.1 g, n = 4; P = 0.90, t(6) = 0.1279) and by body composition (fat mass: SF1-CB1-WT, 5.1 ± 0.5 g vs. SF1-CB1-KO, 5.5 ± 0.8 g; P = 0.68, t(6) = 0.4267; lean mass: SF1-CB1-WT, 23.8 ± 0.7 g vs. SF1-CB1-KO, 23.2 ± 1.2 g, n = 4; P = 0.65, t(6) = 0.4636) in order to study the action of leptin in the absence of any possible confounding effect due to differences in adiposity. Under these conditions, SF1-CB1-KO mice displayed a significantly decreased leptin-induced phospho-STAT3 response in the VMN as compared with SF1-CB1-WT littermates (Figure 7A,B). Thus, exposure to HFD blunts molecular leptin sensitivity in the VMN of SF1-CB1-KO before they overtly gain weight.
Figure 7.
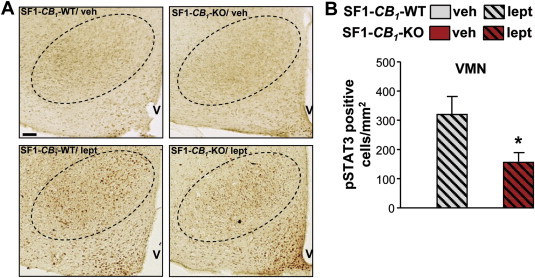
SF1-CB1-KO mice have impaired VMN leptin signaling in response to HFD consumption. (A) Representative staining for phospho-STAT3 (dark brown nuclear dots) in the VMN (dotted line) of male SF1-CB1-WT and SF1-CB1-KO mice fed with HFD for 2 weeks and then treated with leptin (5 mg/kg) or its vehicle; (B) quantification of phospho-STAT3 staining in the VMN (n = 4). Scale bar in (A): 50 μm. Data are represented as mean ± SEM. *P < 0.05.
4. Discussion
What we eat critically influences the biological mechanisms regulating energy balance. Besides obviously providing different amounts of energy, diets with different nutrient composition affect a plethora of metabolic responses, and specific nutrients can work as signals able to modulate well-identified intracellular pathways [3,4]. However, less is known about how diet-induced metabolic changes are integrated and coordinated at the CNS level.
The present study demonstrates the critical role of CB1 receptors in the VMN in regulating metabolic flexibility and in determining behavioral and metabolic responses to leptin.
During consumption of a standard chow, lack of CB1 in SF1-positive neurons heightens SNS outflow and lipolysis, thus decreasing adiposity, while improving glucose tolerance and insulin sensitivity. Conversely, during exposure to an HFD, deletion of the CB1 gene from the VMN blunts peripheral use of lipid substrates and causes hyperphagia, body weight gain and glucose intolerance. Importantly, the diet-dependent, bidirectional regulation of energy balance observed when CB1 is deleted from VMN neurons is closely intertwined with the effects of leptin. Thus, an enhanced sensitivity to the anorectic and metabolic action of the hormone is observed in mutant mice under chow, whereas lack of CB1 in the VMN of animals fed with an HFD for only 2 weeks determines the appearance of molecular leptin resistance in the VMN.
When exposed to a diet with a prevalent carbohydrate content, such as the regular chow routinely used in animal studies, SF1-CB1-KO mice did not show changes in body weight or food intake, but had a small, significant decrease in fat mass. This was associated with improved peripheral glucose metabolism and resulted from enhanced SNS activity, leading to increased metabolism in the BAT, lipolysis in the WAT and in vivo lipid oxidation. Overall, these findings demonstrate that CB1 receptors in the VMN affect peripheral metabolic responses by modulating VMN-SNS outflow.
Studies in the ‘80s had initially proposed that VMN neurons exert their metabolic functions on the periphery by modulating the activity of the SNS [42,43]. For instance, VMN lesions reduce SNS outflow in a number of tissues, including the WAT and the BAT [42,43]. Conversely, administration of leptin directly into the VMN produces elevations of plasma epinephrine and norepinephrine [44]. However, although evidence has clearly suggested that excitation of VMN neurons evokes SNS outflow, the anatomical and mechanistic bases underlying VMN-mediated autonomic functions have remained for a long time unclear. Only very recent studies have finally demonstrated the neuroanatomical connection between the VMN and the SNS. In particular, it has been shown that SF1-positive neurons of the dorsomedial subdivision of the VMN project to autonomic centers of both the hypothalamus and hindbrain [15]. Taking into account that SF1-positive neurons are largely glutamatergic and that CB1 receptor agonists inhibit the firing of action potentials of SF1-positive neurons [20], deletion of CB1 on this neuronal population is expected to facilitate SF1-positive neurons activation and to increase SNS activity. Our in vivo and ex-vivo findings fully support this conclusion.
