Abstract
The transcription factor FoxO1 regulates multiple physiological processes. Here, we show that FoxO1 is highly expressed in neurons of the locus coeruleus and of various sympathetic ganglions, but not in the adrenal medulla. Consistent with this pattern of expression, mice lacking FoxO1 only in sympathetic neurons (FoxO1Dbh−/−) display a low sympathetic tone without modification of the catecholamine content in the adrenal medulla. As a result, FoxO1Dbh−/− mice demonstrate an increased insulin secretion, improved glucose tolerance, low energy expenditure, and high bone mass. FoxO1 favors catecholamine synthesis because it is a potent regulator of the expression of Dbh that encodes the initial and rate-limiting enzyme in the synthesis of these neurotransmitters. By identifying FoxO1 as a transcriptional regulator of the sympathetic tone, these results advance our understanding of the control of some aspects of metabolism and of bone mass accrual.
Keywords: FoxO1, Locus coeruleus, Sympathetic tone
1. Introduction
In the last 15 years, the interactions taking place between bone and other organs, most notably the brain, have become a topic of growing interest [1]. One reason for this interest is that the study of these interactions has provided a better understanding of the regulation of bone mass accrual and energy metabolism [2–4]. An obvious question raised by the cross-talk between bone and the brain has been to decipher its molecular underpinning. This has been achieved in part for sympathetic signaling in osteoblasts [5,6], but only begins to be done for the central control of bone mass [7,8].
Biochemical and molecular studies conducted in cells have shown that the transcription factor FoxO1 often lies downstream of AKT and that phosphorylation of FoxO1 by AKT hampers its nuclear translocation and therefore its transactivation activity. This cascade of molecular events explains why for instance FoxO1 plays a critical role downstream of insulin signaling in various cell types [9,10]. This work raises the prospect that FoxO1 lies downstream of other hormones and growth factors that may signal, in cell types where FoxO1 is expressed, through a receptor tyrosine kinase.
Along these lines, we recently provided indirect evidence suggesting that the positive regulation of bone mass accrual that the hormone adiponectin exerts by signaling in neurons of the sympathetic system may rely on the activity of FoxO1 in these neurons [11]. If this were the case, then one would expect that FoxO1 could be a long sought after transcriptional regulator of the synthesis of catecholamines through molecular mechanisms that would need to be deciphered.
In an effort to determine if it is the case, we first performed a systematic analysis of FoxO1 expression in catecholamine-producing cells. We then used a cell-specific gene deletion approach and generated mice lacking FoxO1 only in Dbh-expressing cells which are responsible for catecholamine production (FoxO1Dbh−/−). We show here that, among all catecholamine-producing cell types, FoxO1 expression is detected in neurons of the locus coeruleus, of the dorsal root ganglion, and of the superior cervical ganglion, but not in the postsynamptic ganglion in the ileum, or in the adrenal medulla. FoxO1Dbh−/− mice are characterized by a marked decrease in the activity of the sympathetic tone as determined by catecholamines production, Ucp1 expression in brown fat, low energy expenditure, increased insulin secretion, and high bone mass. Additional experiments provide molecular and genetic evidences that this is explained by the fact that FoxO1 regulates the expression of Dbh, the gene encoding the initial and rate-limiting enzyme in the biosynthesis of catecholamines. This study identifies a novel function and mechanism of action for FoxO1 and in so doing enhances our molecular understanding of the central control of bone mass and energy metabolism.
2. Material and method
2.1. Mice
FoxO1Dbh−/− mice were generated by crossing FoxO1fl/+ mice with FoxO1fl/+; Dbh-cre mice as previously described in Refs. [11–13]. For all the analyses, C57BL/6J background, 9 month-old male mice were used except for deletion specificity, which was done in 9 month-old male and female FoxO1Dbh−/− mice (Figure 1C and Supplemental Figure 1B), and FoxO1 and Dbh expression, which was done in 6 week-old and P10 male mice for locus coeruleus, and 6 week-old male mice for adrenal gland, the dorsal root ganglion, superior cervical ganglion, and ileum (Figures 1A, D, and 2E and Supplemental Figures 1A and 2). All procedures involving animals were approved by CUMC IACUC and conform to the relevant regulatory standards.
Figure 1.
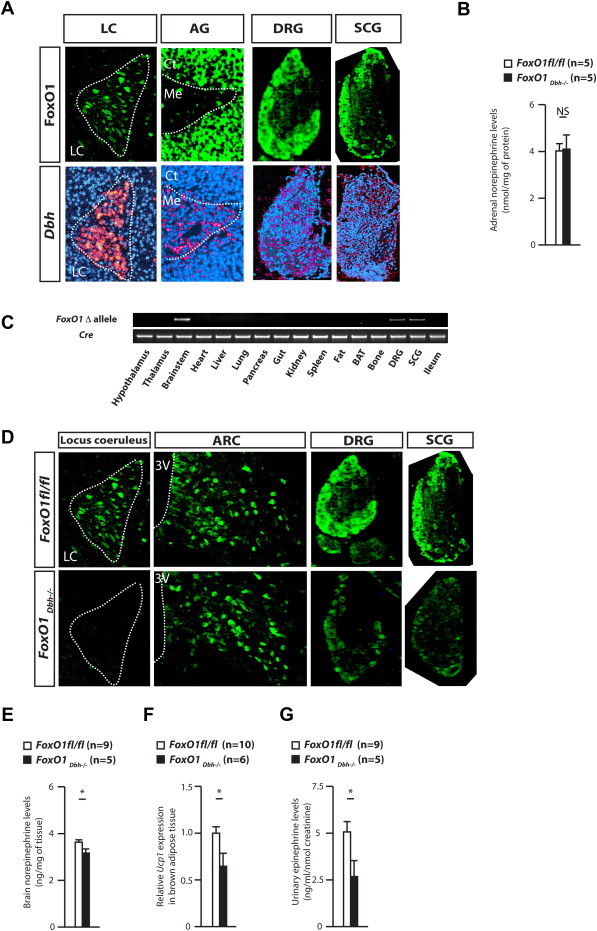
(A) FoxO1 and Dbh expression in cells of the locus coeruleus (LC), adrenal gland (AG), the dorsal root ganglion (DRG), and superior cervical ganglion (SCG). Ct: Adrenal cortex. Me: Adrenal medulla. (B) Norepinephrine contents in FoxO1fl/fl and FoxO1Dbh−/− adrenal glands. (C) Detection of FoxO1 mutant allele in genomic DNA isolated from tissues from FoxO1fl/fl Dbh-cre (FoxO1Dbh−/−) male mouse. (D) Immunohistochemistry in the locus coeruleus, arcuate neurons, the dorsal root ganglion, the superior cervical ganglion of FoxO1fl/fl and FoxO1Dbh−/− mice using anti-FoxO1 antibody. 3V: Third ventricle. ARC: Arcuate nucleus. (E) Norepinephrine content in the brainstem of 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice. (F) Ucp1 expression in 36 week-old FoxO1fl/fl and FoxO1Dbh−/− brown fat. (G) Urinary epinephrine elimination of 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice.
Figure 2.