The relationship between leptin and hypothalamic endocannabinoid signaling was established more than a decade ago [45], but the functional consequences of this interaction have been only partially addressed [22,46,47]. SF1-positive neurons express leptin receptors [27] and are the target of the action of leptin [16]. Our data now demonstrate that leptin's ability to regulate food intake and peripheral lipid metabolism depend upon CB1 receptors expressed on SF1-positive neurons. In particular leptin engages, under the modulatory control of endocannabinoid signaling, the above-mentioned VMN-SNS output to exert its metabolic actions. In 2008, Buettner and colleagues demonstrated that direct administration of leptin in the rat medio-basal hypothalamus stimulated WAT lipolysis by increasing SNS outflow [36]. However, that study did not provide any information about the exact neuronal population or the cellular signaling pathways involved in determining the effects of leptin on the WAT. Our present findings reveal that such population is the SF1-positive neurons, which regulate SNS outflow via endocannabinoid signaling.
Our data also reveal that lack of CB1 in SF1-positive neurons facilitates molecular leptin sensitivity specifically within the VMN, as assessed by leptin-induced phospho-STAT3 expression, the gold standard method used to study hypothalamic leptin signaling [41]. This finding could be explained by taking into account that SF1-positive neurons form a local circuit with extensive axonal fibers and terminals particularly in the dorsomedial part of the VMN [15]. Thus, this local circuit is the likely anatomical substrate used by the endocannabinoid signaling to fine-tune VMN responses to leptin.
As opposed to what was observed in chow, when SF1-CB1-KO mice were chronically exposed to a mild HFD, they gained more weight and fat mass than their WT littermates. This was due to the presence of hyperphagia, likely due to decreased hypothalamic expression of POMC. This molecular change is of interest, particularly considering that VMN neurons provide excitatory input to POMC neurons in the ARC, thus facilitating the activation of this anorexigenic neuronal pathway [48].
Moreover, obese SF1-CB1-KO mice showed decreased in vivo lipid oxidation and lipolysis in the WAT. These metabolic changes were related to reduced SNS outflow, as suggested by the decreased activity of SNS-dependent lipolytic markers and β3-adrenergic receptor expression in the WAT. Interestingly, long-term (over 10 years) changes in body weight in humans are inversely correlated with β3-adrenergic receptor induced lipolysis in white adipocytes, so that subjects with low basal β3-adrenergic receptor function gain weight, while those with high β3-adrenergic receptor function are protected from weight gain, implying a critical role for adipocyte β3-adrenergic receptor activity in body weight regulation [49].
Leptin receptor signaling, as assessed by STAT3 phosphorylation, was impaired in the VMN of SF1-CB1-KO mice consuming an HFD, even before the occurrence of changes in body weight and adiposity, thus suggesting that CB1 receptors on VMN neurons may actually protect from the development of HFD-induced leptin resistance. Moreover, in view of the link among central leptin action, modulation of SNS outflow and WAT lipolysis [36], the present findings hint that decreased WAT β3-adrenergic receptor expression and activity found in obese SF1-CB1-KO mice might be the result of the decreased leptin signaling described in the VMN of HFD-fed SF1-CB1-KO mice before they phenotypically differ from HFD-fed SF1-CB1-WT.
The molecular, behavioral and metabolic changes found in SF1-CB1-KO mice on HFD, are likely the result of blunted activity of VMN neurons projecting to autonomic centers regulating the SNS, to the ARC and to the VMN itself. Thus, while in chow endocannabinoid signaling on VMN neurons favors body weight gain, in HFD it protects from DIO. The reason for this opposite, diet-dependent effect may reside in the ability of nutrients to directly affect both VMN neurons activity and endocannabinoid signaling. Indeed, VMN neurons use both glucose and long-chain fatty acids as signaling molecules to alter their activity [50], whilst endocannabinoid-mediated neuronal function and plasticity are affected by dietary fat content [8].
Lastly, future studies will have to clarify the molecular cascade downstream the CB1 receptor. Recent evidence suggests that modulation of the cellular fuel sensor AMP-activated protein kinase (AMPK) directly in the VMN regulates SNS outflow and BAT function [51]. Cannabinoids stimulate AMPK activity in the hypothalamus [52]. Thus, AMPK may be a likely downstream target of CB1 receptors in VMN neurons.