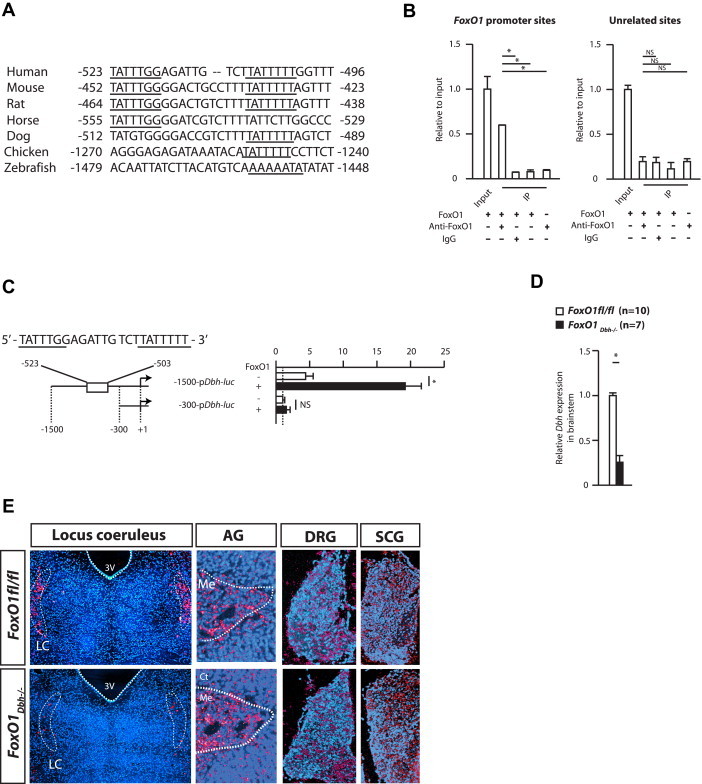
(A) Putative FoxO1 binding sites in the Dbh promoter in human, mouse, rat, horse, dog, chicken and zebrafish. (B) Binding of FoxO1 to the Dbh promoter detected by Chromatin immuno-precipitation (ChIP) assay. (C) DNA cotransfections assays in HEK cells using a FoxO1 expression vector and reporter vectors containing fragments of Dbh promoter fused to the Luciferase gene. (D) Dbh expression in FoxO1fl/fl and FoxO1Dbh−/− brainstem. (E) In situ hybridization analysis of the Dbh expression in the locus coeruleus, adrenal gland, the dorsal root ganglion, and superior cervical ganglion.
2.2. Molecular studies
In situ hybridization, and immunohistochemistry were performed as previously described in Ref. [11]. Briefly, mice were anesthetized, perfused with PBS followed by fixation in 4% paraformaldehyde. Brains were dissected, dehydrated, and embedded in paraffin. 5 μm sections were made and either hybridized against 35S-labeled probe for Dbh, or incubated with anti-FoxO1 or anti-Neuronal Nuclei (NeuN) antibody (cell signaling) followed by fluorescent conjugated secondary antibody. RNA isolation, cDNA preparation and real-time PCR analyses were carried out following standard protocols. For chromatin immuno-precipitation (ChIP) assay, HEK cells were transfected with flag-tagged FoxO1 expression vector. 48 h after transfection, cells were cross-linked with formaldehyde, lysed and sonicated to obtain sheared DNA fragments. Samples were immunoprecipitated with anti-FoxO1 antibody (Cell signaling), incubated with blocked beads, washed in low salt buffer, twice in high salt, twice in LiCl buffer and twice in TE buffer. Washed beads were then eluted in elution buffer, the DNA was reverse cross-linked and PCR analysis performed on ChIPed material with specific primers designed to detect the FoxO1 binding sites within the Dbh promoter. For DNA co-transfection assays, human Dbh 5′ promoter fragments corresponding to −1500 to +1(−1500-pDbh-luc) or −300 to +1 (−300-pDbh-luc) were cloned into pGL3 Luciferase reporter vector (Promega). HEK cells were transfected with flag-tagged FoxO1 expression vector, β-gal reporter vectors and −1500-pDbh-luc, or −300-pDbh-luc luciferase reporter vectors. 48 h after DNA transfections, luciferase and β-gal assays were performed using standard procedure [11].
2.3. Metabolic analyses
For glucose tolerance test (GTT), mice were fasted 16 h, glucose (2 g/kg) was administrated by intraperioneal (i.p.) Injection, and blood glucose was monitored at the indicated time points. Insulin tolerance test was performed after 4 h of fasting, insulin (Humulin, Lilly: 0.5 units/kg) was injected i.p., and blood glucose monitored at the indicated time points. For the glucose-stimulated insulin secretion test, mice were fasted 16 h, glucose (3 g/kg) was injected i.p., and serum was collected from tail veins at the indicated time points. Insulin levels were measured using an ELISA according to the manufacture's instruction (Crystal Cehm). For food intake study, mice were housed individually in metabolic cages (Nalgene) and fed ad libitum. Food consumption was determined by the weight change of the powdered chow before and after 24 h of measurement. The energy expenditure measurements were performed with Oxymax system (Columbus instruments) as previously described in Ref. [11].