Taken together, these data demonstrate that CB1 receptors in the VMN determine metabolic and molecular adaptations to different environmental dietary conditions. In order to do so, CB1 receptors in VMN neurons orchestrate peripheral use of substrates and fine-tune sensitivity (or resistance) to the actions of leptin.
Acknowledgments
We thank the personnel of the animal and genotyping facilities of the NeuroCentre Magendie for mouse caring and genotyping.
Supported by INSERM/AVENIR (D.C., G.M.), INSERM/interface (D.C.), Aquitaine Region (D.C., G.M.), European Foundation for the Study of Diabetes-Sanofi Aventis (D.C., G.M.), Labex BRAIN ANR-10-LABX-43 (D.C.), ANR-10-EQPX-08 OPTOPATH (D.C.), ANR Blanc ANR-2010-1414-01 (D.C.), Ajinomoto 3ARP programme (D.C.), INSERM/Aquitaine Region PhD fellowship (P.C.), EU-FP7 Marie Curie IRG n°224757 (D.C.), EU-FP7 HEALTH-F2-2008-223713 Reprobesity (G.M., U.P.), EU-FP7 ENDOFOOD ERC-2010-StG (G.M.) and Fondation Recherche Medicale (M.M. and G.M.).
Conflict of interest
The authors declare no competing financial interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Popkin B.M. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nature Reviews Cancer. 2007;7(1):61–67. doi: 10.1038/nrc2029. [DOI] [PubMed] [Google Scholar]
- 2.Ryan K.K., Woods S.C., Seeley R.J. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metabolism. 2012;15(2):137–149. doi: 10.1016/j.cmet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cota D., Proulx K., Seeley R.J. The role of CNS fuel sensing in energy and glucose regulation. Gastroenterology. 2007;132(6):2158–2168. doi: 10.1053/j.gastro.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Ryan K.K., Seeley R.J. Physiology. Food as a hormone. Science. 2013;339(6122):918–919. doi: 10.1126/science.1234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez-Silva F.J., Cardinal P., Cota D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. Journal of Psychopharmacology. 2012;26(1):114–124. doi: 10.1177/0269881111408458. [DOI] [PubMed] [Google Scholar]
- 6.Quarta C., Mazza R., Obici S., Pasquali R., Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends in Molecular Medicine. 2011;17(9):518–526. doi: 10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.DiPatrizio N.V., Astarita G., Schwartz G., Li X., Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafourcade M., Larrieu T., Mato S., Duffaud A., Sepers M., Matias I. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nature Neuroscience. 2011;14(3):345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 9.Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsicano G., Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. European Journal of Neuroscience. 1999;11(12):4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 11.King B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology & Behavior. 2006;87(2):221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda Y., Shen W.H., Ingraham H.A., Parker K.L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular Endocrinology. 1994;8(5):654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda Y., Luo X., Abbud R., Nilson J.H., Parker K.L. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Molecular Endocrinology. 1995;9(4):478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 14.Majdic G., Young M., Gomez-Sanchez E., Anderson P., Szczepaniak L.S., Dobbins R.L. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143(2):607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg D., Chen P., Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. Journal of Comparative Neurology. 2013;521(14):3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Hill J.W., Fukuda M., Gautron L., Sohn J.W., Kim K.W. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metabolism. 2010;12(1):88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R., Dhillon H., Yin H., Yoshimura A., Lowell B.B., Maratos-Flier E. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149(11):5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamshidi N., Taylor D.A. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. British Journal of Pharmacology. 2001;134(6):1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.W., Jo Y.H., Zhao L., Stallings N.R., Chua S.C., Jr., Parker K.L. Steroidogenic factor 1 regulates expression of the cannabinoid receptor 1 in the ventromedial hypothalamic nucleus. Molecular Endocrinology. 2008;22(8):1950–1961. doi: 10.1210/me.2008-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellocchio L., Soria-Gómez E., Quarta C., Metna-Laurent M., Cardinal P., Binder E. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardinal P., Bellocchio L., Clark S., Cannich A., Klugmann M., Lutz B. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153(9):4136–4143. doi: 10.1210/en.2012-1405. [DOI] [PubMed] [Google Scholar]
- 23.Cota D., Matter E.K., Woods S.C., Seeley R.J. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. Journal of Neuroscience. 2008;28(28):7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galie M., Farace P., Nanni C., Spinelli A., Nicolato E., Boschi F. Epithelial and mesenchymal tumor compartments exhibit in vivo complementary patterns of vascular perfusion and glucose metabolism. Neoplasia. 2007;9(11):900–908. doi: 10.1593/neo.07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder E., Bermúdez-Silva F.J., Elie M., Leste-Lasserre T., Belluomo I., Clark S. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity (Silver Spring) 2013;22(3):713–720. doi: 10.1002/oby.20578. [DOI] [PubMed] [Google Scholar]