2.4. Bioassays
Serum PINP, CTx (IDS), Urinary epinephrine (ALPICO) were measured with ELISA kit according to manufactures' instructions. Osteocalcin levels were measured by ELISA [11]. Norepinephrine levels in brainstem, adrenal gland and dopamine levels in the brainstem were measured by HPLC as described previously in Ref. [4,11].
2.5. Bone histomorphometry analyses
Bone histomorphometry analysis was performed as previously described in Ref. [11]. Briefly, lumber vertebrate were dissected, fixed for 24 h, dehydrated with ethanol, and embedded in MMA. Von Kossa/von Gieson staining, toluidine blue staining, and tartrate-resistant acid phosphatase (TRAP) staining were used to measure bone volume over tissue volume (BV/TV), osteoblast number, and osteoclast surface per bone surface, respectively. Bone formation rate was analyzed by calcein double-labeling method. Histomorphometric analyses were performed by using the Osteomeasure analysis sytem (Osteometrics).
2.6. Statistics
Results are given as means ± standards error of the mean (SEM). Statistical analyses were performed using unpaired Student's t-test. In all figures, *P < 0.05, NS: not significant.
3. Results
3.1. Study of the pattern of expression of FoxO1
Prior to embark in the study of the functions of FoxO1 in catecholamine-producing cells, we asked whether it was expressed in these cells. We used as a marker of the identity of catecholamine-producing cells expression of dopamine β-hydroxylase (Dbh) [14]. An immunohistochemical analysis showed that FoxO1 was highly expressed in Dbh-expressing neurons of the locus coeruleus in the brain and in neurons of the dorsal root and superior cervical ganglions that relay the sympathetic tone (Figure 1A). In contrast, FoxO1 could not be detected in neurons of the myenteric or submcosal ganglions in the ileum, or in catecholamine-producing cells of the adrenal medulla in the adrenal glands (Figure 1A and Supplemental Figure 1A). In agreement with this latter result, catecholamine content in adrenal is normal in FoxO1Dbh−/− mice (Figure 1B). These results indicate that FoxO1 is expressed in some neurons of the sympathetic nervous system.
To delete FoxO1 only in catecholamine-producing cells, we bred mice expressing a floxed allele of FoxO1 [15] with transgenic mice expressing Cre under the control of the regulatory elements of the Dbh gene (FoxO1Dbh−/−). Dbh, which encodes the initial enzyme in the biogenesis of catecholamines [14], is expressed in all cells that are responsible for catecholamine production, and these deletor mice have already been shown by others and us to delete genes in those cells [11,12]. A PCR-based analysis showed that in the brain of male mice, FoxO1 had been efficiently deleted only in neurons of the brainstem in which the locus coeruleus is located, but not in any other parts of the brain. In the periphery, FoxO1 deletion was observed in the dorsal root ganglia, and the superior cervical ganglion, but not in the ileum (Figure 1C).
We next examined, in cells where FoxO1 and Dbh are co-expressed, whether FoxO1 had been deleted appropriately in FoxO1Dbh−/− mice. FoxO1 expression has essentially vanished in the neurons of the locus coeruleus of FoxO1Dbh−/− mice (Figure 1D and Supplemental Figure 2). This was specific since FoxO1 was normally expressed in other parts of the brain such as the arcuate nucleus (ARC) of the hypothalamus in FoxO1Dbh−/− mice (Figure 1D). In contrast, FoxO1 expression in the dorsal root ganglion, or the superior cervical ganglion was reduced, but still detectable (Figure 1D). For all subsequent experiments, we used as controls FoxO1fl/fl mice since we have already demonstrated that Dbh-cre mice do not display any abnormality of the sympathetic tone, energy metabolism or bone mass [11].