- 26.Proulx K., Cota D., Woods S.C., Seeley R.J. Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes. 2008;57(12):3231–3238. doi: 10.2337/db07-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M. Leptin targets in the mouse brain. Journal of Comparative Neurology. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn S.E., Prigeon R.L., Schwartz R.S., Fujimoto W.Y., Knopp R.H., Brunzell J.D. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. Journal of Nutrition. 2001;131(2):354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- 29.Bartness T.J., Shrestha Y.B., Vaughan C.H., Schwartz G.J., Song C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and Cellular Endocrinology. 2010;318(1–2):34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueiras R., Lopez M., Dieguez C. Regulation of lipid metabolism by energy availability: a role for the central nervous system. Obesity Reviews. 2010;11(3):185–201. doi: 10.1111/j.1467-789X.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 31.Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L.J. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metabolism. 2010;11(4):273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Ruffin M., Nicolaidis S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Research. 1999;846(1):23–29. doi: 10.1016/s0006-8993(99)01922-8. [DOI] [PubMed] [Google Scholar]
- 33.Anthonsen M.W., Rönnstrand L., Wernstedt C., Degerman E., Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. The Journal of Biological Chemistry. 1998;273(1):215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 35.Myers M.G., Cowley M.A., Munzberg H. Mechanisms of leptin action and leptin resistance. Annual Review of Physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 36.Buettner C., Muse E.D., Cheng A., Chen L., Scherer T., Pocai A. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nature Medicine. 2008;14(6):667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherer T., Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Reviews in Endocrine and Metabolic Disorders. 2011;12(3):235–243. doi: 10.1007/s11154-011-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm C., Osterlund T., Laurell H., Contreras J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annual Review of Nutrition. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 39.Ravinet Trillou C., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. International Journal of Obesity and Related Metabolic Disorders. 2004;28(4):640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 40.Kim K.W., Zhao L., Donato J., Jr., Kohno D., Xu Y., Elias C.F. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers M.G., Jr., Leibel R.L., Seeley R.J., Schwartz M.W. Obesity and leptin resistance: distinguishing cause from effect. Trends in Endocrinology & Metabolism. 2010;21(11):643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi T., Bray G.A., Eddlestone G. Sympathetic activity following paraventricular or ventromedial hypothalamic lesions in rats. Brain Research Bulletin. 1988;20(4):461–465. doi: 10.1016/0361-9230(88)90135-9. [DOI] [PubMed] [Google Scholar]
- 43.Vander Tuig J.G., Knehans A.W., Romsos D.R. Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sciences. 1982;30(11):913–920. doi: 10.1016/0024-3205(82)90619-1. [DOI] [PubMed] [Google Scholar]
- 44.Satoh N., Ogawa Y., Katsuura G., Numata Y., Tsuji T., Hayase M. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 45.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 46.Jo Y.H., Chen Y.J., Chua S.C., Jr., Talmage D.A., Role L.W. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48(6):1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malcher-Lopes R., Di S., Marcheselli V.S., Weng F.J., Stuart C.T., Bazan N.G. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. Journal of Neuroscience. 2006;26(24):6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sternson S.M., Shepherd G.M., Friedman J.M. Topographic mapping of VMH--> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience. 2005;8(10):1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 49.Andersson D., Wahrenberg H., Lofgren P. Beta3-adrenoceptor function and long-term changes in body weight. International Journal of Obesity (London) 2009;33(6):662–668. doi: 10.1038/ijo.2009.54. [DOI] [PubMed] [Google Scholar]
- 50.Le Foll C., Irani B.G., Magnan C., Dunn-Meynell A.A., Levin B.E. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. The American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2009;297(3):R655–R664. doi: 10.1152/ajpregu.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López M., Varela L., Vázquez M.J., Rodríguez-Cuenca S., González C.R., Velagapudi V.R. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nature Medicine. 2010;16(9):1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kola B., Hubina E., Tucci S.A., Kirkham T.C., Garcia E.A., Mitchell S.E. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. The Journal of Biological Chemistry. 2005;280(26):25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