3.2. Low sympathetic tone in FoxO1Dbh−/− mice
Male FoxO1Dbh−/− mice were born at the expected mendelian ratio, had a normal life expectancy and no overt physical abnormalities. Since there was evidence that FoxO1 may lie downstream of adiponectin signaling in the brain, we analyzed FoxO1Dbh−/− mice at the same age and the same sex of Adiponectin−/− mice showed the most overt disruption of the central control of bone mass, i.e., 9 months of age [11]. Three lines of evidence indicated that the deletion of FoxO1 from sympathetic neurons affected the activity of the sympathetic nervous system. First, brain norepinephrine content was decreased in FoxO1Dbh−/− compared with FoxO1fl/fl mice (Figure 1E). Of note, dopamine contents were normal in FoxO1Dbh−/− mice (Supplemental Figure 1C). Second, expression of Ucp1 in brown fat, a biomarker of the activity of the sympathetic nervous system, was decreased approximately 50% in FoxO1Dbh−/− compared with FoxO1fl/fl mice (Figure 1F). Third, urinary epinephrine elimination was also decreased by 50% in FoxO1Dbh−/− mice compared with control animals (Figure 1G). Together, these data established that FoxO1, through its expression in sympathetic neurons, is necessary for the activity of the sympathetic nervous system.
3.3. FoxO1 regulates Dbh expression
Next, we searched for molecular mechanisms that would account for the FoxO1 regulation of the activity of the sympathetic nervous system. In view of the decrease in the norepinephrine content in FoxO1Dbh−/− brain, we studied the 5′ regulatory sequences of the Dbh gene. This analysis identified two putative FoxO1 binding sites in the promoter of this gene in the mouse that are located close to each other between −428 and −434, and between −446 and −452. These two putative FoxO1 binding sites are conserved in the human and rat Dbh promoter, and at least one of them is conserved in the promoter regions of the horse, dog, chicken and zebrafish Dbh gene (Figure 2A).
We first tested if FoxO1 binds to these putative binding sites in the Dbh promoter through a Chromatin Immuno-Precipitation assay (ChIP). This experiment showed that FoxO1 was able to bind to the Dbh promoter region (Figure 2B). Next, we assessed the biological relevance of these binding sites in cell culture and in vivo. In DNA co-transfection assays performed in HEK cells that do not express the Dbh gene, FoxO1 could trans-activate a vector containing a 1.5 kb-long fragment of Dbh promoter fused to the Luciferase gene (−1500-pDbh-luc), which includes the two FoxO1 binding sites. In contrast FoxO1 failed to transactivate a vector containing a shorter fragment of Dbh promoter that lacks these FoxO1 binding sites (−300-pDbh-luc) (Figure 2C). To establish in vivo that FoxO1 is necessary for Dbh expression, we analyzed expression of this gene in the brainstem of FoxO1Dbh−/− and control mice by quantitative PCR, and observed a decrease in Dbh expression in the FoxO1Dbh−/− brainstem (Figure 2D). More precisely, an in situ hybridization analysis showed that Dbh expression in neurons of the locus coeruleus has essentially disappeared in FoxO1Dbh−/− mice (Figure 2E). In contrast, there was no overt decrease of Dbh expression in neurons of the dorsal root ganglion, or superior cervical ganglion in FoxO1Dbh−/− compared with control mice, the same was true for medulla of the adrenal gland (Figure 2E). Taken together, these results demonstrate that FoxO1 enhances the activity of the sympathetic nervous system by favoring Dbh expression in sympathetic neurons.
3.4. Decreased energy expenditure in FoxO1Dbh−/− mice
To assess the consequences on the entire animal of the FoxO1-dependent regulation of the sympathetic nervous system, we analyzed, in FoxO1Dbh−/− and control mice fed a normal diet, physiological processes that are regulated by the sympathetic nervous system.
First, we noticed that FoxO1Dbh−/− mice have a significantly lower energy expenditure than control animals as determined by decreased oxygen consumption, carbon dioxide production and heat generation measurements (Figure 3A). In contrast, appetite was normal in FoxO1Dbh−/− mice (Figure 3B). This decrease in energy expenditure in the face of a normal appetite explained, in part, why FoxO1Dbh−/− mice have higher fat pad weights compared with control mice (Figure 3C). Since insulin secretion is regulated by the sympathetic tone [16], we measured insulin secretion and glucose tolerance in FoxO1Dbh−/− and control mice. Glucose-stimulated insulin secretion test showed that insulin secretion was enhanced in FoxO1Dbh−/− compared with FoxO1fl/fl mice (Figure 3D), this is consistent with the decreased activity of the sympathetic nervous system noted in FoxO1Dbh−/− mice. As a result, a glucose tolerance test (GTT) showed that FoxO1Dbh−/− mice exhibited improved glucose tolerance, whereas insulin sensitivity as measured by an insulin tolerance test (ITT) remained normal in FoxO1Dbh−/− mice (Figure 3E,F). These results indicate that FoxO1, through its expression in sympathetic neurons, because it regulates positively the sympathetic tone, affects energy expenditure and insulin secretion.
Figure 3.
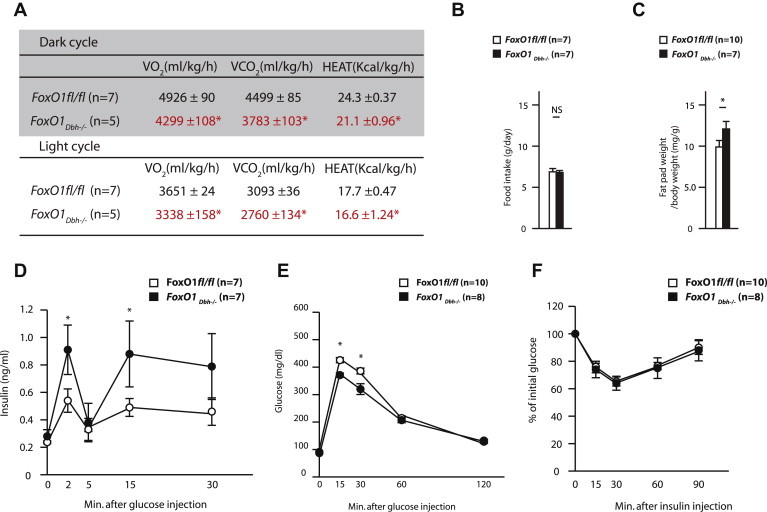
(A) Energy expenditure of 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice. (B) Food intake of 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice. (C) Fat pad weight of 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice. (E and F) Glucose-stimulated insulin secretion test (GSIS), glucose tolerance test (GTT), and insulin tolerance test (ITT) of FoxO1fl/fl and FoxO1Dbh−/− mice.
3.5. High bone mass in FoxO1Dbh−/− mice
Since the sympathetic tone regulates bone mass accrual [5,17], we analyzed bone mass in FoxO1Dbh−/− and control mice. A bone histomorphometric analysis revealed the existence of a high bone mass phenotype in 9 month-old FoxO1Dbh−/− mice (Figure 4A). As expected, given the cellular nature of the sympathetic regulation of bone mass [18], this phenotype had two cellular bases. First, there was an increase in bone formation parameters as measured by the osteoblast number and bone formation rate (BFR). Serum PINP levels verified the existence of an increase in bone formation whereas analysis of Cyclin gene expressions confirmed that there was an increase in osteoblast proliferation in FoxO1Dbh−/− mice (Figure 4B,C). Serum osteocalcin levels were higher in FoxO1Dbh−/− mice (Supplemental Figure 3). Second, there was also a decrease in bone resorption parameters in FoxO1Dbh−/− mice as determined by osteoclast surface (Figure 4A) and serum Ctx levels, a byproduct of collagen degradation and a biomarker of bone resorption (Figure 4D). The same was true for Rankl expression (Figure 4E). Thus, FoxO1, through its expression in sympathetic neurons, exerts a negative regulation of bone mass accrual because it favors the activity of the sympathetic nervous system.
Figure 4.
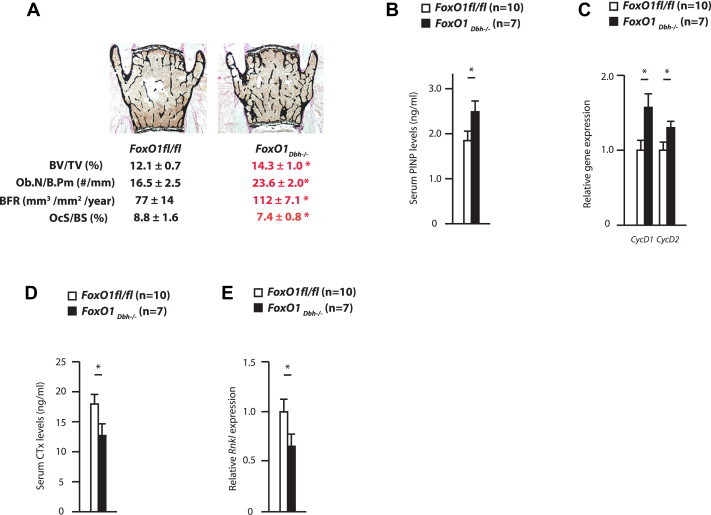
(A) Bone histomorophometric analysis of 36 week-old FoxO1fl/fl (n = 10) and FoxO1Dbh−/− (n = 7) mice. BV/TV: Bone volume per tissue volume. Ob.N/B.Pm: Number of osteoblasts per bone perimeter. BFR: Bone formation rate. OcS/BS: Osteoclast surface per bone surface. (B) Serum PINP levels in 36 week-old FoxO1 FoxO1fl/fl and FoxO1Dbh−/− mice. (C) Cyclin expression in 36 week-old FoxO1fl/fl and FoxO1Dbh−/− bone. (D) Serum Ctx levels in 36 week-old FoxO1fl/fl and FoxO1Dbh−/− mice. (E) Rankl expression in 36 week-old FoxO1fl/fl and FoxO1Dbh−/− bones.
4. Discussion
The results presented in this study demonstrate that, in the mouse, the transcription factor FoxO1, is expressed in neurons of the locus coeruleus and that it favors Dbh expression and catecholamine synthesis. As a result, FoxO1 regulates physiological processes such as energy expenditure, glucose metabolism, and bone mass accrual that are under the influence of the sympathetic nervous system.
FoxO1 can exert multiple functions in a given cell type; it can, for instance, hamper proliferation and favor apoptosis [9]. In addition, FoxO1 is involved in multiple developmental, physiological and pathological processes such as angiogenesis, organismal growth, insulin signaling, bone mass, and tumorgenesis [13,19,20]. FoxO1 can also be phosphorylated by AKT, an event that results in the accumulation of FoxO1 into the cytoplasm and therefore prevents its transactivating function [21]. Given the number of cellular events it affects or are affecting it, and its broad spectrum of expression, it comes as no surprise that FoxO1 activity is affected by several extracellular cues chief among them being insulin [22]. In essence, FoxO1 can be seen as a molecular bridge between several extracellular cues and the genome of a large number of cell types [9,10].
In the nervous system for instance, FoxO1 has been proposed to influence appetite through its expression in hypothalamic neurons [23], and energy balance [24]. Results presented here indicate that a major function of FoxO1 is to favor the synthesis of catecholamines through its regulation of Dbh expression in sympathetic neurons of the locus coeruleus and sympathetic ganglion. These results were observed in male mice because the Dbh-cre deletor mice delete genes in catecholamine-producing cells only in male mice (Figure 1C and Supplemental Figure 1B). That female Dbh-cre mice do not delete genes with the same specificity than their male counterparts may be explained by the site of integration of the transgene. Because the locus coeruleus and the hypothalamus are not the only structure where FoxO1 is expressed in the brain, it is likely that FoxO1 may have other functions in the brain. Immunohistochemical results presented in Figure 1D suggest that FoxO1 deletion was more effective in the locus coeruleus. This is also suggested by the fact that Dbh expression was more severely decreased in the locus coeruleus than in sympathetic ganglions in FoxO1dbh−/− mice. Nevertheless, there is a need of Cre deleter mouse strains with an even more restricted pattern of expression than the Dbh-Cre mice to precisely delineate the contribution of FoxO1 expression in the locus coeruleus and in the dorsal root and superior cervical ganglions in modulating the sympathetic tone.
In recent years, the transcriptional control of the activity of the sympathetic nervous system began to be investigated. It was shown for instance that CREB, through its expression in neurons of the ventromedial hypothalamic nuclei inhibits the activity of the sympathetic tone [7]. This study however, did not address the nature of the transcriptional events that take place in neurons of the locus coeruleus and that determine the output of the sympathetic tone. Our results identify FoxO1 as such a transcription factor in vivo. To further improve our understanding of this central control of energy metabolism and bone mass accrual, it will now be important to determine if Creb, through its hypothalamic expression, affects FoxO1 expression in neurons of the locus coeruleus and of other catecholamine-producing neurons.
The possible involvement of FoxO1 in the regulation of the sympathetic tone, and thereby, of energy expenditure and bone mass accrual was only suggested by the fact that FoxO1 phosphorylation, i.e., its inactivation, was induced when treating cells with adiponectin, a negative regulator of the activity of the sympathetic nervous system [11]. The clear regulation of the activity of the sympathetic nervous system exerted by FoxO1 demonstrated here together with the regulation of its activity by adiponectin in neurons of the locus coeruleus naturally raise a question of the nature of the receptor used by adiponectin to mediate its function in osteoblasts and neurons of the locus coeruleus. Identifying this receptor is the next step in our investigation.
Acknowledgments
This work is supported by grant AG032959-01A1, DK067936 from National Institutes of Health and grant AG-SS-2730-11 from the Ellison Foundation (G.K.).
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Karsenty G., Oury F. Biology without walls: the novel endocrinology of bone. Annual Review of Physiology. 2012;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducy P., Amling M., Takeda S., Priemel M., Schilling A.F., Beil F.T. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 3.Karsenty G., Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oury F., Khrimian L., Denny C.A., Gardin A., Chamouni A., Goeden N. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elefteriou F., Ahn J.D., Takeda S., Starbuck M., Yang X., Liu X. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 6.Fu L., Patel M.S., Bradley A., Wagner E.F., Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Oury F., Yadav V.K., Wang Y., Zhou B., Liu X.S., Guo X.E. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes & Development. 2010;24:2330–2342. doi: 10.1101/gad.1977210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav V.K., Oury F., Suda N., Liu Z.W., Gao X.B., Confavreux C. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgering B.M., Kops G.J. Cell cycle and death control: long live Forkheads. Trends in Biochemical Sciences. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 10.Coffer P.J., Burgering B.M. Forkhead-box transcription factors and their role in the immune system. Nature Reviews Immunology. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 11.Kajimura D., Lee H.W., Riley K.J., Arteaga-Solis E., Ferron M., Zhou B. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metabolism. 2013;17:901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K., Takahashi M., Matsushita N., Miyazaki J., Koike M., Yaginuma H. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. The Journal of Neuroscience. 2004;24:3480–3488. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rached M.T., Kode A., Xu L., Yoshikawa Y., Paik J.H., Depinho R.A. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metabolism. 2010;11:147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas S.A., Matsumoto A.M., Palmiter R.D. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 15.Paik J.H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonogai K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 17.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 18.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metabolism. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Dansen T.B., Burgering B.M. Unravelling the tumor-suppressive functions of FOXO proteins. Trends in Cell Biology. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Rached M.T., Kode A., Silva B.C., Jung D.Y., Gray S., Ong H. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. The Journal of Clinical Investigation. 2010;120:357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 22.Gross D.N., van den Heuvel A.P., Birnbaum M.J. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 23.Ren H., Orozco I.J., Su Y., Suyama S., Gutierrez-Juarez R., Horvath T.L. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K.W., Donato J., Jr., Berglund E.D., Choi Y.H., Kohno D., Elias C.F. FOXO1 in the ventromedial hypothalamus regulates energy balance. The Journal of Clinical Investigation. 2012;122:2578–2589. doi: 10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


